Structural and functional alterations of the hippocampal subfields in T2DM with mild cognitive impairment and insulin resistance: A prospective study
Chen Yang
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorHuiyan Zhang
School of Clinical Medicine, Ningxia Medical University, Yinchuan, China
Search for more papers by this authorZihan Ma
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorYanjun Fan
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorYanan Xu
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorJian Tan
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorJing Tian
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorJiancang Cao
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorWenwen Zhang
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorGang Huang
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorCorresponding Author
Lianping Zhao
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Correspondence
Lianping Zhao, Department of Radiology, Gansu Provincial Hospital, No. 204#, Donggang West Road, Lanzhou 730000, China.
Email: [email protected]
Search for more papers by this authorChen Yang
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorHuiyan Zhang
School of Clinical Medicine, Ningxia Medical University, Yinchuan, China
Search for more papers by this authorZihan Ma
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorYanjun Fan
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorYanan Xu
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorJian Tan
The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Gansu University of Chinese Medicine, Lanzhou, China
Search for more papers by this authorJing Tian
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorJiancang Cao
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorWenwen Zhang
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorGang Huang
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Search for more papers by this authorCorresponding Author
Lianping Zhao
Department of Radiology, Gansu Provincial Hospital, Lanzhou, China
Correspondence
Lianping Zhao, Department of Radiology, Gansu Provincial Hospital, No. 204#, Donggang West Road, Lanzhou 730000, China.
Email: [email protected]
Search for more papers by this authorAbstract
Background
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance (IR) and is often accompanied by mild cognitive impairment (MCI). The detrimental effects of T2DM and IR on the hippocampus have been extensively demonstrated. Few studies have examined the effects of IR on structure and function of hippocampal subfields in T2DM-MCI patients.
Method
A total of 104 T2DM patients were recruited in this prospective study and divided into four groups (T2DM-MCI-higherIR, n = 17; T2DM-MCI-lowerIR, n = 32; T2DM-nonMCI-higherIR, n = 19; T2DM-nonMCI-lowerIR, n = 36). Structure and function MRI data were captured. Clinical variables and neuropsychological scores were determined for all participants. Hippocampal subfield volume and functional connectivity were compared among four groups. Partial correlation analysis was performed between imaging indicators, clinical variables, and neuropsychological scores in all patients.
Results
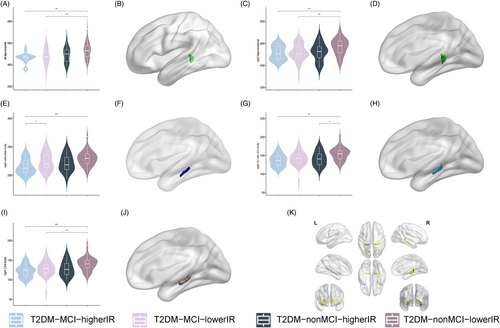
T2DM-MCI-higher IR group had the smallest volumes of bilateral hippocampal tail, right subiculum-body, right GC-ML-DG-body, and right CA4-body. IR in right hippocampal tail, right subiculum-body, and right GC-ML-DG-body exerted main effect. Furthermore, elevated functional connectivity was found between right subiculum-body and bilateral dorsolateral prefrontal cortex and right anterior cingulate–medial prefrontal cortex. Hippocampal subfield volume positively correlates with total cholesterol and triglycerides and negatively correlates with fasting insulin.
Conclusion
The present study found that T2DM-MCI patients have structural and functional alterations in hippocampal subfields, and IR is a negative factor influencing the alteration of hippocampal subfields volume. These findings support the importance of IR in T2DM-MCI patients and might be potential neuroimaging biomarkers of cerebral impairment in T2DM-MCI patients.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting Information
| Filename | Description |
|---|---|
| jdb70029-sup-0001-Supinfo1.docxWord 2007 document , 19.9 KB | Data S1. Supporting Information. |
| jdb70029-sup-0002-Supinfo2.docxWord 2007 document , 14.6 KB | Data S2. Supporting Information. |
| jdb70029-sup-0003-Supinfo3.docxWord 2007 document , 17.3 KB | Data S3. Supporting Information. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Colagiuri S, Guariguata L, Motala A, Ogurtsova KJ. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9 (th) edition. Diabetes Res Clin Pract. 2019; 157:107843.
- 2Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015; 1:15018.
- 3Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020; 37:101799.
- 4Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021; 17(7): 400-420.
- 5Lu Y, Jiang H, Zhang H, et al. Serum oxidized low density lipoprotein serves as a mediator for the inverse relationship between serum d-ribose and cognitive performance in type 2 diabetic patients. Free Radic Biol Med. 2021; 171: 91-98.
- 6Ehtewish H, Arredouani A, El-Agnaf O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int J Mol Sci. 2022;23(11):6144.
- 7Rizzo MR, di Meo I, Polito R, et al. Cognitive impairment and type 2 diabetes mellitus: focus of SGLT2 inhibitors treatment. Pharmacol Res. 2022; 176:106062.
- 8Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022; 18(9): 525-539.
- 9Michailidis M, Moraitou D, Tata DA, Kalinderi K, Papamitsou T, Papaliagkas V. Alzheimer's disease as type 3 diabetes: common pathophysiological mechanisms between Alzheimer's disease and type 2 diabetes. Int J Mol Sci. 2022;23(5):2687.
10.3390/ijms23052687 Google Scholar
- 10de Felice FG, Gonçalves RA, Ferreira ST. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat Rev Neurosci. 2022; 23(4): 215-230.
- 11Agrawal R, Reno CM, Sharma S, Christensen C, Huang Y, Fisher SJ. Insulin action in the brain regulates both central and peripheral functions. Am J Physiol Endocrinol Metab. 2021; 321(1): E156-E163.
- 12Scherer T, Sakamoto K, Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021; 17(8): 468-483.
- 13Milstein JL, Ferris HA. The brain as an insulin-sensitive metabolic organ. Mol Metab. 2021; 52:101234.
- 14Rajasekar N, Nath C, Hanif K, Shukla R. Intranasal insulin administration ameliorates Streptozotocin (ICV)-induced insulin receptor dysfunction, Neuroinflammation, Amyloidogenesis, and memory impairment in rats. Mol Neurobiol. 2017; 54(8): 6507-6522.
- 15Kazkayasi I, Telli G, Nemutlu E, Uma S. Intranasal metformin treatment ameliorates cognitive functions via insulin signaling pathway in ICV-STZ-induced mice model of Alzheimer's disease. Life Sci. 2022; 299:120538.
- 16Vogrinc D, Gregorič Kramberger M, Emeršič A, Čučnik S, Goričar K, Dolžan V. Genetic polymorphisms in oxidative stress and inflammatory pathways as potential biomarkers in Alzheimer's disease and dementia. Antioxidants (Basel). 2023; 12(2):316.
- 17Martinez-Valbuena I, Valenti-Azcarate R, Amat-Villegas I, et al. Amylin as a potential link between type 2 diabetes and alzheimer disease. Ann Neurol. 2019; 86(4): 539-551.
- 18Akhtar A, Sah SP. Insulin signaling pathway and related molecules: role in neurodegeneration and Alzheimer's disease. Neurochem Int. 2020; 135:104707.
- 19O'keefe J, Nadel L. Précis of O'Keefe & Nadel'sThe hippocampus as a cognitive map. Behav Brain Sci. 1979; 2(4): 487-494.
- 20Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000; 1(1): 41-50.
- 21Wei L, Zhang Y, Wang J, et al. Parietal-hippocampal rTMS improves cognitive function in Alzheimer's disease and increases dynamic functional connectivity of default mode network. Psychiatry Res. 2022; 315:114721.
- 22Carey G, Görmezoğlu M, de Jong JJA, et al. Neuroimaging of anxiety in Parkinson's disease: a systematic review. Mov Disord. 2021; 36(2): 327-339.
- 23Lucini-Paioni S, Squarcina L, Cousins DA, Brambilla P. Lithium effects on hippocampus volumes in patients with bipolar disorder. J Affect Disord. 2021; 294: 521-526.
- 24Binnewies J, Nawijn L, van Tol M-J, van der Wee NJA, Veltman DJ, Penninx BWJH. Associations between depression, lifestyle and brain structure: a longitudinal MRI study. NeuroImage. 2021; 231:117834.
- 25Liu S, Cao L, Li H, et al. Trait anxiety mediates the association between hippocampal-insula functional connectivity and anxiety symptom severity in adults with and without generalized anxiety disorder. J Affect Disord. 2024; 344: 1-7.
- 26Tang W, Li Y, He S, et al. Caveolin-1 alleviates diabetes-associated cognitive dysfunction through modulating neuronal Ferroptosis-mediated mitochondrial homeostasis. Antioxid Redox Signal. 2022; 37(13–15): 867-886.
- 27Gao Y, Sui C, Chen B, et al. Voxel-based morphometry reveals the correlation between gray matter volume and serum P-tau-181 in type 2 diabetes mellitus patients with different HbA1c levels. Front Neurosci. 2023; 17:1202374.
- 28van der Meer D, Rokicki J, Kaufmann T, et al. Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Mol Psychiatry. 2020; 25(11): 3053-3065.
- 29Constantinides VC, Tentolouris-Piperas V, Paraskevas GP, et al. Hippocampal subfield volumetry in corticobasal syndrome of diverse underlying pathologies. J Neurol. 2023; 270(4): 2059-2068.
- 30Weis CN, Webb EK, Huggins AA, et al. Stability of hippocampal subfield volumes after trauma and relationship to development of PTSD symptoms. NeuroImage. 2021; 236:118076.
- 31de Flores R, Mutlu J, Bejanin A, et al. Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Hum Brain Mapp. 2017; 38(10): 4922-4932.
- 32Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018; 14(3): 168-181.
- 33Kullmann S, Kleinridders A, Small DM, et al. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020; 8(6): 524-534.
- 34Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage. 2015; 115: 117-137.
- 35Yan C-G, Wang X-D, Lu B. DPABISurf: data processing & analysis for brain imaging on surface. Sci Bull (Beijing). 2021; 66(24): 2453-2455.
- 36Ghasemi A, Tohidi M, Derakhshan A, Hasheminia M, Azizi F, Hadaegh F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran lipid and glucose study. Acta Diabetol. 2015; 52(5): 905-915.
- 37Saber-Ayad M, Manzoor S, El Serafi A, et al. The FTO rs9939609 “a” allele is associated with impaired fasting glucose and insulin resistance in Emirati population. Gene. 2019; 681: 93-98.
- 38Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27(6): 1487-1495.
- 39Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53(4): 695-699.
- 40Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Reproducibility of single-subject functional connectivity measurements. AJNR Am J Neuroradiol. 2011; 32(3): 548-555.
- 41Birn RM, Molloy EK, Patriat R, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013; 83: 550-558.
- 42Samann PG, Iglesias JE, Gutman B, et al. FreeSurfer-based segmentation of hippocampal subfields: a review of methods and applications, with a novel quality control procedure for ENIGMA studies and other collaborative efforts. Hum Brain Mapp. 2022; 43(1): 207-233.
- 43Cui Y, Tang T-Y, Lu C-Q, Ju S. Insulin resistance and cognitive impairment: evidence from neuroimaging. J Magn Reson Imaging. 2022; 56(6): 1621-1649.
- 44Shen Z, Li Z-Y, Yu M-T, Tan K-L, Chen S. Metabolic perspective of astrocyte dysfunction in Alzheimer's disease and type 2 diabetes brains. Biomed Pharmacother. 2023; 158:114206.
- 45Shpakov AO, Zorina II, Derkach KV. Hot spots for the use of intranasal insulin: cerebral ischemia, brain injury, diabetes mellitus, endocrine disorders and postoperative delirium. Int J Mol Sci. 2023; 24(4):3278.
10.3390/ijms24043278 Google Scholar
- 46Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014; 63(7): 2253-2261.
- 47Haukvik UK, Westlye LT, Mørch-Johnsen L, et al. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2015; 77(6): 581-588.
- 48Hainmueller T, Bartos M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat Rev Neurosci. 2020; 21(3): 153-168.
- 49Mizuseki K, Kitanishi T. Oscillation-coordinated, noise-resistant information distribution via the subiculum. Curr Opin Neurobiol. 2022; 75:102556.
- 50Huang Y, Huang L, Wang Y, Liu Y, Lo C-YZ, Guo Q. Differential associations of visual memory with hippocampal subfields in subjective cognitive decline and amnestic mild cognitive impairment. BMC Geriatr. 2022; 22(1): 153.
- 51Park AJ, Harris AZ, Martyniuk KM, et al. Reset of hippocampal-prefrontal circuitry facilitates learning. Nature. 2021; 591(7851): 615-619.
- 52Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci. 2017; 18(9): 547-558.
- 53Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022; 91(12): 1061-1069.
- 54Márquez-Valadez B, Rábano A, Llorens-Martín M. Progression of Alzheimer's disease parallels unusual structural plasticity of human dentate granule cells. Acta Neuropathol Commun. 2022; 10(1): 125.
- 55Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998; 4(11): 1313-1317.
- 56Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008; 11(3): 309-317.
- 57van Dijk MT, Fenton AA. On how the dentate gyrus contributes to memory discrimination. Neuron. 2018; 98(4): 832-845.e5.
- 58Nogovitsyn N, Muller M, Souza R, et al. Hippocampal tail volume as a predictive biomarker of antidepressant treatment outcomes in patients with major depressive disorder: a CAN-BIND report. Neuropsychopharmacology. 2020; 45(2): 283-291.
- 59Lambert C, Benjamin P, Zeestraten E, Lawrence AJ, Barrick TR, Markus HS. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain. 2016; 139(Pt 4): 1136-1151.
- 60Marchesi VT. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011; 25(1): 5-13.
- 61Fang F, Cao R, Luo Q, et al. The silent occurrence of cerebral small vessel disease in nonelderly patients with type 2 diabetes mellitus. J Diabetes. 2021; 13(9): 735-743.
- 62Klosovskii BN. Fundamental principles of the development, structure and function of the vaso-capillary network of the brain. The Development of the Brain and its Disturbance by Harmful Factors. Pergamon; 1963.
- 63Watt MJ, Miotto PM, de Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. 2019; 40(5): 1367-1393.
- 64Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology. 2021; 160(2): 573-599.
- 65Zhou X, Shin S, He C, et al. Qki regulates myelinogenesis through Srebp2-dependent cholesterol biosynthesis. elife. 2021; 10:e60467.
- 66Civeira F, Arca M, Cenarro A, Hegele RA. A mechanism-based operational definition and classification of hypercholesterolemia. J Clin Lipidol. 2022; 16(6): 813-821.