Trends in clinical characteristics, medication use, and glycemic control in insulin-treated patients with type 1 and type 2 diabetes in Finland in 2012–2019: Nationwide real-world evidence study
Abstract
Aims
To describe the clinical characteristics and medication purchases of insulin-treated adults in Finland at index (January 1, 2012 or first insulin purchase) and December 31, 2019. Additionally, to describe basal insulin (BI) treatment patterns and associated changes in hemoglobin A1c (HbA1c) values.
Materials and Methods
In this descriptive study using nationwide registries, we included adults with at least two reimbursed insulin purchases within 12 months of the first purchase between January 1, 2012 and December 31, 2019. We formed four study groups: type 1 diabetes (T1D) and type 2 diabetes (T2D)-diagnosed people who were further divided into prevalent or naïve users (start of insulin use before or after January 1, 2012). Insulin treatment patterns were estimated from medication purchase data and glycemic control from HbA1c results.
Results
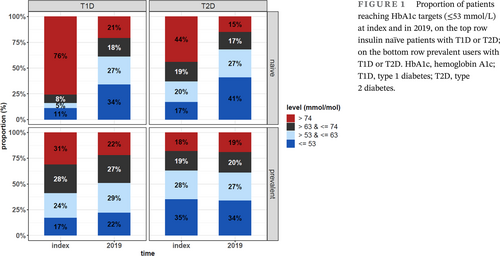
Out of 145 020 people included, 34 359 had T1D and 110 661 T2D. By 2019, in parallel with the adaptation of new noninsulin medications, second-generation basal insulin (BI) analogues were adopted by 45.9% and 21.1% of prevalent T1D and T2D users. At index, HbA1c target (≤53 mmol/mol) was reached by 17% and 35% of T2D naïve and prevalent users, respectively, and by 17% of T1D prevalent users. At study end, the target was reached respectively by 41%, 34%, and 22% of insulin users. Insulin initiation improved and discontinuation worsened glycemic control in T2D, with lesser effects seen after treatment gaps or switches between BIs.
Conclusions
Our study showed that glycemic control in insulin users has remained stable or improved between 2012 and 2019 despite aging population and in parallel with introduction of new treatment options, providing valuable insight into Finnish national diabetes care.
DISCLOSURE
Leo Niskanen has received speaker honoraria from Amgen, Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Sanofi, MSD, and AstraZeneca; research support from Novo Nordisk to the hospital; and has participated in the scientific advisory boards of Amgen, Boehringer Ingelheim, Eli Lilly AstraZeneca, MSD, and Novo Nordisk. Timo T. Valle has received speaker honoraria from AstraZeneza, BMS, Boehringer Ingelheim, SK, Eli Lilly, Takeda, MSD, Mundipharma, Mylan, Novartis, Novo Nordisk, Orion, Sanofi, and Schering Plough and has provided consultation and participated in the scientific advisory boards of AstraZeneca, BMS, Eli Lilly, Janssen/J&J, MSD, Mundipharma, Mylan, Novartis, Novo Nordisk, and Sanofi. Kai Kysenius, Saara Kaijala, and Mariann I. Lassenius are employees of Medaffcon Oy. Minna Hannula is an employee of Sanofi Oy.