Lipocalin 2—A bone-derived anorexigenic and β-cell promoting signal: From mice to humans
Yuying Yang
Department of Endocrine and Metabolic Diseases, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Institute of Endocrine and Metabolic Diseases, and Shanghai Clinical Center for Endocrine and Metabolic Diseases, Shanghai, China
Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Clinical Research Center for Metabolic Diseases, Shanghai National Center for Translational Medicine, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Search for more papers by this authorJianmin Liu
Department of Endocrine and Metabolic Diseases, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Institute of Endocrine and Metabolic Diseases, and Shanghai Clinical Center for Endocrine and Metabolic Diseases, Shanghai, China
Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Clinical Research Center for Metabolic Diseases, Shanghai National Center for Translational Medicine, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Search for more papers by this authorCorresponding Author
Stavroula Kousteni
Department of Physiology and Cellular Biophysics, Columbia University Medical Center, New York, New York, USA
Correspondence
Stavroula Kousteni, Department of Physiology and Cellular Biophysics, Columbia University Medical Center, New York, NY 10032, USA.
Email: [email protected]
Search for more papers by this authorYuying Yang
Department of Endocrine and Metabolic Diseases, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Institute of Endocrine and Metabolic Diseases, and Shanghai Clinical Center for Endocrine and Metabolic Diseases, Shanghai, China
Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Clinical Research Center for Metabolic Diseases, Shanghai National Center for Translational Medicine, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Search for more papers by this authorJianmin Liu
Department of Endocrine and Metabolic Diseases, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Institute of Endocrine and Metabolic Diseases, and Shanghai Clinical Center for Endocrine and Metabolic Diseases, Shanghai, China
Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Clinical Research Center for Metabolic Diseases, Shanghai National Center for Translational Medicine, Rui-jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Search for more papers by this authorCorresponding Author
Stavroula Kousteni
Department of Physiology and Cellular Biophysics, Columbia University Medical Center, New York, New York, USA
Correspondence
Stavroula Kousteni, Department of Physiology and Cellular Biophysics, Columbia University Medical Center, New York, NY 10032, USA.
Email: [email protected]
Search for more papers by this authorAbstract
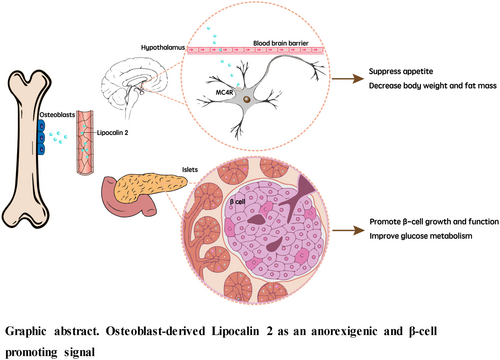
The skeleton is traditionally known for its structural support, organ protection, movement, and maintenance of mineral homeostasis. Over the last 10 years, bone has emerged as an endocrine organ with diverse physiological functions. The two key molecules in this context are fibroblast growth factor 23 (FGF23), secreted by osteocytes, and osteocalcin, a hormone produced by osteoblasts. FGF23 affects mineral homeostasis through its actions on the kidneys, and osteocalcin has beneficial effects in improving glucose homeostasis, muscle function, brain development, cognition, and male fertility. In addition, another osteoblast-derived hormone, lipocalin 2 (LCN2) has emerged into the researchers' field of vision. In this review, we mainly focus on LCN2's role in appetite regulation and glucose metabolism and also briefly introduce its effects in other pathophysiological conditions, such as nonalcoholic fatty liver disease, sarcopenic obesity, and cancer-induced cachexia.
REFERENCES
- 1Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab. 2012; 23(12): 610-618. doi:10.1016/j.tem.2012.07.002
- 2Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130(3): 456-469. doi:10.1016/j.cell.2007.05.047
- 3Khrimian L, Obri A, Karsenty G. Modulation of cognition and anxiety-like behavior by bone remodeling. Mol Metab. 2017; 6(12): 1610-1615. doi:10.1016/j.molmet.2017.10.001
- 4Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016; 5(10): 1042-1047. doi:10.1016/j.molmet.2016.07.002
- 5Oury F, Khrimian L, Denny CA, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013; 155(1): 228-241. doi:10.1016/j.cell.2013.08.042
- 6Karsenty G. Update on the biology of OSTEOCALCIN. Endocr Pract. 2017; 23(10): 1270-1274. doi:10.4158/EP171966.RA
- 7Yang YY, Zheng SC, Wang WC, et al. Osteocalcin levels in male idiopathic hypogonadotropic hypogonadism: relationship with the testosterone secretion and metabolic profiles. Front Endocrinol (Lausanne). 2019; 10:687. doi:10.3389/fendo.2019.00687
- 8Hou Y-F, Shan C, Zhuang S-Y, et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease. Microbiome. 2021; 9(1): 34. doi:10.1186/s40168-020-00988-6
- 9Liu D-M, Mosialou I, Liu J-M. Bone: another potential target to treat, prevent and predict diabetes. Diabetes Obes Metab. 2018; 20(8): 1817-1828. doi:10.1111/dom.13330
- 10Flower DR. The lipocalin protein family: a role in cell regulation. FEBS Lett. 1994; 354(1): 7-11. doi:10.1016/0014-5793(94)01078-1
- 11Chu ST, Lin HJ, Huang HL, Chen YH. The hydrophobic pocket of 24p3 protein from mouse uterine luminal fluid: fatty acid and retinol binding activity and predicted structural similarity to lipocalins. J Pept Res. 1998; 52(5): 390-397. doi:10.1111/j.1399-3011.1998.tb00663.x
- 12Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993; 268(14): 10425-10432.
- 13Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000; 1482(1–2): 9-24. doi:10.1016/s0167-4838(00)00148-5
- 14Mosialou I, Shikhel S, Liu JM, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017; 543(7645): 385-390. doi:10.1038/nature21697
- 15Petropoulou P-I, Mosialou I, Shikhel S, et al. Lipocalin-2 is an anorexigenic signal in primates. Elife. 2020; 9: 9. doi:10.7554/eLife.58949
- 16Paton CM, Rogowski MP, Kozimor AL, Stevenson JL, Chang H, Cooper JA. Lipocalin-2 increases fat oxidation in vitro and is correlated with energy expenditure in normal weight but not obese women. Obesity (Silver Spring). 2013; 21(12): E640-E648. doi:10.1002/oby.20507
- 17Lim WH, Wong G, Lim EM, et al. Circulating Lipocalin 2 levels predict fracture-related hospitalizations in elderly women: a prospective cohort study. J Bone Miner Res. 2015; 30(11): 2078-2085. doi:10.1002/jbmr.2546
- 18Liu D-M, Zhao H-Y, Zhao L, et al. The relationship among serum lipocalin 2, bone turnover markers, and bone mineral density in outpatient women. Endocrine. 2018; 59(2): 304-310. doi:10.1007/s12020-017-1504-1
- 19Rucci N, Capulli M, Piperni SG, et al. Lipocalin 2: a new mechanoresponding gene regulating bone homeostasis. J Bone Miner Res. 2015; 30(2): 357-368. doi:10.1002/jbmr.2341
- 20Capulli M, Rufo A, Teti A, Rucci N. Global transcriptome analysis in mouse calvarial osteoblasts highlights sets of genes regulated by modeled microgravity and identifies a “mechanoresponsive osteoblast gene signature”. J Cell Biochem. 2009; 107(2): 240-252. doi:10.1002/jcb.22120
- 21Veeriah V, Zanniti A, Paone R, et al. Interleukin-1β, lipocalin 2 and nitric oxide synthase 2 are mechano-responsive mediators of mouse and human endothelial cell-osteoblast crosstalk. Sci Rep. 2016; 6:29880. doi:10.1038/srep29880
- 22Park K-A, Jin Z, An HS, et al. Effects of caloric restriction on the expression of lipocalin-2 and its receptor in the brown adipose tissue of high-fat diet-fed mice. Korean J Physiol Pharmacol. 2019; 23(5): 335-344. doi:10.4196/kjpp.2019.23.5.335
- 23Wang Y, Lam KSL, Kraegen EW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007; 53(1): 34-41. doi:10.1373/clinchem.2006.075614
- 24Mosialou I, Shikhel S, Luo N, et al. Lipocalin-2 counteracts metabolic dysregulation in obesity and diabetes. J Exp Med. 2020; 217(10):e20191261. doi:10.1084/jem.20191261
- 25Law IKM, Xu A, Lam KSL, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010; 59(4): 872-882. doi:10.2337/db09-1541
- 26Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008; 22(6): 1416-1426. doi:10.1210/me.2007-0420
- 27Chen T, Zhang Y, Liu Y, et al. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging (Albany NY). 2019; 11(18): 7510-7524. doi:10.18632/aging.102263
- 28Li X, Zhang D, Vatner DF, et al. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc Natl Acad Sci U S A. 2020; 117(51): 32584-32593. doi:10.1073/pnas.1922169117
- 29Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995; 269(5223): 546-549. doi:10.1126/science.7624778
- 30Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022; 10(4): 284-296. doi:10.1016/S2213-8587(22)00003-1
- 31Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021; 397(10290): 2212-2224. doi:10.1016/S0140-6736(20)32511-3
- 32Xin Z, Huang J, Cao Q, et al. Nonalcoholic fatty liver disease in relation to the remission and progression along the glycemic continuum. J Diabetes. 2022; 14(9): 606-619. doi:10.1111/1753-0407.13314
- 33Su W, Wang Y, Chen K, et al. Association between nonalcoholic fatty liver and increased low-level albuminuria in postmenopausal women in China: a cross-sectional study. J Diabetes. 2021; 13(6): 494-505. doi:10.1111/1753-0407.13134
- 34Auguet T, Terra X, Quintero Y, et al. Liver lipocalin 2 expression in severely obese women with non alcoholic fatty liver disease. Exp Clin Endocrinol Diabetes. 2013; 121(2): 119-124. doi:10.1055/s-0032-1331696
- 35Xu Y, Zhu Y, Jadhav K, et al. Lipocalin-2 protects against diet-induced nonalcoholic fatty liver disease by targeting hepatocytes. Hepatol Commun. 2019; 3(6): 763-775. doi:10.1002/hep4.1341
- 36Lambertz J, Berger T, Mak TW, van Helden J, Weiskirchen R. Lipocalin-2 in fructose-induced fatty liver disease. Front Physiol. 2017; 8: 964. doi:10.3389/fphys.2017.00964
- 37Wen X, Su B, Gao M, et al. Obesity-associated up-regulation of lipocalin 2 protects gastric mucosa cells from apoptotic cell death by reducing endoplasmic reticulum stress. Cell Death Dis. 2021; 12(2): 221. doi:10.1038/s41419-021-03512-2
- 38Borkham-Kamphorst E, Van de Leur E, Haas U, Weiskirchen R. Liver parenchymal cells lacking Lipocalin 2 (LCN2) are prone to endoplasmic reticulum stress and unfolded protein response. Cell Signal. 2019; 55: 90-99. doi:10.1016/j.cellsig.2019.01.001
- 39Yong J, Parekh VS, Reilly SM, et al. Chop/Ddit3 depletion in β cells alleviates ER stress and corrects hepatic steatosis in mice. Sci Transl Med. 2021; 13(604):eaba9796. doi:10.1126/scitranslmed.aba9796
- 40Ye D, Yang K, Zang S, et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J Hepatol. 2016; 65(5): 988-997. doi:10.1016/j.jhep.2016.05.041
- 41Kim KE, Lee J, Shin HJ, et al. Lipocalin-2 activates hepatic stellate cells and promotes nonalcoholic steatohepatitis in high-fat diet-fed Ob/Ob mice. Hepatology. 2023; 77(3): 888-901. doi:10.1002/hep.32569
- 42Ponzetti M, Aielli F, Ucci A, et al. Lipocalin 2 increases after high-intensity exercise in humans and influences muscle gene expression and differentiation in mice. J Cell Physiol. 2022; 237(1): 551-565. doi:10.1002/jcp.30501
- 43Sousa-Victor P, García-Prat L, Muñoz-Cánoves P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol. 2022; 23(3): 204-226. doi:10.1038/s41580-021-00421-2
- 44Rebalka IA, Monaco CMF, Varah NE, et al. Loss of the adipokine lipocalin-2 impairs satellite cell activation and skeletal muscle regeneration. Am J Physiol Cell Physiol. 2018; 315(5): C714-C721. doi:10.1152/ajpcell.00195.2017
- 45Choi EB, Jeong JH, Jang HM, et al. Skeletal Lipocalin-2 Is associated with iron-related oxidative stress in Ob/Ob mice with sarcopenia. Antioxidants. 2021; 10(5):758. doi:10.3390/antiox10050758
- 46Santiago-Sánchez GS, Pita-Grisanti V, Quiñones-Díaz B, Gumpper K, Cruz-Monserrate Z, Vivas-Mejía PE. Biological functions and therapeutic potential of Lipocalin 2 in cancer. Int J Mol Sci. 2020; 21(12):4365. doi:10.3390/ijms21124365
- 47Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996; 38(3): 414-420. doi:10.1136/gut.38.3.414
- 48Lee E-K, Kim H-J, Lee K-J, et al. Inhibition of the proliferation and invasion of hepatocellular carcinoma cells by lipocalin 2 through blockade of JNK and PI3K/Akt signaling. Int J Oncol. 2011; 38(2): 325-333. doi:10.3892/ijo.2010.854
- 49Tong Z, Kunnumakkara AB, Wang H, et al. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008; 68(15): 6100-6108. doi:10.1158/0008-5472.CAN-08-0540
- 50Moniaux N, Chakraborty S, Yalniz M, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008; 98(9): 1540-1547. doi:10.1038/sj.bjc.6604329
- 51Xu B, Zheng W-Y, Feng J-F, Huang X-Y, Ge H. One potential oncolytic adenovirus expressing Lipocalin-2 for colorectal cancer therapy. Cancer Biother Radiopharm. 2013; 28(5): 415-422. doi:10.1089/cbr.2012.1352
- 52Olson B, Zhu X, Norgard MA, et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun. 2021; 12(1): 2057. doi:10.1038/s41467-021-22361-3
- 53Lemecha M, Chalise JP, Takamuku Y, et al. Lcn2 mediates adipocyte-muscle-tumor communication and hypothermia in pancreatic cancer cachexia. Mol Metab. 2022; 66:101612. doi:10.1016/j.molmet.2022.101612
- 54Wang D, Li X, Jiao D, et al. LCN2 secreted by tissue-infiltrating neutrophils induces the ferroptosis and wasting of adipose and muscle tissues in lung cancer cachexia. J Hematol Oncol. 2023; 16(1): 30. doi:10.1186/s13045-023-01429-1
- 55Talbert EE, Guttridge DC. Emerging signaling mediators in the anorexia-cachexia syndrome of cancer. Trends Cancer. 2022; 8(5): 397-403. doi:10.1016/j.trecan.2022.01.004
- 56McLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr Rev. 2021; 42(2): 101-132. doi:10.1210/endrev/bnaa032
- 57Xing X, Wang Y, Pan F, Cai G. Osteoarthritis and risk of type 2 diabetes: a two-sample Mendelian randomization analysis. J Diabetes. July. 2023. doi:10.1111/1753-0407.13451. Online ahead of print.
- 58Yao F, Deng Y, Zhao Y, et al. A targetable LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun. 2021; 12(1): 7333. doi:10.1038/s41467-021-27452-9