Neurological and metabolic related pathophysiologies and treatment of comorbid diabetes with depression
Abstract
Background
The comorbidity between diabetes mellitus and depression was revealed, and diabetes mellitus increased the prevalence of depressive disorder, which ranked 13th in the leading causes of disability-adjusted life-years. Insulin resistance, which is common in diabetes mellitus, has increased the risk of depressive symptoms in both humans and animals. However, the mechanisms behind the comorbidity are multi-factorial and complicated. There is still no causal chain to explain the comorbidity exactly. Moreover, Selective serotonin reuptake inhibitors, insulin and metformin, which are recommended for treating diabetes mellitus-induced depression, were found to be a risk factor in some complications of diabetes.
Aims
Given these problems, many researchers made remarkable efforts to analyze diabetes complicating depression from different aspects, including insulin resistance, stress and Hypothalamic–Pituitary–Adrenal axis, neurological system, oxidative stress, and inflammation. Drug therapy, such as Hydrogen Sulfide, Cannabidiol, Ascorbic Acid and Hesperidin, are conducive to alleviating diabetes mellitus and depression. Here, we reviewed the exact pathophysiology underlying the comorbidity between depressive disorder and diabetes mellitus and drug therapy.
Methods
The review refers to the available literature in PubMed and Web of Science, searching critical terms related to diabetes mellitus, depression and drug therapy.
Results
In this review, we found that brain structure and function, neurogenesis, brain-derived neurotrophic factor and glucose and lipid metabolism were involved in the pathophysiology of the comorbidity. Obesity might lead to diabetes mellitus and depression through reduced adiponectin and increased leptin and resistin. In addition, drug therapy displayed in this review could expand the region of potential therapy.
Conclusions
The review summarizes the mechanisms underlying the comorbidity. It also overviews drug therapy with anti-diabetic and anti-depressant effects.
1 INTRODUCTION
Depression is one of the comorbidities of diabetes mellitus (DM). The prevalence of depressive disorders in patients with type 2 diabetes mellitus (T2DM) has increased from 0.70 per cent in 2000 to 1.25 per cent in 2010.1 T2DM has a 1.35% relative risk of depressive disorder despite symptom severity.2 28.2% had increased their HbA1c since the pandemic began and 18.2% had uncontrolled T2DM.3 Among the top 25 causes of disability-adjusted life years (DALYs), depressive disorders ranked 13th, according to statistics from the Global Burden of Disease (GBD) 2019.4 It is estimated that major depressive disorder caused 49.4 million DALYs globally due to the COVID-19 pandemic in 2020.5
People experience increased levels of depressive and anxiety disorders in a highly stressful society.6, 7 Developments in neuropsychiatry have identified depression as a risk factor for some diseases, such as Alzheimer's.8, 9 Diabetes and depression have a bidirectional relationship, increasing each other's risk. Individuals with T2DM are at higher risk of experiencing depressive symptoms and displaying elevated levels of hyperglycaemic markers, as evidenced by the Maastricht Study.10 Conversely, it is feasible that depression could lead to the development of diabetes due to an elevated incidence of central obesity. Thus, depression appears to contribute to the progression of diabetes.11 Depression is a notable factor contributing to dementia and cognitive impairment in individuals with diabetes.12, 13 A cohort study has pointed out that people with diabetes and depressive disorder tend to develop hyperglycemic crisis episodes (HCE).14
The current understanding of depression is primarily centered around receptor theory. This includes the N-methyl-D-aspartate receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, glucocorticoid receptor, 5-hydroxytryptamine receptor, GABAA receptor α2, and dopamine receptor. Thus, the mainstream therapy for depression in DM is SSRIs, which remain 5-HT at the receptor sites to exert an anti-depression effect.15 However, the explicit mechanisms behind the comorbidity of DM and depression are more complicated than depression, as much research has revealed that emotions affected our capacity to control our body.16, 17 The recent attention towards the pathophysiology underlying DM with depression focused on insulin resistance, oxidative stress, inflammation and the nervous system.18 For instance, high insulin resistance in patients with T2DM had a close association with depressive symptoms.19 Furthermore, a higher systemic immune-inflammation (SII) index had been found in patients with DM and depressive disorder.20 The numerous articles exploring other mechanisms, such as the HPA axis and adipokine, suggest the diversity of mechanisms behind comorbidity. Higher depression severity was found to correlate positively with higher midnight cortisol levels.21 Patients with diabetes presented the worst glycemia control, the most obese and the worst executive functions, and the reduction of executive functions resulted from depressive symptoms.22 Also, the relationship between uncontrolled diabetes and depression had been possibly mediated by central adiposity.11 The main goal of treatment in comorbidity is to improve glycemic control and alleviate depression symptoms. However, the above findings suggested that the mainstream therapy for depression, such as SSRIs,23 focusing on receptor therapy, found it hard to meet the multi-faceted pathology of depression in DM. It was found that not all treatments, which are adequate to attenuate depressive symptoms, can control blood glucose levels.24 For example, sertraline has been proven to alleviate depression but does not affect blood glucose.25 Analyses about the drug with both anti-diabetic and anti-depressant effects increased. For instance, CBD treatment reduced depressive symptoms, as evidenced by the altered level of 5-HT, NA and/or DA, and reduced glycemia, as evidenced by a remarkable increase in weight gain and the level of insulin.26, 27
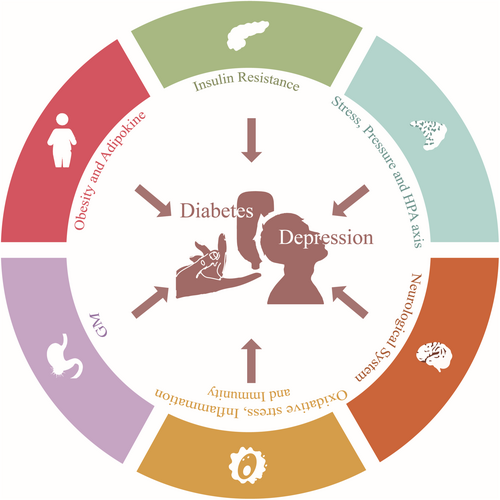
Therefore, it is urgent to discover the exact pathogenesis underlying the comorbidity between depressive disorder and diabetes mellitus and explore alternative drug therapy that is more effective and has fewer adverse effects. Given the significant research investigating the mechanisms and potential drugs for treatment, this article reviews the pathophysiology (Figure 1) and drug alternatives of depression with comorbid diabetes mellitus.
2 METHODS
The current review refers to the available literature in PubMed and Web of Science. The critical terms related to diabetes mellitus, depression and drug therapy. Full-length articles and abstracts were screened for the collection of information included in the paper. We included clinical studies discovering the mechanisms of DM with depression in human subjects and experimental studies exploring the pathology of the comorbidity and drug therapy in animal models. Articles informed by observational studies and clinical trials, review or meta-analysis articles relevant to the topic, and other publications cross-referenced for additional published articles were included. Studies were excluded if they failed to discuss the mechanisms and therapy in this field; unpublished data and conference abstracts were also excluded. The main concern in the treatment in this review is natural products for the following two reasons. On the one hand, compared to the traditional therapy against depression and diabetes, such SSRIs, SNRIs (Serotonin and norepinephrine reuptake inhibitors), insulin, metformin and pioglitazone, many natural products were found to have anti-diabetic and anti-depressant effects and have an ameliorative effect on complications in DM. SSRIs are clinically used in treating major depressive disorder28 and depression in DM but are a risk factor for some complications in DM. On the other hand, insulin resistance, HPA axis, neurological system, GM oxidative stress, inflammation and obesity contributed to the pathology of the comorbidity of DM and depression. Much research has discovered the performance of natural products on these mechanisms. Therefore, we summarize SSRIs, SNRIs, insulin, metformin, pioglitazone and natural products to find potential drugs for comorbidity.
3 MECHANISMS
3.1 Insulin resistance
Individuals with higher insulin resistance in T2DM display more depressive symptoms, such as irritability, anhedonia, fatigue, and hypersomnia.19 Additionally, rats exposed to HDF followed by STZ injection showed induced insulin resistance.29 The association between markers of hyperglycemia and insulin resistance and prevalent depressive symptoms was common.10 The interaction between insulin and its receptor leads to the stimulation of insulin receptor substrate (IRS) proteins, which then activate two principal insulin signaling pathways: the phosphatidylinositol 3-kinase (PI3K)-AKT/protein kinase B (PKB) pathway and the ras-mitogen-activated protein kinase (MAPK) pathway. Research findings have indicated that insulin resistance is linked to the IRS and PI3K-AKT/PKB pathways. The correlation between IRS1 and Langerhans' islets was weakened due to a number of indirect intermediate steps involved in the connection between reduced phosphorylated IRS1 level and destruction of β-cells.30 In diabetic mice and renal cells exposed to high glucose, reduced IRS-1 levels caused significant up-regulation of tumor protein 63 (TP63), which demonstrated the potential of TP63 in regulating IRS-1 and insulin resistance.31 Furthermore, PI3K-generated phosphatidylinositol-(3,4,5)-triphosphate facilitated the activation of various serine/threonine kinases dependent on phosphatidylinositols-(3,4,5)-triphosphate, particularly AKT/PKB.32 Both angptl7 and secreted frizzled-related protein (sFRP) 4 promoted insulin resistance by inhibiting the activation of Akt.33, 34 Exercising increased glucose uptake rate in T2DM by PI3K and AS160 activity.35
The impairment of Insulin receptors also leads to DM and depression. There are two primary insulin receptor (IR) isoforms, A and B. Insulin-like growth factor-2 (IGF-2) activated IR-A, which was exclusively expressed by neurons and displayed high binding affinity.36 In mice lacking astrocyte IR, brain slices showed impaired dopamine release, and the latter increased depression symptoms. The situation was mainly because astrocytic insulin signaling regulated the phosphorylation of Munc18c and the exocytosis of ATP via syntaxin-4, which modulated the activity of presynaptic dopaminergic neuronal and the release of dopamine.37 Research showed that the minor alleles of both rs2245649 and rs2229429 in the insulin receptor gene (INSR) were risk factors for poor glycaemic control in type 1 diabetes mellitus (T1DM) and were associated strongly with the absence of anti-insulin antibodies (IAs) in T1DM.38 Experimental research revealed that reducing the amount of 5′-nucleotidase, cytosolic II (NT5C2), and CD36 inhibited insulin signaling by suppressing insulin receptors.39 Insulin and insulin-like growth factor-1 (IGF-1) may contribute to antidepressive-like behavior, yet the antidepressive-like effect was hindered by JB-1, an IGF receptor antagonist.40 A study demonstrated that DM mice could be induced anxiety- and depressive-like behaviors by knock-out IR on astrocytes.37
3.2 Stress and hypothalamic–pituitary–adrenal (HPA) axis
It has been noted that the messed up HPA axis plays a part in the emergence of depression. The boost in serum corticosterone among diabetic patients and rats suggests that the HPA axis is too active.41-43 Among patients with T2DM, there was an association between low fasting levels of 2-h C-peptide and higher levels of midnight cortisol and higher severity of depression.21 Research revealed that stressor demands and stress perceptions were associated with depressive symptoms. According to the longitudinal pattern, hair cortisol responded to contextual features related to anticipation, novelty/familiarity, and social evaluative threat.44 Another study found that morning cortisol levels were inversely correlated with the frequency of using effective prosocial coping strategies.45 Both suggest that although stress increased cortisol levels and depressive symptoms, they followed distinct trajectories.44 Depressive symptoms were associated with blunted and exaggerated cortisol responses to and recovery from stress, indicating the high risk of HPA axis dysregulation.46 It was found that hsa_circ_0111707, mediated a negative correlation between scores of “demands at work” and “insecurity at work,” was associated with the risk of type 2 diabetes mellitus via sponging miR-144-3p.47 According to the neuroplasticity hypothesis, glucocorticoids (GC) negatively affected neural development. According to the neurogenesis hypothesis, GC negatively affected neural precursor cell proliferation. This phenomenon was proven by the fact that indirectly, anti-depressants reversed GC's adverse effects on neural precursor cells via the neuron–noradrenaline–cAMP response element-binding protein (CREB) pathway and/or the astrocytes–fibroblast growth factor 2 (FGF2) pathway.48
The hyperactivity of the HPA axis has an association with DM. In patients with T2DM, GC resistance resulted in the hyperactivity of the HPA axis and hypercortisolism. High GCs led to hyperglycemia. In GC-sensitive peripheral tissues, the glucocorticoid receptor (GR) regulated glucose production, uptake, and insulin signaling.49 Rat hepatocytes lacking GR inhibited hyperglycemia development, indicating that liver-specific GC antagonists may help control hyperglycemia.50 GLP-1 receptor (GLP-1R) agonists activated the acute neuroendocrine responses to stress. Diabetes mellitus, as a stressful metabolic situation for cells, induced chronic activation of the HPA axis in the long term, perhaps contributing to insulin resistance.51 After inhibiting FoxO1, insulin regulates adrenal steroidogenesis by increasing the expression and activity of steroidogenic factor 1 (SF-1).52
3.3 Neurological system
3.3.1 Brain structure and function
It was proven that there was a close association between abnormality of the fornix, hippocampus and prefrontal cortex and depression with comorbid DM. In T2DM, hippocampal atrophy occurred asymmetrically, with more significant atrophy occurring on the right than the left, probably leading to cognitive impairment.53 In the hippocampal CA1 region, diabetes increased the number of apoptotic cells, astrocytes, and mitotic activity and stimulated apoptosis and cell proliferation54 (Figure 2). The research found that in mice with T2DM, fewer new neurons were formed in the hippocampus. There was a deficit in the clone formation capacity of neural progenitor cells. In hippocampal neurospheres, insulin and epidermal growth factor receptors decreased.55 Similarly, many neuropsychiatric diseases had abnormalities in tissues and organs56 like the brain.57 For example, MDD also had hippocampal volume reductions, especially for those with more severe depression.58 Furthermore, in the depression subject, neurons' and glial cells' volume and number were decreased by 20% to 35% across all hippocampal regions.59 The reduction of systemic vascular health, considering diabetes mellitus, had a close relationship with loss of microstructural integrity in the fronix and decline of FA in the frontix and the hippocampal cingulum.60, 61 The myelin integrity disruption in the fornix was also found in patients with rMDD.62 It was revealed that in individuals with T2DM and MDD, cognition and brain structural connectivity were involved in a polygenic risk of T2DM.63 It was discovered that the prefrontal–hippocampal circuits originated from the fornix, output fibers to the hippocampus and went back to the prefrontal cortex.64
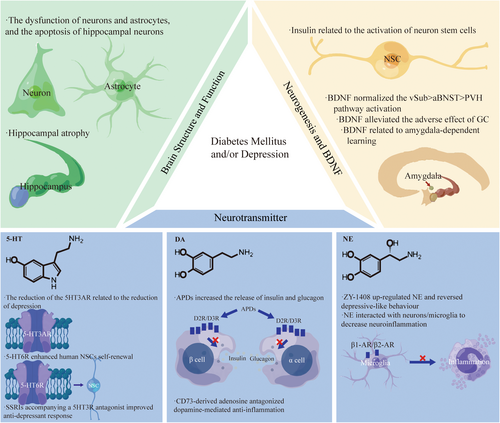
Much research had explored the molecular regulation of the neurological system in neuropsychiatric diseases, such as mitochondrial impairment.65 Although the molecular regulation of prefrontal-hippocampal circuits remains unclear, many studies focused on the relationship between the Glu-Gln cycle and these brain regions. Glu-Gln cycle inhibition, anaerobic glycolysis increase, and defects in the lactate-alanine shuttle may be associated with diabetic depression (DD) development in rats. The latter was also linked to the dysfunction of neurons and astrocytes.66 DD rats demonstrated depressive-like behavior and monoamine neurotransmitter deficiency due to mitophagy-induced apoptosis of hippocampal neurons through aberrant Glu-GluR2-Parkin pathways.67 The deep brain stimulation in the frontix could up-regulate the hippocampus's glucose metabolism by down-regulating the old mice's extracellular glutamate levels.68 In addition, the changes in Glu metabolism in the hippocampus and prefrontal cortex were associated with cognitive impairment and depressive symptoms linked to early life stress.69
3.3.2 Neurogenesis and BDNF
An experimental study showed that hyperglycemia in diabetes led to apoptosis and pyroptosis of hippocampal neuron cells, thus resulting in depressive phenotypes.70 Islet Antigen-2 (Ia-2) loss and over-expression, which was required for insulin secretion of neurons, induced the proliferation of glia and production of neural stem cells (NSC) from glia by regulating Drosophila insulin-like peptide 6 (Dilp-6).71 The evolutionarily conserved pseudokinase Tribbles (Trbl) promoted the degradation of Cdc25String to induce the quiescence of G2 NSCs. Trbl inhibited the activation of Akt subsequently to maintain quiescence. But insulin signaling allowed neural stem cells (NSCs) to exit quiescence by overriding repression of Akt and silencing trbl transcription.72 The phosphorylation of cAMP response element-binding protein (CREB) and the CREB target genes expression in the hippocampus was inhibited by a high-fat diet (HFD). Still, NSC-derived exosomes (exo-NSC) induced the CREB recruitment to restore Sirt1, nNOS, BDNF, RelA genes, and Egr3 transcription.73 Mob4, Cka, and Hsp83 reactivated NSCs by activating insulin receptor(InR)/PI3K/Akt pathways74, 75 (Figure 2).
It has been shown that there is increased serum cortisol and miR-128 and decreased levels of shortened telomeres and BDNF in patients with T2DM and depression.29, 76 The over-expression of BDNF promoted the polarization of M2 macrophage, thus repressing diabetes mellitus-accelerated atherosclerosis (DMAS) development by the inactivation of the STAT3 pathway.77 In the intact and diabetic brain, BDNF-driven hippocampal activity was significant in maintaining the vulnerability towards stress vulnerability, because BDNF over-expression (BDNFOE) could normalize the ventral subiculum (vSub) > anterior bed nucleus of stria terminalis (aBNST) > paraventricular hypothalamus (PVH) pathway activation.78 In addition, the research found that the increase in platelet BDNF levels had significant correction with left amygdala responses, suggesting the importance of BDNF in amygdala-dependent learning in MDD.79 According to the neuroplasticity hypothesis, anti-depressants and BDNF could alleviate the adverse effects of GC on neural morphology. The phenomenon was because ketamine improved neural morphology through the BDNF–mammalian target of rapamycin (mTOR) pathway48 (Figure 2).
3.3.3 Neurotransmitter
Serum serotonin (5-HT) was found to have a robust correlation with blood glucose and depression.80 Depression individuals had higher platelet 5-HT than the healthy group, whose Hamilton Depression (HAMD) scores were significantly decreased by the administration of SSRIs for 4 weeks.81 However, patients with recurrent depressive disorder (RDD) showed higher plasma serotonin concentration and lower platelet serotonin content, which was associated with psychotic symptoms.82 The level of 5-HT in the hippocampus, the expression of the brainstem's 5HT3A receptor, and tryptophan hydroxylase two were found to be reduced in Neuroplastin 65 knock-out (Np65 KO) mice, displaying reduced depressive-like behaviors partially.83 The administration of SSRIs accompanying a 5-HT3 receptor (5HT3R) antagonist improved anti-depressant response.84 However, the activation of the 5-HT6 receptor (5-HT6R) enhanced human NSC's self-renewal to induce human cerebral organoids' expansion and folding, and the mice without the 5-HT6R gene showed depression-like behaviors.85 In addition, the hippocampus in SDT fatty had a significant decrease in 5-HT concentrations86 (Figure 2).
Problems with the brain's dopamine system correlate with DM and depression. In STZ mice, dopaminergic neurotransmission was downregulated in the amygdala.87 Dopamine (DA) was α2A-adrenergic receptors' biased agonist, so antipsychotic drugs (APDs), which blocked DA D2-like receptors in islets, remarkably increased the release of insulin and glucagon.88 The reduction of β-cell functional insulin secretion was accompanied by the decrease in endocrine dopamine D2 receptor and dopamine D3 receptor.89 Furthermore, because inflammation plays a vital role in the pathogenesis underlying diabetes and comorbid depression, which will be mentioned in the following article, CD73-derived adenosine activated A2A to antagonize dopamine-mediated anti-inflammation, thus enhancing inflammation.90 Exercise increased DA serum levels, which enhanced the anti-inflammation of activity in return by inhibiting tumor necrosis factor (TNF) production of splenocytes.91 In diabetic septic mice, dopaminergic agonist type-1 inhibited splenic p65NF-kB phosphorylation to attenuate systemic inflammation and hyperglycemia.92 In addition, HFD exposure reduced TH levels and increased TH phosphorylation at serine 40 in the ventral tegmental area. These effects were associated with insulin resistance, increased tumor necrosis factor-α levels, oxidative stress, astrogliosis and microglia activation.93 Blunted central nervous system insulin receptor signaling through a HF diet could impair DA homeostasis, disrupting cognitive and reward circuitry in regulating hedonic feeding94 (Figure 2).
ZY -1408 remarkably up-regulated the hippocampus's norepinephrine (NE) and 5-HT extracellular concentrations and reversed depressive-like behavior.95 In the spinal cord and brain, remarkable regression of the locus coeruleus (LC)-NE system was associated with an increase in neuroinflammation and the activation of microglia.96 Neurons/microglia may interact with NE via β1-AR and β2-AR.97 CUMS caused a significant depletion of the norepinephrine transporter (NET).98 The interaction between depression and hypertension might be affected by the NET gene differential expression with the target towards TNF-α and IL-6.99 Chronically elevated NE decreased rates of net glucose uptake in the fetus through insulin resistance100 (Figure 2).
3.4 Oxidative stress, inflammation and immunity
A wide variety of dysfunctions in oxidative stress, inflammation, and immunity affect the comorbidity between depression and DM. A high level of oxidative stress, expressed by more elevated serum PAB (prooxidant-antioxidant balance) values and high serum C-reactive protein (CRP) levels, was found to be associated with depressive symptoms.101-103 MDD was associated with lower non-enzymatic antioxidants, rising pro-inflammatory pathways' activity and other apoptotic mediators' activity, like Caspase-3, resulting in neuronal death.104, 105 Because the brain increased oxygen consumption and lipid content and decreased anti-oxidative defense, it was more vulnerable to oxidative stress (OS), contributing to neurodegeneration.104 In addition, the higher plasma NO levels correlated with MDD106-108 and depressive symptoms, such as fatigue, weight loss, psychomotor retardation, sexual dysfunction, irritability, and indecisiveness.107 Consistently, NO blockers were proven to have anti-depressant effects in MDD patients.107 Furthermore, the nitric oxide synthase (NOS) genotype may be necessary in mediating peripheral NOx- concentration.108 NOS, especially nNOS and eNOS, contributed to the damage of uterine tissue in DM patients.109
Consistent with that the expression of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor–α (TNF-α) in T2DM rats increased.29 Patients with DM and depressive disorder also suffered from a higher level of the systemic immune-inflammation (SII) index and a lower level of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK).20, 110 In diabetes-depressed conditions, hippocampus microglia chemokine I receptor (CX3CR1) expression and several pro-inflammatory factors secretion, especially TNF-α, IL-6, IL-8, and IL-1β were up-regulated.111 Consistent with these researches, the TNFα inhibitor etanercept alleviated impaired recovery induced by prolonged learned helplessness and improved the blood–brain barrier (BBB).112 In addition, the evidence that the visual cortex anatomy in depressed patients was affected by a genetic variation, which selectively raised TNF-α expression, supported the notion that the anatomical changes were partially regulated by the genetic determinants within the activity of inflammation.113 Similarly, T2DM has also been found to have a high frequency of the G allele in the TNF-α -308G/A polymorphisms.114 Research also found that DN development in T2DM possibly resulted from the synergies of T-lymphocytes and the TNF-α signaling pathway.115
In MDD, there was an increase in the Th17:Treg ratio and elevated circulating T helper 17 (Th17) cell percentage.116 Moreover, patients with a higher risk of suicide showed a robust increase in Th17 cells.117 The microbiota was shown to increase Th17 cell production to promote depressive-like behaviors by producing the quorum sensing molecule autoinducer-2 (AI-2) and promoting the production of serum amyloid protein-1 (SAA1) and SAA2 by the host.118 In T2DM, disease-predictive inflammation was promoted by long-chain acylcarnitine combined with compromised β oxidation through activating Th17 inflammation.119 In T1DM with an SNP (rs12150220) in NLRP1, the levels of IL-17 and memory Th17 cells were decreased in peripheral blood mononuclear cells, proving that NLRP1 is a potential therapy target.120 In lean T2D patients, Th17-like CD4 + CXCR5+ T cells increased, which was associated with the positivity of autoantibody.121 The memory T and B cells induced an up-regulation of antibodies towards glutamic acid decarboxylase 65 (GAD65) of peripheral blood mononuclear cells (PBMCs) in T1DM. The memory T and B cells were selectively activated by EVs in PBMCs and human islet EVs.122
3.5 Gastrointestinal microbiome (GM)
Mechanism studies verified that the gastrointestinal microbiome is linked to the development of DM and depression. There was an association between a smaller decrease in Bacteroides fragilis and a higher increase in insulin sensitivity.123 Intermittent hypoxia (IH) exposures induced changes in GM, increased gut permeability, and altered plasma exosome cargo, the latter causing adipocyte dysfunction (increased IR).124 In overweight and obese participants, the associations between trimethylamine N-oxide (TMAO) changes and increased insulin sensitivity and glycemia were regulated by dietary fat intake.125 Dorea, Oscillospira, and Ruminococcus, which fermented polysaccharide into short-chain fatty acids (SCFAs), was abundant. Increased inflammation exhibited a low level of Turicibacter and a high level of Lactococcus, all of which suggest that the development of obesity may result from the alteration of gut microbiome via increasing systemic inflammation and insulin resistance.126 PHZ supplementation alleviates insulin resistance and attenuates gut microbiota alterations induced by HFD.124 The administration of fecal microbiota transplantation (FMT) improved insulin resistance and repaired impaired islets by inhibiting β-cell apoptosis.127
In the hippocampus, pro-inflammatory cytokines were regulated by GM through the dysfunction of the microbiota–gut–brain axis, aggravating the phenotypes of anxiety and depression.128 It was generally recognized that probiotics exerted anti-depressant and anxiolytic effects, but these effects were minor and concentrated on microbial diversity profile.129-131 Therefore, the pooled results of probiotics should be confirmed by trials with clinical samples.130
3.6 Obesity and adipokine
It was pointed out that central adiposity may mediate the relationship between uncontrolled diabetes and depression.11 Depression had synergistic effects with obesity on diabetes incidence in Chinese adults.132 Likewise, patients with diabetes and obesity had an increased risk of depression.133 Moreover, another research found that the link between insulin resistance and depression possibly resulted from the over-adjustment of central obesity.10 Furthermore, it was demonstrated that obesity, depression, and DM were associated with low adiponectin levels, high leptin levels, leptin resistance, and high resistin levels134-140 (Figure 3).
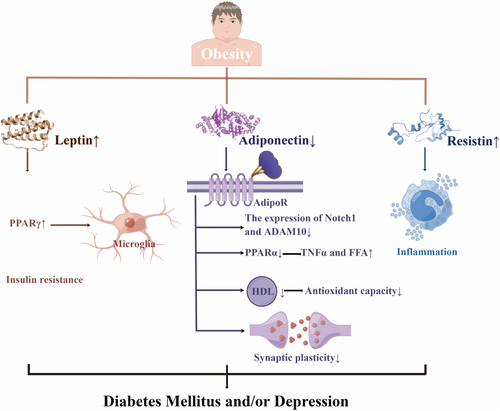
Adiponectin could increase fat metabolism, control insulin sensitivity, modify homeostasis, and regulate glucose tolerance to protect individuals from diabetes.135 Not only lower antioxidant capacity but also a worse effect of type 2 diabetes mellitus on high-density lipoprotein (HDL) functionality by rising APO AI, particle size, and cholesterol content, correlated with higher adiponectin.141 Adiponectin knock-out (Adp−/−) mice increased concentrations of TNFα and free fatty acid (FFA) by down-regulating peroxisome proliferator-activated receptor (PPAR)α levels, thus leading to insulin resistance.142 Adiponectin receptors (AdipoR) 1 and AdipoR2, respectively had a region-specific effect on anxiety-like behavior and fear memory extinction. This situation was proven by AdipoR1/2-dependent synaptic plasticity modulation as well as neuronal excitability.143 Chronic stress-induced hippocampal neurogenesis impairment and cognitive dysfunction may partially result from the inhibition of the Adiponectin-Notch pathway, which can be reversed by physical exercise. Adiponectin increased Notch1 and ADAM10144 (Figure 3).
Ob/ob mice, which are Leptin-related gene-deficient mice, showed depressive symptoms after CUMS, possibly by reducing the expression of PPARγ to down-regulate the activation phenotype of microglia.145 The reduction of leptin was associated with alleviating depressive symptoms in obese teenagers after weight loss therapy.146 Moreover, the Jackson heart study revealed that insulin resistance was crucial in mediating the correlation between leptin and T2DM.147 Endoplasmic reticulum (ER) stress, which was observed to inhibit leptin signaling, and the saturable leptin signaling pathways were involved in developing leptin resistance148 (Figure 3).
In the high-sucrose diet group, anxiety symptoms correlated with metabolic syndrome, which showed hyperglycemia and the reduction of leptin and resistin.149 The association between high resistin and reduced T2DM survival may partially result from the pro-inflammatory nature of resistin.150 Hyperglycemia in obesity and DM might enhance the expression of resistin from human mononuclear cells, and resistin might impair insulin sensitivity and promote systemic inflammation in return151 (Figure 3).
3.7 Metabolic problems due to atypical antipsychotics
In atypical antipsychotic-naive patients, the T2DM prevalence was 2.9%.152 According to a systematic review and meta-analysis, all antipsychotics were significantly associated with more weight-gain and a higher risk for a ≥ 7% clinically relevant weight-gain.153 Olanzapine lowered high-density lipoprotein cholesterol levels. It increased blood prolactin levels and body weight.154 Also, in bipolar disorder, olanzapine worsened HbA1c, weight gain and total cholesterol. In the bipolar depression group, olanzapine+fluoxetine worsened total cholesterol and weight gain.155 58.3% of registered clozapine clinic attenders were metabolic syndrome. 79.6% were overweight or obese. 46.6% had elevated fasting blood glucose and 55.2% had elevated blood triglycerides.156 The clozapine-treated group had higher level of diabetes mellitus, diagnosed hypertension and dyslipidemia.157 Risperidone-treated obese mice exhibited increased insulin resistance and glucose intolerance but decreased serum insulin levels. Risperidone could increase serum inflammatory cytokines, reduce pancreatic antioxidant enzyme activity and causes β-cell damage. Moreover, In the risperidone group, impaired glucose tolerance, increased IR and decreased IS were associated with reduced GLUT4 expression and Akt phosphorylation in insulin signaling.158 Potentially clinically significant elevations in clinical chemistry values included triglycerides.159 Quetiapine proved to be a relatively safe drug with the most common side effects: headache, somnolence, gastric upset, weight gain, and increased triglyceride levels.160
4 TREATMENT
4.1 SSRIs
According to an observational study, sertraline was conducive to ameliorating depression, and had no aggravating effect on HbA 1c in patients with T2DM.28 Likewise, FLX exerted an ameliorative impact on depressive phenotypes and systemic glucose dyshomeostasis, mainly because it down-regulated PPARγ and adipose triglyceride lipase (ATGL) in visceral white adipose tissue (vWAT), decreased leptin and up-regulated insulin signaling in the same tissue.161 Moreover, SSRIs had been found to reduce the risk of mortality among metabolic syndrome, including diabetes mellitus, complicating depression.162, 163 Polypharmacy with antipsychotics and psychotropics in bipolar disorder is associated with an increased risk of diabetes mellitus. Although SSRIs mono-class therapy had an association with a lower risk of DM, the risk of multi-drug combinations containing SSRIs was relatively high and largely depended on what the other drugs in that combination were.164 In skeletal muscle tissue, SSRIs could exert influence on the metabolism of energy, structure properties and electrical muscle activity.165 Therefore, reducing the administration of SSRIs could moderate the risk of first falls and fractures among individuals who have diabetes.166 Multivariate analysis suggested that SSRIs were the risk factor for CAN (cardiovascular autonomic neuropathy) in T1DM.167 In summary, SSRIs have the ability to ameliorate depressive-like behaviors in DM without exacerbating glucose dyshomeostasis, but are a risk factor for some complications in DM (Table 1).
| Natural compounds | Mechanisms and targets | Experiment type | Experiment subject | Insulin resistance | Neurological system | Oxidative stress, inflammation and immunity | Obesity and adipokine | Reference |
|---|---|---|---|---|---|---|---|---|
| Selective serotonin reuptake inhibitors | Down-regulated PPARγ and ATGL in vWAT | In vivo | C57BL/6N mice | √ | 34198047 | |||
| Decreased leptin | In vivo | C57BL/6N mice | √ | 34198047 | ||||
| Up-regulated insulin signaling | In vivo | C57BL/6N mice | √ | 34198047 | ||||
| Alter energy metabolism, structure properties and electrical muscle activity | In vivo | Patients and rodents | 30196103 | |||||
| Insulin | Modulate the dynamic synaptic transmission and plasticity in the hippocampus of the mouse | In vivo | C57BL/6 mice | √ | 31146008 | |||
| Metformin | Corrected abnormal glutamatergic transmission | In vivo | C57BL/6 mice | √ | 33114529 | |||
| Decreased circulating branched-chain amino acids levels to favor serotonergic neurotransmission in the hippocampus | In vivo | C57Bl6/j mice | √ | 31160539 | ||||
| Regulated Lcn-2 and inflammation-related molecules expression level | In vivo | C57BL/6 mice | √ | 31977586 | ||||
| Enhanced the expression of BDNF by activating AMPK/CREB-mediated histone acetylation | In vivo | C57BL/6J mice | √ | 31521867 | ||||
| Pioglitazone | Ameliorated upregulation of ventral hippocampal GFAP, the HFD-induced glucose-metabolic dysfunctions | In vivo | C57BL/6N | √ | 34022606 | |||
| Reversed the abnormality of the ventral hippocampal CA1 GFAP-immunoreactive astrocytes | √ | |||||||
| Activated the microglia to decrease the susceptibility of stressed ob/ob mice to develop depression | In vivo | wt mice or ob/ob mice | √ | 32422516 | ||||
| Improved the imbalance of M1 and M2 inflammatory cytokines | in vitro | Murine N9 microglial cells | √ | 27716270 | ||||
| Increased neural apoptosis via up-regulating the PI3K/AKT pathway and down-regulating the JNK/p38 pathway | In vivo | C57BL/6 mice | √ | 29359338 | ||||
| Modulated NF-κB/IL-6/STAT3 and CREB/BDNF pathways | In vivo | C57BL/6 mice | 28595081 | |||||
| Hydrogen sulfide | Reduced iron deposition and oxidative stress | In vivo | C57BL/6J mice | √ | 33945828 | |||
| Increased the expression of GPX4 and SLC7A11 | ||||||||
| Modulated Sirt6 | ||||||||
| Upregulated the expressions of BDNF and p-TrkB proteins | In vivo | SD rats | √ | 31660632 | ||||
| Activated the PI3K/AKT pathway | In vivo | SD rats | √ | √ | 34087334 | |||
| Reversed hippocampal neurogenesis | ||||||||
| Reduced malondialdehyde and 4-hydroxynonenal | In vivo | SD rats | √ | 25932716 | ||||
| Elevated superoxide dismutase and reduced glutathione | ||||||||
| Cannabidiol | Increased 5-HT, NA and/or DA | In vivo | Wistar rats | √ | √ | 32360935 | ||
| Increased weight gain and the insulin levels | ||||||||
| Interacted with 5-HT1A, CB1, or CB2 receptor | In vivo | Wistar rats | 33464458 | |||||
| Ascorbic acid | Increased insulin and monoamines | In vivo | Albino rats | √ | √ | 28827076 | ||
| Hesperidin | Ameliorated hyperglycaemia, oxidative stress and inflammation | In vivo | Albino Wistar rats | √ | √ | 25358020 | ||
| Enhanced neurogenesis | ||||||||
| Increased monoamines | ||||||||
| Activated the Nrf2/ ARE pathway | In vivo | SD rats | √ | 32982741 |
4.2 Serotonin and norepinephrine reuptake inhibitors (SNRIs)
According to the glucose consumption assay, venlafaxine caffeic acid salt showed strong hypoglycemic activity in human liver cells of HL-7702, suggesting its potential therapeutic effect for the comorbidity of T2DM and depression.168 A retrospective study showed that the mean ratio of HbA1c for Venlafaxine was numerically lower than Citalopram, however there is no statistically significant difference.169 A study indicated that the risk of T2DM increased because of the exposure of antidepressants, and that Duloxetine and Venlafaxine were the antidepressants most associated with T2DM.170 Venlafaxine hydrochloride markedly elevated the consumption of glucose in the glucose consumption test in vitro.171 Results from a survey aimed at psychiatrists illustrated that duloxetine was widely applied in fibromyalgia and DM due to its alleviation of neuropathic pain.172 However, the administration of SNRIs, tricyclic antidepressants, SSRIs, and other antidepressants was associated with increased risk of diabetes.173 Furthermore, treatment with SSRIs or SNRIs correlated with a rise in the risk of T2DM, and this association intensified with increasing cumulative dose, duration of use and average daily dose.174, 175
4.3 Insulin
According to an observational study and meta-analysis, insulinized patients with DM were found to present remarkable impairment of depressive symptoms.176-178 The high relation between insulin use and depression resulted from two conditions. On the one hand, it was revealed that adolescents with T1DM were much more likely to reject insulin use because of diabetes distress and reduced self-care.179, 180 On the other hand, patients with diabetes mellitus and depression suffered from the uneffective utilization of insulin. Insulin resistance as a cross-disorder mechanism contributed to the higher somatic comorbidity and shortened lifespan in diabetes and depression. Compared to healthy people, elevated insulin was found in patients with MDD.181 Dietary insulin index (DII) and Dietary insulin load (DIL) were discovered to have a positive correlation with the incidence of depression in women.182 In addition, depressive symptoms have been implicated in the upregulated production of antibodies against estrogenic insulin in patients with T1DM, which may be partly mediated by depression-induced inflammation.183 However, the improvement of insulin sensitivity could alleviate depression. The progress of glycemic control and reduction of depressive symptoms were related to the higher mealtime insulin bolus score behavior.184 The antidepressive effect of insulin and IGF-1 in rats with DM was mediated by IGF-1 receptor in brain.40 An experimental study revealed that insulin could improve learning and memory by modulating the dynamic synaptic transmission and plasticity in the hippocampus of the mouse, thus improving cognitive impairment185 (Table 1). Therefore, insulin administration was not recommended in the therapy of the comorbidity of DM and depression, while the therapy of up-regulating insulin sensitivity should be recommended.
4.4 Metformin
A nationwide population-based study revealed an association between metformin in continued use and combinations of drugs and decreased rates of incident depression, suggesting a positive effect of metformin on depression rates.186 Compared to elderly diabetic patients taking no medication, patients taking metformin showed a lower risk of depression, indicating that metformin was a protective factor against depression in those patients.187 Metformin users with T2DM in Korea had a significantly lower prevalence of malignancy and depression.188 However, a research found an association between low doses of metformin and lower depression risk in diabetes, while high doses of metformin correlated with increased risk of depression.189 In terms of the mechanism underlying the antidepressant effect of metformin, a study found that metformin recovered the glutamatergic transmission, which was elevated in depressive mouse. The result suggested that metformin ameliorated depression via modifying abnormal glutamatergic transmission.190 By decreasing circulating BCAA levels in the hippocampus, metformin might serve as an antidepressant in diabetic mice to favor serotonergic neurotransmission in the hippocampus.191 Metformin regulated Lcn-2 and inflammation-related molecules expression level to down-regulate depression symptom.192 Metformin activated the AMPK-CREB mediated histone acetylation and up-regulated the level of BDNF promoter to increase the expression of BDNF, thus alleviating the depression in diabetes. The metformin administration could not only reverse depression in mice but also strengthen the antidepressant effect of fluoxetine in combination with fluoxetin193 (Table 1).
4.5 Pioglitazone
The pioglitazone treatment could ameliorate the upregulation of ventral hippocampal GFAP (glial fibrillary acidic protein), the glucose-metabolic dysfunctions and reverse the abnormality of the ventral hippocampal CA1 GFAP-immunoreactive astrocytes, and alleviate the depression phenotypes.194 There were severe impairment of spatial memory, behavioral disorders, an increase in pro-inflammatory cytokines, and a decrease in antiinflammatory cytokines and Peroxisome proliferator-activated receptor gamma (PPARγ) in the prefrontal cortex (PFC) and hippocampus in diabetic mice with CUMS. It was revealed that PPARγ activated the microglia, so depression was more likely to develop in stressed ob/ob mice when PPARγ was reduced. As a result, the Pioglitazone, a PPARγ 1 activator, alleviated depressive symptom in mice.145 In an in vitro experiment, the administration of pioglitazone alleviated depression by rebalance of M1 and M2 inflammatory cytokines. The imbalanced level of M1 and M2 inflammatory cytokines inhibited nuclear factor kB activation and is expressed in lipopolysaccharide (LPS)-stimulated N9 microglial cells.195 Furthermore, pioglitazone's antidepressant effect was due to an increase of neural apoptosis in the PFC, associated with down-regulation of the Jun N-terminal kinase (JNK)/p38 pathway and up-regulation of the PI3K/AKT pathway.196 Pioglitazone had anti-depressant effect, as indicated by the improvement of behaviors in several tests like open field, elevated plus maze and forced swimming tests.197 Pioglitazone's antidepressant effect on depression-like behaviors was dependent on PPAR-γ, with the modulation of the nuclear factor kappa B/interleukin 6/signal transducer and activator of transcription 3 (NF-κB/IL-6/STAT3) and CREB/BDNF pathways198 (Table 1). However, according to a double-blind, placebo-controlled trial, pioglitazone was not found to be more effective than placebo at treating depression based on inflammatory and metabolic markers.199
4.6 Hydrogen sulfide (H2S)
Many mechanisms are involved in the effect of hydrogen sulfide, a gaseous mediator, on depression with comorbid DM. For instance, sodium hydrosulfide (NaHS), a donor of H2S, remarkably down-regulated the PFC ferroptosis via decreasing oxidative stress and iron deposition. NaHS reduced anxiety-like and depressive-like behaviors by modulating sirtuin 6 (Sirt6) to inhibit inflammation and reducing the PFC ferroptosis in the BV2 cells and T1DM.200 In the hippocampus of STZ-induced diabetic rats, BDNF and p-tropomyosin-related kinase B (TrkB) proteins were up-regulated by H2S.201 In the hippocampus, NaHS triggered PI3K/AKT pathway and promoted neurogenesis induced by STZ, displaying an anti-depressant effect.202 H2S exerted an ameliorative influence against depression and anxiety by inhibiting oxidative stress in the hippocampus of diabetes203 (Table 1). Exogenous H2S inhibited autophagy via activating the PI3K/AKT1 signaling pathway to mitigate diabetes-induced myocardial fibrosis. Exogenous H2S not only promoted autophagy via activating the BDNF/TrkB pathway to improve diabetic depression but also inhibited autophagy via the Nrf2-ROS-AMPK signaling pathway to improve endothelial cell density (ECD) in diabetic rats.204
4.7 Cannabidiol (CBD)
Many experiments support that the administration of cannabidiol helps alleviate depression and diabetes mellitus. CBD treatment (high dose) not only reduced the depressive symptoms in diabetic rats, as evidenced by the altered level of 5-HT, NA and/or DA, but also caused a remarkable increase in weight gain and the level of insulin, thus reducing glycemia.26 Sub-chronic treatment with cannabidiol (high dose) induced a mild effect against depression.27 The impact of CBD against depression was blocked by the antagonists −5-HT1A, cannabinoids type-1 (CB1), or CB2. The effect of CBD against glycemia was blocked by the blockade of CB2 in diabetic animals. These phenomenons indicated that cannabidiol induced different effects through multiple sites of action205 (Table 1). CBD caused a strain-dependant antidepressant-like effect, as evidenced by the improvement of the tail suspension test in Swiss rats exclusively. CBD exerted a sex-dependant anti-depressant effect, as proven by the improvement of the tail suspension test in the male Swiss rats exclusively, and had a time-dependant antidepressant-like effect, as evidenced by the effect against depression in male Flinders Sensitive Lines (FSL) rats but an effect with bimodality in female FSL.206 In MIN6 as well as Beta-TC-6, cells abnormal cannabidiol (Abn-CBD) down-regulated apoptosis induced by ER stress via the CREB phosphorylation of β-cells.207
4.8 Ascorbic acid (AA)
It has been demonstrated that ascorbic acid could have an ameliorative effect on DM and depression. Ascorbic acid exerted a dose-dependent influence on reducing the immobility period, hyperglycemia, and hypoinsulinemia.208 The treatment of metformin combined with AA was shown to down-regulate glucose, immobility period, and corticosterone levels, up-regulate the insulin and monoamine levels and decrease pro-inflammatory cytokines as well as oxidative stress in diabetic rats with comorbid depression209 (Table 1). Also, in CUS-induced rats, AA and ketamine in single sub-effective doses have alleviated depressive-like behavior.210 Ascorbic acid not only ameliorated 24 h as well as postprandial glycemia but also down-regulated blood pressure in T2DM.211 Pre-exercise and postexercise blood pressure (BPs) in patients with T2DM decreased after 6 weeks of Vitamin C supplementation, possibly resulting from improved oxidative stress and NO release.212 Glucose transporter 10 (GLUT10) regulated adipogenesis, and prevented mice from HFD-induced metabolic dysregulation by maintaining AA-dependent DNA demethylation.213
4.9 Hesperidin
Many studies indicated that hesperidin has the potential to cure depression and DM. Hesperidin had an anti-depressant effect in diabetic rats, possibly because it alleviated hyperglycemia, oxidative stress, and inflammation, enhanced neurogenesis, and changed the levels of monoamines in the brain.214 Hesperidin alleviated the symptoms of depression and anxiety in diabetic animals via activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/ARE/Glyoxase 1 pathway215 (Table 1). Also, in mTBI-induced mice, hesperidin reduced depression by decreasing TNF-α, IL-1β and malondialdehyde (MDA), and increasing BDNF levels in the hippocampus.216 In CUMS-induced diabetes, hesperidin treatment alleviated depression by inhibiting inflammation and microglia, as evidenced by the decreased expression of IL-6, IL-1β, NLRP3, TNF-a, ASC, and caspase-1 in the microglia and prefrontal cortex.217 Hesperidin protected pancreatic β cells and improved their function in diabetic rats, possibly by inhibiting ER stress as well as oxidative stress, along with the effects against oxidation, inflammation, and apoptosis.218 Systolic blood pressure (SBP), mean arterial blood pressure, IL-6 and hs-CRP were reduced in the hesperidin group, suggesting that hesperidin may have effects against hypertension and inflammation in T2DM.219
5 CONCLUSION AND LIMITATION
The potential of this review was presented in the following aspects. First, this review demonstrated that stress contributed to the hyperactivity of the HPA axis, and then resulted in depression, indicating that medical personnel should pay attention to diabetes distress to avoid depression. Second, it was revealed that prefrontal-hippocampal circuits had structural and functional abnormalities in the comorbidity, suggesting the potential to explore these circuits' molecular regulation. Third, obesity might lead to diabetes mellitus and depression through reduced adiponectin and increased leptin and resistin, indicating the importance of weight control.
To reach the ultimate goal of finding anti-diabetic and anti-depressant drug therapy, more efforts should be made to solve limitations and conduct more studies in the future.
First, the performance of drug therapy in terms of the HPA axis and adipokine was insufficient. Before exploring drug therapy in clinical trials, there is an urge to fully discover the drug therapy in comprehensive mechanisms related to comorbidity to avoid their side effects.
Second, although this review explored the alternative therapy for DM with depression based on different natural products, it is also inspiring to discover the treatment from the perspective of different pathophysiological hypotheses. For example, an animal experiment suggested that agmatine could alleviate insulin resistance by remarkably inhibiting T2DM-induced depression, anxiety, and neuroinflammatory markers in rats.29 Animal research found that Quercetin and Rutin improve glucose control and alleviate depressive symptoms in rats mainly by inhibiting the HPA axis response.43 From the oxidative stress perspective, date seed extract could reduce blood glucose and oxidative stress to prevent the brain regions from lipid peroxidation products, thus exhibiting an anxiolytic effect.220 Metformin attenuated depressive symptoms mainly because it increased brain-derived neurotrophic factor (BDNF) by up-regulating the histone acetylation and the BDNF promoter.193 From the aspect of GM, the banana starch diet ameliorated depression in diabetic rats mainly due to the improvement of the gut-microbiota-brain axis.221 In adipokines, plant and marine sources of n-3 PUFAs have been proved to increase adiponectin and decrease leptin levels in patients with type 2 diabetes.222
Third, many animal models and cell models were discovering the comorbidity of DM and depression. However, effective models have not been utilized generally. Therefore, it is necessary to establish effective models in vivo and in vitro to represent the uniqueness of the comorbidity.
There are some weaknesses worth noting in this study. First, since this review is not a systematic review, it cannot discover the comprehensive mechanisms of the comorbidity of DM and depression. Second, the main subjects of literature in this review are animals, leading to the decreased exploration of mechanisms and drug therapies in human subjects. Third, evidence on the antidepressant effects of exercise and diet therapy is increasing, which also played an important role in the treatment of comorbidity of depression and metabolic disturbances. However, this review only included the drug therapy.
Experiments in vivo and in vitro had discovered many meaningful findings in this field. Their scientific value gave researchers further insight into the mechanisms and treatment of comorbidity, thus providing an important theoretical basis. However, there are still some challenges faced by this field. The generally recognized standards of models in vivo and in vitro have not been built up. To find out the well-recognized models, research should be conducted to identify the critical characteristics of the main models regarding anatomy and physiology of the brain, diabetes mellitus modeling, depression modeling and implementation aspects. In addition, It is necessary to carry out more experiments and research on human subjects to confirm the findings in animal and cell models. Due to the anti-diabetic and anti-depressant effects of drug therapy, it is worthwhile to find out the translation value of these experiments in bedside research on human subjects. Therefore, it is of great help to establish large-scale cohorts of people with DM and depression. The longitudinal follow-up could investigate the history, mechanisms, treatment response, and prognosis. The underlying pathogenesis could be uncovered after the application of the high throughput techniques in omics.
Researchers should pay much attention to the comorbidity of DM and depression regarding its high prevalence and social burden. Considering that most of the reviews did not provide a thought-provoking and comprehensive insight into the interface of DM and depression, we summarized the mechanisms underlying the comorbidity and drug therapy with anti-diabetic and anti-depressant effects. Previous studies have deeply explored and summarized the contribution of insulin resistance, neurotransmitters, oxidative stress and inflammation towards the comorbidity. This review also summarized numerous studies about stress, the HPA axis, prefrontal-hippocampus circuits and adipokines. It was found that prefrontal-hippocampal circuits played a critical role in the comorbidity as a result of dysfunction of the Glu-Gln cycle. Weight control is significant because of the reduced adiponectin and increased leptin and resistin in comorbidity. But what internal and external elements can trigger the molecular pathways and how the bioactive compounds interact remain unclear in comorbidity. The genetic background should be explored to get further insight into the environmental factors, molecular pathways and effective targets. Since most of the evidence came from animal and cell studies, more research such as clinical research in human subjects, meta-analysis, omics technologies, and network pharmacology should be conducted. In addition, although the medicines displayed in this review could expand the potential therapy region, exploring their mechanisms of action was recently based on animal experiments. Conducting a large-scale cohort is a good way to investigate the natural history, pathogenesis and treatment response. We strongly advise that experiments on human subjects should be conducted to establish a novel and effective pharmacological therapy.
AUTHOR CONTRIBUTIONS
Sixin Li, Dong Yang and Xuhui Zhou discussed the subject matter, drafted the manuscript and prepared the figures. Lu Chen, Lini Liu, Ruoheng Lin, Xinyu Li, Ying Liu and Huiwen Qiu collected the related references and participated in the critical revision of the article. Hui Cao, Jian Liu and Quan Cheng designed, revised and funded the review. All authors contributed to this manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This study was supported by the Hunan Provincial Natural Science Foundation of China (NO.2023JJ40362 and NO.2022JJ30451), Changsha City Natural Science Foundation of China (NO. kq2208103), National Natural Science Foundation of China (NO.82104793) and Hunan Youth Science and Technology Talent Project (NO.2022RC1226).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




