Comparing Glucagon-like peptide-1 receptor agonists versus metformin in drug-naive patients: A nationwide cohort study
Abstract
Background
Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are increasingly being prescribed in drug-naive patients. We aimed to contrast add-on therapy, adherence, and changes in biomarkers, 1 year after treatment initiation with GLP-1 RA or metformin.
Methods
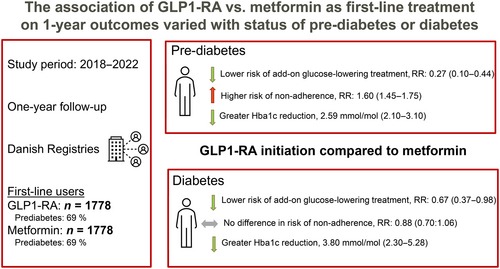
Using Danish nationwide registers, we included incident GLP-1 RA or metformin users from 2018 to 2021 with glycated hemoglobin (HbA1c) ≥ 42 mmol/mol. GLP-1 RA initiators were matched to metformin initiators in a ratio of 1:1 to assess outcomes in prediabetes and diabetes. Main outcomes analyzed were 1-year risk of add-on glucose-lowering medication and 1-year risk of nonadherence. One-year risks were estimated with multiple logistic regression and standardized. Multiple linear regression was used to estimate the average differences in biomarker changes.
Results
In total, 1778 individuals initiating GLP-1 RA and metformin were included. After standardizing for various factors, GLP-1 RA compared with metformin was associated with reduced 1-year risk of add-on glucose-lowering treatment in patients with prediabetes (1-year risk ratio [RR]: 0.27, 95% confidence interval [CI]: 0.10–0.44) and diabetes (RR: 0.67, 95% CI: 0.37–0.98). GLP-1 RA was associated with higher 1-year risk of nonadherence among patients with prediabetes (RR: 1.60, 95% CI: 1.45–1.75), but no difference in patients with diabetes (RR: 0.88, 95% CI: 0.70–1.06). Compared to metformin, GLP-1 RA was associated with greater HbA1c reduction (prediabetes: −2.59 mmol/mol 95% CI: −3.10 to −2.09, diabetes: −3.79 mmol/mol, 95% CI: −5.28 to −2.30).
Conclusions
GLP-1 RA was associated with a reduced risk of additional glucose-lowering medication, achieving better glycated hemoglobin control overall. However, among patients with prediabetes, metformin was associated with better adherence.
CONFLICT OF INTEREST STATEMENT
CT-P reports grants from Bayer and Novo Nordisk unrelated to the current study. UPB has served on advisory boards for Novo Nordisk, Sanofi, and Vertex and has received lecture fees from Novo Nordisk and Sanofi. LK reports speakers' honorariums from AstraZeneca, Bayer, Boehringer, Novartis, and Novo. All other authors declare no competing interests.