Ubiquitin-conjugating enzyme E2 for regulating autophagy in diabetic cardiomyopathy: A mini-review
Yueran Zhou
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Search for more papers by this authorZequn Zheng
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Search for more papers by this authorShenglin Wu
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Search for more papers by this authorCorresponding Author
Jinxiu Zhu
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Longgang Maternity and Child Institute of Shantou University Medical College (Longgang District Maternity & Child Healthcare Hospital of Shenzhen City), Shenzhen, China
Correspondence
Jinxiu Zhu, Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, NO.57 Changping Road, Jiinping District, Shantou, Guangdong, 515041, China.
Email: [email protected]
Search for more papers by this authorYueran Zhou
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Search for more papers by this authorZequn Zheng
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Search for more papers by this authorShenglin Wu
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Search for more papers by this authorCorresponding Author
Jinxiu Zhu
Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Longgang Maternity and Child Institute of Shantou University Medical College (Longgang District Maternity & Child Healthcare Hospital of Shenzhen City), Shenzhen, China
Correspondence
Jinxiu Zhu, Institute of Clinical Electrocardiology, First Affiliated Hospital of Shantou University Medical College, NO.57 Changping Road, Jiinping District, Shantou, Guangdong, 515041, China.
Email: [email protected]
Search for more papers by this authorYueran Zhou is the first author of this study.
Abstract
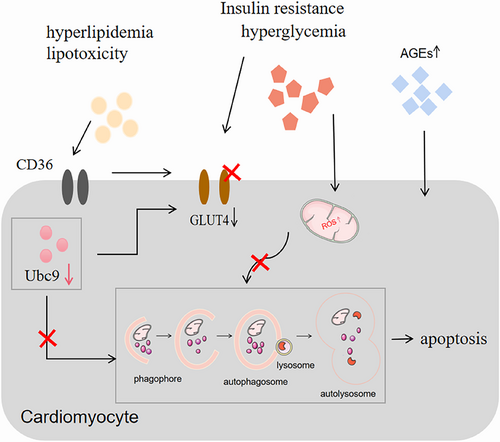
The prevalence of diabetic cardiomyopathy (DCM) increases year by year with the increase in the prevalence of diabetes mellitus (DM), which is one of the most serious cardiovascular complications of DM and a major cause of death in diabetic patients. Although the pathological molecular features of DCM have not been fully elucidated, increasing evidence suggests that impaired autophagy in cardiomyocytes plays a nonnegligible role in the development of DCM. It has been shown that SUMOylation [SUMO = small ubiquitin-like modifier], a post-translational modification of proteins, and its associated ubiquitin-proteasome system mediates protein quality control in the heart and plays an important role in the proteotoxic environment of the heart. Specifically, the expression of ubiquitin-conjugating enzyme E2 (Ubc9), the only SUMO-E2 enzyme, exerts a positive regulatory effect on autophagy in cardiomyocytes with potential cardioprotective effects. This review focuses on the role that autophagy plays in DCM and the potential for Ubc9-regulated autophagy pathways to ameliorate DCM, highlighting the potential of Ubc9 as an interventional target in DCM and providing new insights into the pathogenesis of the disease.
REFERENCES
- 1Zheng P-F, Xiong Z, Liao C-Y, et al. In vitro and in silico studies of bis (indol-3-yl) methane derivatives as potential α-glucosidase and α-amylase inhibitors. J Enzyme Inhib Med Chem. 2021; 36: 1938-1951.
- 2Ikegami H, Hiromine Y, Noso S. Insulin-dependent diabetes mellitus in older adults: current status and future prospects. Geriatr Gerontol Int. 2022; 22: 549-553.
- 3Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016; 12: 144-153.
- 4Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014; 142: 375-415.
- 5Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019; 124: 121-141.
- 6Lu Q-B, Ding Y, Liu Y, et al. Metrnl ameliorates diabetic cardiomyopathy via inactivation of cGAS/STING signaling dependent on LKB1/AMPK/ULK1-mediated autophagy. J Adv Res. 2022; 51: 161-179.
- 7Riehle C, Abel ED. Insulin regulation of myocardial autophagy. Circ J. 2014; 78: 2569-2576.
- 8Dewanjee S, Vallamkondu J, Kalra RS, John A, Reddy PH, Kandimalla R. Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 2021; 68:101338.
- 9Gupta MK, McLendon PM, Gulick J, James J, Khalili K, Robbins J. UBC9-mediated Sumoylation favorably impacts cardiac function in compromised hearts. Circ Res. 2016; 118: 1894-1905.
- 10Li N, Wang L-J, Jiang B, et al. Recent progress of the development of dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Eur J Med Chem. 2018; 151: 145-157.
- 11Ruze R, Liu T, Zou X, et al. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne). 2023; 14:1161521.
- 12Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013; 36(Suppl 1): S67-S74.
- 13Demir S, Nawroth PP, Herzig S, Ekim ÜB. Emerging targets in type 2 diabetes and diabetic complications. Adv Sci (Weinh). 2021; 8:e2100275.
- 14Dillmann WH. Diabetic cardiomyopathy. Circ Res. 2019; 124: 1160-1162.
- 15Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018; 122: 624-638.
- 16Bouthoorn S, Valstar GB, Gohar A, et al. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: a systematic review and meta-analysis. Diab Vasc Dis Res. 2018; 15: 477-493.
- 17Haji M, Erqou S, Fonarow GC, Echouffo-Tcheugui JB. Type 1 diabetes and risk of heart failure: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2023; 202:110805.
- 18Murtaza G, Virk HUH, Khalid M, et al. Diabetic cardiomyopathy-a comprehensive updated review. Prog Cardiovasc Dis. 2019; 62: 315-326.
- 19Chen Y, Hua Y, Li X, Arslan IM, Zhang W, Meng G. Distinct types of cell death and the implication in diabetic cardiomyopathy. Front Pharmacol. 2020; 11: 42.
- 20Zang H, Wu W, Qi L, et al. Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes. 2020; 69: 2720-2734.
- 21Parmar UM, Jalgaonkar MP, Kulkarni YA, Oza MJ. Autophagy-nutrient sensing pathways in diabetic complications. Pharmacol Res. 2022; 184:106408.
- 22Hsuan C-F, Teng SIF, Hsu C-N, et al. Emerging therapy for diabetic cardiomyopathy: from molecular mechanism to clinical practice. Biomedicine. 2023; 11: 662.
- 23Wang H, Wang L, Hu F, et al. Neuregulin-4 attenuates diabetic cardiomyopathy by regulating autophagy via the AMPK/mTOR signalling pathway. Cardiovasc Diabetol. 2022; 21: 205.
- 24Liu D, Xing R, Zhang Q, et al. The CREG1-FBXO27-LAMP2 axis alleviates diabetic cardiomyopathy by promoting autophagy in cardiomyocytes. Exp Mol Med. 2023; 55: 2025-2038.
- 25Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023; 24: 167-185.
- 26Li W, He P, Huang Y, et al. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 2021; 11: 222-256.
- 27Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011; 147: 728-741.
- 28Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021; 17: 2706-2733.
- 29Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021; 40:e108863.
- 30Yamamoto H, Zhang S, Mizushima N. Autophagy genes in biology and disease. Nat Rev Genet. 2023; 24: 382-400.
- 31Fleming A, Bourdenx M, Fujimaki M, et al. The different autophagy degradation pathways and neurodegeneration. Neuron. 2022; 110: 935-966.
- 32Sun-Wang JL, Yarritu-Gallego A, Ivanova S, Zorzano A. The ubiquitin-proteasome system and autophagy: self-digestion for metabolic health. Trends Endocrinol Metab. 2021; 32: 594-608.
- 33Migneault F, Hébert M-J. Autophagy, tissue repair, and fibrosis: a delicate balance. Matrix Biol. 2021; 100-101: 182-196.
- 34Gonzalez CD, Lee M-S, Marchetti P, et al. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy. 2011; 7: 2-11.
- 35Xiao Y, Wu QQ, Duan MX, et al. TAX1BP1 overexpression attenuates cardiac dysfunction and remodeling in STZ-induced diabetic cardiomyopathy in mice by regulating autophagy. Biochim Biophys Acta Mol Basis Dis. 1864; 2018: 1728-1743.
- 36Tong M, Saito T, Zhai P, et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. 2019; 124: 1360-1371.
- 37Lim H, Lim Y-M, Kim KH, et al. A novel autophagy enhancer as a therapeutic agent against metabolic syndrome and diabetes. Nat Commun. 2018; 9: 1438.
- 38Zhang H, Ge S, He K, et al. FoxO1 inhibits autophagosome-lysosome fusion leading to endothelial autophagic-apoptosis in diabetes. Cardiovasc Res. 2019; 115: 2008-2020.
- 39Lee JM, Hammarén HM, Savitski MM, Baek SH. Control of protein stability by post-translational modifications. Nat Commun. 2023; 14: 201.
- 40Chang E, Abe J-I. Kinase-SUMO networks in diabetes-mediated cardiovascular disease. Metabolism. 2016; 65: 623-633.
- 41Kroonen JS, Vertegaal ACO. Targeting SUMO signaling to wrestle cancer. Trends Cancer. 2021; 7: 496-510.
- 42Chang H-M, Yeh ETH. SUMO: from bench to bedside. Physiol Rev. 2020; 100: 1599-1619.
- 43Gupta MK, Robbins J. Making the connections: autophagy and post-translational modifications in cardiomyocytes. Autophagy. 2016; 12: 2252-2253.
- 44Zhang Y-Q, Sarge KD. Sumoylation regulates Lamin a function and is lost in Lamin a mutants associated with familial cardiomyopathies. J Cell Biol. 2008; 182: 35-39.
- 45Bian X, Xu J, Zhao H, et al. Zinc-induced SUMOylation of dynamin-related protein 1 protects the heart against ischemia-reperfusion injury. Oxid Med Cell Longev. 2019; 2019:1232146.
- 46Sadeghi M, Dehnavi S, Shohan M, Jamialahmadi T, Sathyapalan T, Sahebkar A. Potential role of SUMO and SUMOylation in the pathogenesis of diabetes mellitus. Curr Med Chem. 2023; 30: 1623-1637.
- 47Wang F, Sun F, Luo J, et al. Loss of ubiquitin-conjugating enzyme E2 (Ubc9) in macrophages exacerbates multiple low-dose streptozotocin-induced diabetes by attenuating M2 macrophage polarization. Cell Death Dis. 2019; 10: 892.
- 48Li M, Guo D, Isales CM, et al. SUMO wrestling with type 1 diabetes. J Mol Med (Berl). 2005; 83: 504-513.
- 49Kampmann U, Christensen B, Nielsen TS, et al. GLUT4 and UBC9 protein expression is reduced in muscle from type 2 diabetic patients with severe insulin resistance. PLoS ONE. 2011; 6:e27854.
- 50Liu L-B, Omata W, Kojima I, Shibata H. The SUMO conjugating enzyme Ubc9 is a regulator of GLUT4 turnover and targeting to the insulin-responsive storage compartment in 3T3-L1 adipocytes. Diabetes. 2007; 56: 1977-1985.
- 51Bertrand L, Auquier J, Renguet E, et al. Glucose transporters in cardiovascular system in health and disease. Pflugers Arch. 2020; 472: 1385-1399.
- 52Hou N, Mai Y, Qiu X, et al. Carvacrol attenuates diabetic cardiomyopathy by modulating the PI3K/AKT/GLUT4 pathway in diabetic mice. Front Pharmacol. 2019; 10: 998.
- 53Wende AR, Schell JC, Ha C-M, et al. Maintaining myocardial glucose utilization in diabetic cardiomyopathy accelerates mitochondrial dysfunction. Diabetes. 2020; 69: 2094-2111.
- 54Gupta MK, Gulick J, Liu R, Wang X, Molkentin JD, Robbins J. Sumo E2 enzyme UBC9 is required for efficient protein quality control in cardiomyocytes. Circ Res. 2014; 115: 721-729.
- 55Xiao Q, Chen X-H, Jiang R-C, et al. Ubc9 attenuates myocardial ischemic injury through accelerating Autophagic flux. Front Pharmacol. 2020; 11:561306.