Traditional and emerging strategies using hepatocytes for pancreatic regenerative medicine
Shuang Liu
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Search for more papers by this authorYuYing Zhang
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Search for more papers by this authorYunFei Luo
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Search for more papers by this authorCorresponding Author
JianPing Liu
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Correspondence
JianPing Liu, Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang 330006, China, China.
Email: [email protected]
Search for more papers by this authorShuang Liu
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Search for more papers by this authorYuYing Zhang
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Search for more papers by this authorYunFei Luo
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Search for more papers by this authorCorresponding Author
JianPing Liu
Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Correspondence
JianPing Liu, Department of Metabolism and Endocrinology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang 330006, China, China.
Email: [email protected]
Search for more papers by this authorAbstract
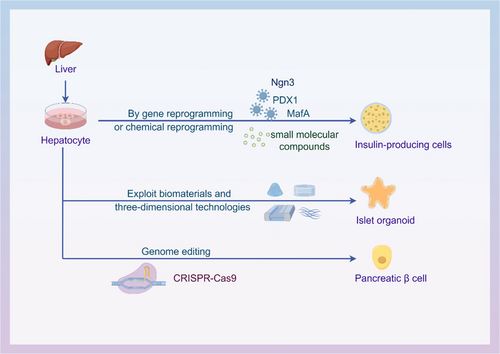
Although pancreas and islet cell transplantation are the only ways to prevent the late complications of insulin-dependent diabetes, a shortage of donors is a major obstacle to tissue and organ transplantation. Stem cell therapy is an effective treatment for diabetes and other pancreatic-related diseases, which can be achieved by inducing their differentiation into insulin-secreting cells. The liver is considered an ideal source of pancreatic cells due to its similar developmental origin and strong regenerative ability as the pancreas. This article reviews the traditional and emerging strategies using hepatocytes for pancreatic regenerative medicine and evaluates their advantages and challenges. Gene reprogramming and chemical reprogramming technologies are traditional strategies with potential to improve the efficiency and specificity of cell reprogramming and promote the transformation of hepatocytes into islet cells. At the same time, organoid technology, as an emerging strategy, has received extensive attention. Biomaterials provide a three-dimensional culture microenvironment for cells, which helps improve cell survival and differentiation efficiency. In addition, clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 gene editing technology has brought new opportunities and challenges to the development of organoid technology.
DISCLOSURE
The authors declare that they have no competing interests.
REFERENCES
- 1Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022; 183:109119. doi:10.1016/j.diabres.2021.109119
- 2Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent Progress and future directions. Endocr Rev. 2019; 40(2): 631-668. doi:10.1210/er.2018-00154
- 3Shapiro AMJ, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2016; 13(5): 268-277. doi:10.1038/nrendo.2016.178
- 4Siehler J, Blochinger AK, Meier M, Lickert H. Engineering islets from stem cells for advanced therapies of diabetes. Nat Rev Drug Discov. 2021; 20(12): 920-940. doi:10.1038/s41573-021-00262-w
- 5Yamanaka S. Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell. 2020; 27(4): 523-531. doi:10.1016/j.stem.2020.09.014
- 6Ruzittu S, Willnow D, Spagnoli FM. Direct lineage reprogramming: harnessing cell plasticity between liver and pancreas. Cold Spring Harb Perspect Biol. 2020; 12(7):a035626. doi:10.1101/cshperspect.a035626
- 7Rajaei B, Shamsara M, Amirabad LM, Massumi M, Sanati MH. Pancreatic endoderm-derived from diabetic patient-specific induced pluripotent stem cell generates glucose-responsive insulin-secreting cells. J Cell Physiol. 2017; 232(10): 2616-2625. doi:10.1002/jcp.25459
- 8Ogoke O, Maloy M, Parashurama N. The science and engineering of stem cell-derived organoids-examples from hepatic, biliary, and pancreatic tissues. Biol Rev Camb Philos Soc. 2021; 96(1): 179-204. doi:10.1111/brv.12650
- 9Kharbikar BN, Mohindra P, Desai TA. Biomaterials to enhance stem cell transplantation. Cell Stem Cell. 2022; 29(5): 692-721. doi:10.1016/j.stem.2022.04.002
- 10Liu Z, Tang M, Zhao J, Chai R, Kang J. Looking into the future: toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv Mater. 2018; 30(17):e1705388. doi:10.1002/adma.201705388
- 11Yi SA, Zhang Y, Rathnam C, Pongkulapa T, Lee KB. Bioengineering approaches for the advanced organoid research. Adv Mater. 2021; 33(45):e2007949. doi:10.1002/adma.202007949
- 12Garreta E, Kamm RD, de Sousa C, Lopes SM, et al. Rethinking organoid technology through bioengineering. Nat Mater. 2020; 20(2): 145-155. doi:10.1038/s41563-020-00804-4
- 13Jennings RE, Berry AA, Kirkwood-Wilson R, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013; 62(10): 3514-3522. doi:10.2337/db12-1479
- 14Yang L-J. Liver stem cell-derived β-cell surrogates for treatment of type 1 diabetes. Autoimmun Rev. 2006; 5(6): 409-413. doi:10.1016/j.autrev.2005.10.009
- 15Zhu Y, Liu Q, Zhou Z, Ikeda Y. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res Ther. 2017; 8(1): 240. doi:10.1186/s13287-017-0694-z
- 16Campbell SC, Macfarlane WM. Regulation of the pdx1 gene promoter in pancreatic beta-cells. Biochem Biophys Res Commun. 2002; 299(2): 277-284. doi:10.1016/s0006-291x(02)02633-5
- 17Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000; 6(5): 568-572. doi:10.1038/75050
- 18Ma XJ, Lu YK, Zhou ZY, et al. Human expandable pancreatic progenitor-derived β cells ameliorate diabetes. Sci Adv. 2022; 8(8):eabk1826. doi:10.1126/sciadv.abk1826
- 19Conrad E, Stein R, Hunter CS. Revealing transcription factors during human pancreatic β cell development. Trends Endocrinol Metab. 2014; 25(8): 407-414. doi:10.1016/j.tem.2014.03.013
- 20Brandhorst H, Brandhorst D, Brendel MD, Hering BJ, Bretzel RG. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 1998; 7(5): 489-495. doi:10.1177/096368979800700508
- 21Yong HJ, Xie G, Liu C, et al. Gene signatures of NEUROGENIN3+ endocrine progenitor cells in the human pancreas. Front Endocrinol. 2021; 12:736286. doi:10.3389/fendo.2021.736286
- 22Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008; 455(7213): 627-632. doi:10.1038/nature07314
- 23Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008; 237(11): 3270-3279. doi:10.1002/dvdy.21740
- 24Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002; 129(10): 2447-2457. doi:10.1242/dev.129.10.2447
- 25Van de Casteele M, Leuckx G, Baeyens L, et al. Neurogenin 3+ cells contribute to beta-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis. 2013; 4(3): e523. doi:10.1038/cddis.2013.52
- 26Xu X, D'Hoker J, Stange G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008; 132(2): 197-207. doi:10.1016/j.cell.2007.12.015
- 27McGrath PS, Watson CL, Ingram C, Helmrath MA, Wells JM. The basic helix-loop-helix transcription factor NEUROG3 is required for development of the human endocrine pancreas. Diabetes. 2015; 64(7): 2497-2505. doi:10.2337/db14-1412
- 28Kojima H, Fujimiya M, Matsumura K, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003; 9(5): 596-603. doi:10.1038/nm867
- 29Kaneto H, Nakatani Y, Miyatsuka T, et al. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005; 54(4): 1009-1022. doi:10.2337/diabetes.54.4.1009
- 30Yoon S. Co-expressing Pdx1 and Ngn3 induces few beta-like cells in the liver of mice. Biochem Biophys Res Commun. 2007; 362(1): 101-106. doi:10.1016/j.bbrc.2007.07.171
- 31Chang F-P, Cho CH-H, Shen C-R, et al. PDGF facilitates direct lineage reprogramming of hepatocytes to functional β-like cells induced by Pdx1 and Ngn3. Cell Transplant. 2016; 25(10): 1893-1909. doi:10.3727/096368916x691439
- 32Nishizawa M, Kataoka K, Goto N, Fujiwara KT, Kawai S. v-maf, a viral oncogene that encodes a "leucine zipper" motif. Proc Natl Acad Sci U S A. 1989; 86(20): 7711-7715. doi:10.1073/pnas.86.20.7711
- 33Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002; 277(51): 49903-49910. doi:10.1074/jbc.M206796200
- 34Matsuoka TA, Zhao L, Artner I, et al. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003; 23(17): 6049-6062. doi:10.1128/MCB.23.17.6049-6062.2003
- 35Olbrot M, Rud J, Moss LG, Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci U S A. 2002; 99(10): 6737-6742. doi:10.1073/pnas.102168499
- 36Banga A, Akinci E, Greder LV, Dutton JR, Slack JMW. In vivo reprogramming of Sox9 + cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci U S A. 2012; 109(38): 15336-15341. doi:10.1073/pnas.1201701109
- 37Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126(4): 663-676. doi:10.1016/j.cell.2006.07.024
- 38Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015; 16(2): 119-134. doi:10.1016/j.stem.2015.01.013
- 39De D, Halder D, Shin I, Kim KK. Small molecule-induced cellular conversion. Chem Soc Rev. 2017; 46(20): 6241-6254. doi:10.1039/c7cs00330g
- 40Li X, Xu J, Deng H. Small molecule-induced cellular fate reprogramming: promising road leading to Rome. Curr Opin Genet Dev. 2018; 52: 29-35. doi:10.1016/j.gde.2018.05.004
- 41D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006; 24(11): 1392-1401. doi:10.1038/nbt1259
- 42Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014; 159(2): 428-439. doi:10.1016/j.cell.2014.09.040
- 43Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014; 32(11): 1121-1133. doi:10.1038/nbt.3033
- 44Liu J, Liu Y, Wang H, et al. Direct differentiation of hepatic stem-like WB cells into insulin-producing cells using small molecules. Sci Rep. 2013; 3: 1185. doi:10.1038/srep01185
- 45Raya A, Rodriguez-Piza I, Aran B, et al. Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harb Symp Quant Biol. 2008; 73: 127-135. doi:10.1101/sqb.2008.73.038
- 46Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131(5): 861-872. doi:10.1016/j.cell.2007.11.019
- 47Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318(5858): 1917-1920. doi:10.1126/science.1151526
- 48Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020; 21(10): 571-584. doi:10.1038/s41580-020-0259-3
- 49Sahu S, Sharan SK. Translating embryogenesis to generate organoids: novel approaches to personalized medicine. iScience. 2020; 23(9):101485. doi:10.1016/j.isci.2020.101485
- 50Jiang L, Shen Y, Liu Y, Zhang L, Jiang W. Making human pancreatic islet organoids: progresses on the cell origins, biomaterials and three-dimensional technologies. Theranostics. 2022; 12(4): 1537-1556. doi:10.7150/thno.66670
- 51Broutier L, Andersson-Rolf A, Hindley CJ, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016; 11(9): 1724-1743. doi:10.1038/nprot.2016.097
- 52Heller RS. The comparative anatomy of islets. Adv Exp Med Biol. 2010; 654: 21-37. doi:10.1007/978-90-481-3271-3_2
- 53Kahn SE, Chen YC, Esser N, et al. The beta cell in diabetes: integrating biomarkers with functional measures. Endocr Rev. 2021; 42(5): 528-583. doi:10.1210/endrev/bnab021
- 54de Souza BM, Rodrigues M, de Oliveira FS, et al. Improvement of human pancreatic islet quality after co-culture with human adipose-derived stem cells. Mol Cell Endocrinol. 2020; 505:110729. doi:10.1016/j.mce.2020.110729
- 55Dossena M, Piras R, Cherubini A, et al. Standardized GMP-compliant scalable production of human pancreas organoids. Stem Cell Res Ther. 2020; 11(1): 94. doi:10.1186/s13287-020-1585-2
- 56Seymour PA, Freude KK, Tran MN, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007; 104(6): 1865-1870. doi:10.1073/pnas.0609217104
- 57Greggio C, De Franceschi F, Figueiredo-Larsen M, et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013; 140(21): 4452-4462. doi:10.1242/dev.096628
- 58Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011; 43(1): 34-41. doi:10.1038/ng.722
- 59Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci U S A. 2007; 104(25): 10500-10505. doi:10.1073/pnas.0704054104
- 60Sugiyama T, Benitez CM, Ghodasara A, et al. Reconstituting pancreas development from purified progenitor cells reveals genes essential for islet differentiation. Proc Natl Acad Sci U S A. 2013; 110(31): 12691-12696. doi:10.1073/pnas.1304507110
- 61Wang D, Wang J, Bai L, et al. Long-term expansion of pancreatic islet organoids from resident Procr(+) progenitors. Cell. 2020; 180(6): 1198-1211 e19. doi:10.1016/j.cell.2020.02.048
- 62Wang J, Wang D, Chen X, et al. Isolation of mouse pancreatic islet Procr(+) progenitors and long-term expansion of islet organoids in vitro. Nat Protoc. 2022; 17(5): 1359-1384. doi:10.1038/s41596-022-00683-w
- 63Kim Y, Kim H, Ko UH, et al. Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci Rep. 2016; 6:35145. doi:10.1038/srep35145
- 64Chaimov D, Baruch L, Krishtul S, Meivar-levy I, Ferber S, Machluf M. Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery. J Control Release. 2017; 257: 91-101. doi:10.1016/j.jconrel.2016.07.045
- 65Candiello J, Grandhi TSP, Goh SK, et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018; 177: 27-39. doi:10.1016/j.biomaterials.2018.05.031
- 66Tao T, Wang Y, Chen W, et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip. 2019; 19(6): 948-958. doi:10.1039/c8lc01298a
- 67Lee YN, Yi HJ, Goh H, et al. Spheroid fabrication using concave microwells enhances the differentiation efficacy and function of insulin-producing cells via cytoskeletal changes. Cell. 2020; 9(12): 2551. doi:10.3390/cells9122551
- 68Jun Y, Kang AR, Lee JS, et al. 3D co-culturing model of primary pancreatic islets and hepatocytes in hybrid spheroid to overcome pancreatic cell shortage. Biomaterials. 2013; 34(15): 3784-3794. doi:10.1016/j.biomaterials.2013.02.010
- 69Liang J, Ng KY, Cheng Q, Xia Y, Wang CC, Leung PS. Human fetal liver stromal cell Co-culture enhances the differentiation of pancreatic progenitor cells into islet-like cell clusters. Stem Cell Rev Rep. 2014; 10(2): 280-294. doi:10.1007/s12015-013-9491-y
- 70Navarro-Tableros V, Gai C, Gomez Y, et al. Islet-like structures generated in vitro from adult human liver stem cells revert hyperglycemia in diabetic SCID mice. Stem Cell Rev Rep. 2018; 15(1): 93-111. doi:10.1007/s12015-018-9845-6
- 71Lee YN, Yi H-J, Seo EH, et al. Improvement of the therapeutic capacity of insulin-producing cells trans-differentiated from human liver cells using engineered cell sheet. Stem Cell Res Ther. 2021; 12(1): 3. doi:10.1186/s13287-020-02080-0
- 72Tao T, Deng P, Wang Y, et al. Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet Axis in Normal and type 2 diabetes. Adv Sci. 2021; 9(5):e2103495. doi:10.1002/advs.202103495
10.1002/advs.202103495 Google Scholar
- 73Jung EJ, Kim SC, Wee YM, et al. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy. 2011; 13(1): 19-29. doi:10.3109/14653249.2010.518608
- 74Kang S, Park HS, Jo A, et al. Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization. Diabetes. 2012; 61(4): 866-876. doi:10.2337/db10-1492
- 75Lebreton F, Lavallard V, Bellofatto K, et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat Commun. 2019; 10(1): 4491. doi:10.1038/s41467-019-12472-3
- 76Frum T, Spence JR. hPSC-derived organoids: models of human development and disease. J Mol Med. 2021; 99(4): 463-473. doi:10.1007/s00109-020-01969-w
- 77Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011; 146(1): 18-36. doi:10.1016/j.cell.2011.06.030
- 78Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977; 145(1): 204-220. doi:10.1084/jem.145.1.204
- 79Gan Z, Qin X, Liu H, Liu J, Qin J. Recent advances in defined hydrogels in organoid research. Bioact Mater. 2023; 28: 386-401. doi:10.1016/j.bioactmat.2023.06.004
- 80Yin J, Meng H, Lin J, Ji W, Xu T, Liu H. Pancreatic islet organoids-on-a-chip: how far have we gone? J Nanobiotechnol. 2022; 20(1):308. doi:10.1186/s12951-022-01518-2
- 81Liu H, Wang Y, Cui K, Guo Y, Zhang X, Qin J. Advances in hydrogels in organoids and organs-on-a-Chip. Adv Mater. 2019; 31(50):e1902042. doi:10.1002/adma.201902042
- 82Youngblood RL, Sampson JP, Lebioda KR, Shea LD. Microporous scaffolds support assembly and differentiation of pancreatic progenitors into beta-cell clusters. Acta Biomater. 2019; 96: 111-122. doi:10.1016/j.actbio.2019.06.032
- 83Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009; 459(7244): 262-265. doi:10.1038/nature07935
- 84Cras-Meneur C, Elghazi L, Czernichow P, Scharfmann R. Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation. Diabetes. 2001; 50(7): 1571-1579. doi:10.2337/diabetes.50.7.1571
- 85Ahnfelt-Ronne J, Ravassard P, Pardanaud-Glavieux C, Scharfmann R, Serup P. Mesenchymal bone morphogenetic protein signaling is required for normal pancreas development. Diabetes. 2010; 59(8): 1948-1956. doi:10.2337/db09-1010
- 86Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011; 108(28): 11452-11457. doi:10.1073/pnas.1106083108
- 87Huang H, Karanth SS, Guan Y, et al. Oxygenated scaffolds for pancreatic endocrine differentiation from induced pluripotent stem cells. Adv Healthc Mater. 2023; 13:e2302275. doi:10.1002/adhm.202302275
- 88Dong PDS, Munson CA, Norton W, et al. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007; 39(3): 397-402. doi:10.1038/ng1961
- 89Mfopou JK, Chen B, Mateizel I, Sermon K, Bouwens L. Noggin, Retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010; 138(7): 2233-2245.e14. doi:10.1053/j.gastro.2010.02.056
- 90Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell. 2005; 17(6): 855-868. doi:10.1016/j.molcel.2005.02.022
- 91Bhushan A, Itoh N, Kato S, et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001; 128(24): 5109-5117. doi:10.1242/dev.128.24.5109
- 92Meng Y, Ren Z, Xu F, et al. Nicotinamide promotes cell survival and differentiation as kinase inhibitor in human pluripotent stem cells. Stem Cell Rep. 2018; 11(6): 1347-1356. doi:10.1016/j.stemcr.2018.10.023
- 93Modell AE, Lim D, Nguyen TM, Sreekanth V, Choudhary A. CRISPR-based therapeutics: current challenges and future applications. Trends Pharmacol Sci. 2022; 43(2): 151-161. doi:10.1016/j.tips.2021.10.012
- 94Wang X, Li Y, Wang X, et al. Guanidyl-rich poly(β amino Ester)s for universal functional cytosolic protein delivery and clustered regularly interspaced short palindromic repeats (CRISPR) Cas9 ribonucleoprotein based gene editing. ACS Nano. 2023; 17(18): 17799-17810. doi:10.1021/acsnano.3c03269
- 95Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013; 339(6121): 819-823. doi:10.1126/science.1231143
- 96Mali P, Yang L, Esvelt KM, et al. RNA-guided human genome engineering via Cas9. Science. 2013; 339(6121): 823-826. doi:10.1126/science.1232033
- 97Cho EY, Ryu JY, Lee HAR, et al. Lecithin nano-liposomal particle as a CRISPR/Cas9 complex delivery system for treating type 2 diabetes. J Nanobiotechnol. 2019; 17(1): 19. doi:10.1186/s12951-019-0452-8
- 98Lopez Rodriguez M, Kaminska D, Lappalainen K, Pihlajamaki J, Kaikkonen MU, Laakso M. Identification and characterization of a FOXA2-regulated transcriptional enhancer at a type 2 diabetes intronic locus that controls GCKR expression in liver cells. Genome Med. 2017; 9(1): 63. doi:10.1186/s13073-017-0453-x