Transferrin receptor levels and its rare variant are associated with human obesity
Jin Qiu, Zhiyin Zhang, and Yepeng Hu contributed equally to this work.
Abstract
Aim
Iron homeostasis is critical for functional respiratory chain complex of mitochondrial, thus potentially contributing to fat biology and energy homeostasis. Transferrin receptor (Tfrc) binds to transferrin for extracellular iron uptake and is recently reported to be involved in brown fat development and functionality. However, whether TFRC levels and variants are associated with human obesity is unknown.
Methods
To investigate the association of TFRC levels and variants with human obesity, fat biopsies were obtained from surgery. Exon-sequencing and genetic assessments were conducted of a case–control study. For TFRC levels assessment in fat biopsy, 9 overweight and 12 lean subjects were involved. For genetic study, obese (n = 1271) and lean subjects (n = 1455) were involved. TFRC levels were compared in abdominal mesenteric fat of pheochromocytoma patients versus control subjects, and overweight versus lean subjects. For genetic study, whole-exome sequencing of obese and matched control subjects were conducted and analyzed. In addition, the possible disruption in protein stability of TFRC variant was assessed by structural and molecular analysis.
Results
TFRC levels are increased in human browning adipose tissue and decreased in fat of overweight patients. Besides, TFRC levels are negatively correlated with body mass index and positively correlated with uncoupling protein 1 levels. Furthermore, a rare heterozygous missense variant p.I337V in TFRC shows a tendency to enrich in obese subjects. Structural and functional study reveals impaired protein stability of the TFRC variant compared to wild-type.
Conclusions
Reduced TFRC levels and its rare variant p.I337V with protein instability are associated with human obesity.
1 INTRODUCTION
Obesity is manifested as excess accumulation of fat in adipose tissues. Three classes of adipose tissues exist, including white adipose tissue as a major site for energy storage and brown adipose tissue with capability of thermogenesis and energy expenditure.1 Beige adipocytes, a newly discovered type of adipocytes, exist in clusters within certain white fat depots and morphologically resemble white adipocytes in rest state. Upon cold or β-adrenergic activation, beige adipocytes are activated to possess a brown-like appearance and high thermogenic capacity, a process referred to as “browning of white fat.”2-5 Clinically relevant, functional brown and beige fat have recently been shown to exist in adults. Human deep cervical, supraclavicular, and paraspinal areas are demonstrated to be enriched with brown and beige adipocytes as a heterogenous mixture with varying constituents depending on their tissue depths.6 Besides, under physiological or pathological stimulation, white adipocytes in humans can also be induced to browning. For instance, the sustained activation of β3-adrenergic signaling in pheochromocytoma patients causes strong white fat browning and enhanced thermogenesis, resulting a pathologically lean phenotype in these patients.7, 8 Critically, adipocytes undergo metabolic declines in the development of obesity or aging, leading to impaired beige/brown fat thermogenesis and reduced ability of white fat browning, indicating the potential of targeting adipose tissues to prevent and treat obesity or metabolic dysfunctions in humans.4, 9
Iron homeostasis has been shown to play vital roles in various physiological and pathological processes, including metabolic diseases, via molecular and clinical studies.10 For example, serum ferritin levels have been shown to associated with metabolic syndrome in multiple human cohorts.11 Recently, iron levels have been linked to fat biology.12 Genetic manipulation of genes related to iron homeostasis, such as transferrin receptor (TFRC), a membrane protein binding to transferrin for extracellular iron uptake, resulted in impaired adipogenesis and functionality of fat in mice and consequently affected their energy metabolism, suggesting the importance of iron metabolism in obesity and metabolic diseases.13, 14
In the present study, we sought to unveil the association of TFRC levels and TFRC variants with human obesity. Of note, we found that, in abdominal mesenteric regions of fat, TFRC levels were increased in pheochromocytoma patients and decreased in overweight patients, compared to their control counterparts. Besides, TFRC levels negatively correlated with body mass index (BMI) and positively correlated with uncoupling protein 1 (UCP1) levels in fat. In addition, via whole-exome sequencing (WES), we discovered a rare heterozygous missense variant p.I337V in TFRC, which showed a tendency to enrich in obese subjects, indicating its potential contribution to obesity possibly due to its impaired protein stability. Overall, we revealed the association of TFRC with human obesity and proposed TFRC as a promising therapeutic target for obesity and metabolic diseases in human.
2 MATERIALS AND METHODS
2.1 Human adipose tissue biopsy samples
Human fat biopsies were obtained from the abdominal mesenteric region of fat from overweight (BMI ≥ 25 kg/m2) and lean subjects (18 kg/m2 < BMI < 25 kg/m2), as well as pheochromocytoma patients and control subjects who received surgery in the First Affiliated Hospital of Wenzhou Medical University or Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (SJTUSM), with written informed consent, as described before.15
2.2 Immunostaining of adipose tissues
For immunohistochemistry staining, adipose tissues were placed in Bouin fixative, embedded in paraffin, and subsequently cut into 5 μm sections. Sections were deparaffinized hydrated in xylene and graded ethanol and rinsed in PBS for 5 min before being incubated with pepsin (Sigma, USA). The sections were incubated for 10 min in 0.3% H2O2 to quench endogenous peroxidase activity. Sections were blocked and incubated at 4°C overnight with diluted polyclonal antibodies against UCP1 (ab10983, Abcam, UK) and TFRC (ab84036, Abcam, UK) and with horseradish peroxidase conjected goat-anti rabbit IgG for 1 h. DAB chromogen (DAB Substrate Kit, H-2200, Vector Labs, USA) was used for peroxidase detection of immunoreactivity. Images were taken with a Zeiss 710 confocal microscope.
2.3 Human genetic study
For genetic analysis of low-frequency or rare variants in the coding regions of TFRC gene with the threshold of minor allele frequency below 5% (MAF < 5%), we examined our in-home database consisting of the WES data of patients with obesity (BMI ≥30 kg/m2) and matched control subjects.16 Basic information was obtained from the Genetics of Obesity in Chinese Youngs study, which was previously established by Ruijin Hospital and registered in ClinicalTrials.gov (ClinicalTrials reg. no. NCT01084967, http://www.clinicaltrials.gov/).17, 18 The clinical procedures for anthropometric assessment, biochemical measurement, blood glucose, and lipid ascertainment were performed according to previously described protocols.17 Functional prediction of TFRC variants is conducted by SIFT (https://sift.bii.a-star.edu.sg/), PolyPhen2 (http://genetics.bwh.harvard.edu/pph2/), and Mutation taster (http://mutationtaster.org).
2.4 Ethics statement
The human studies were approved by the Institutional Review Board of First Affiliated Hospital of Wenzhou Medical University and Ruijin Hospital, SJTUSM. A written informed consent was obtained from each subject.
2.5 Construction of TFRC wild-type and variant plasmid and transfection in HEK293T cells with or without cycloheximide treatment
The TFRC coding sequences were obtained from cDNA and the polymerase chain reaction (PCR) primers or site mutation primers are listed in Supplementary Table S1. TFRC and mutant plasmids were transfected into HEK293T cells according to the manufacturer's protocol (C4058L1080, Shanghai Life iLab Biotech, China) and proteins were harvested at the indicated time. To further examine the protein stability of TFRC and its mutant, after transfection for 48 h, HEK293T cells were treated with cycloheximide (CHX; C7698, Sigma, USA) for the indicated time.
2.6 Mito-tracker analysis
Mitochondria quality and membrane integrity were assessed by staining with MitoTracker® Red CMXRos (Thermo Fisher, United States). Immortalized beige adipocytes were differentiated to day 4 and overexpressed with wild-type (WT) or mutant (Mut) Tfrc. After 48 h, mature adipocytes were incubated with 100 nM MitotTracker for 20 min and then washed three times with PBS. Cells were fixed with 4% PFA for 10 min and fluorescent images were obtained using the Nikon inverted microscope ECLIPSE Ts2.
2.7 Lentiviral constructions and infection
Tfrc and mutant Tfrc lentiviral particles were constructed and concentrated (GeneChem company, Shanghai). Immortalized beige preadipocytes were infected with lentiviral particles as follows: 2 × 109 PFU lentivirus carrying WT and Mut Tfrc were added to cell culture medium with 4 mg/mL sequa-brene (S2667, Sigma, USA) for 24 h and switched to fresh medium for another 3 days. Samples were collected for real-time PCR and western blot analysis.
2.8 Real time PCR
RNAs from adipose tissues were extracted using Trizol (9109, Takara, Japan). Total RNA (1 μg) was reversely transcribed with PrimeScript RT reagent Kit with gDNA Eraser kit (PR047Q, Takara, Japan) and quantitative PCR was performed using SYBR green (11143ES50, Yeasen, China) on the LightCycler480 system (Roche, Switzerland). Messenger RNA (mRNA) levels were calculated by the ΔΔCT method with the level of 36B4 or Pcdh as the internal control. The primers used for quantitative PCR are in Supplementary Table S2.
2.9 Western blot
For western blot, the protein concentrations were quantified using a BCA Protein Assay kit (P0010, Beyotime Biotechnology, China) and equal amounts of protein were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane (66 485, PALL, USA). After blocking with 5% skimmed milk, the membrane was incubated overnight at 4°C with indicated primary antibodies and subsequently incubated with secondary antibody at room temperature for 1 h. The images of the blots were detected using the Odyssey imaging system (LIed ovBiotechnology, USA).19 Antibodies used for western blot are as follows: anti-TFRC (ab84036, Abcam, UK), anti-GAPDH (sc-32233, Santa Cruz, USA), IRDye 800CW Goat anti-Rabbit IgG (P/N 926-32211, LI-COR Biotechnology, USA), and IRDye 800CW Goat anti-Mouse IgG (P/N 926-32210, LI-COR Biotechnology, USA).
2.10 Statistical analysis
Data are presented as means ± SEM in molecular analysis, and means ± SD in human clinical parameters. All statistical analysis was performed with GraphPad Prism software. The rare missense frequency in TFRC was calculated using the chi-square test, and two-tailed Student's t test was used in other experiments to determine p values and p < .05 was considered as significant.
3 RESULTS
3.1 TFRC levels are increased in activated human thermogenic fat from pheochromocytoma patients
Pheochromocytoma patients are clinically characterized by abnormal high levels of catecholamine secretion. This results in an excess sympathetic tone and strongly induces white fat browning especially in patients with high catecholamines levels.8 We collected fat biopsies from pheochromocytoma patients and control subjects15 and validated enhanced white fat browning in the abdominal mesenteric regions of pheochromocytoma patients but not for controls (Figure 1A). Of clinical relevance, we found that TFRC levels were significantly increased in adipose tissues from pheochromocytoma patients compared to control subjects (Figure 1B). Notably, TFRC staining showed a similar expression pattern and intensity compared to UCP1, a major thermogenic marker (Figure 1B). Thus, these results suggested that TFRC levels in humans were correlated with thermogenic capacity in adipose tissues.
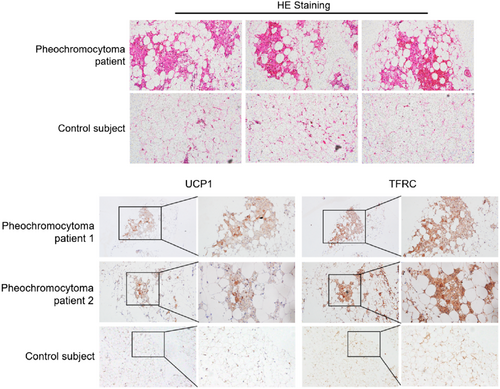
3.2 TFRC levels are decreased in adipose tissue of obese patients, as well as correlated with UCP1 levels and BMI
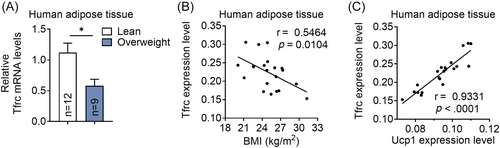
We next assess the association of TFRC levels with obesity in an overweight subjects (BMI ≥25 kg/m2, n = 9) and lean controls (18 kg/m2 < BMI < 24.9 kg/m2, n = 12) (Supplementary Table S3). Importantly, TFRC levels are decreased in abdominal mesenteric fat from overweight subjects compared to controls (Figure 2A). In addition, TFRC levels in fat are negatively correlated with BMI of human participates and positively correlated with thermogenic marker gene UCP1 levels in human biopsies (Figure 2B,C), suggesting the close correlation of TFRC levels with thermogenic adipocytes and obesity progression in human.
3.3 Rare nonsynonymous TFRC variants are enriched in young, severely obese subjects
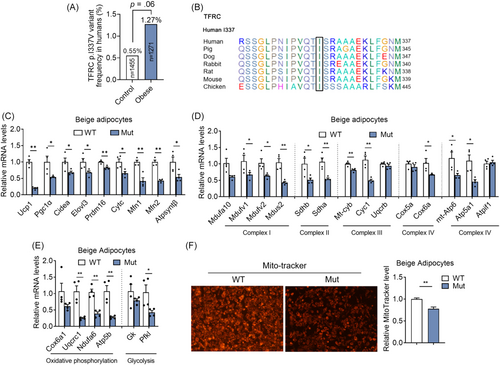
We next examined whether TFRC variants/mutants exist in human and how they impact the development of human obesity. Previous genome-wide association studies have identified the common variations nearby TFRC gene, with MAF >5%, are associated with low mean cell volume (MCV) of erythrocytes in Asian and European population.20, 21 A rare homozygous mutation of p.Y20H in TFRC was first reported in two pedigrees to cause low MCV and combined immunodeficiency by impaired TFRC internalization and consequent intracellular iron deficiency.22 To explore the potential nonsynonymous variants in TFRC, we screened the low-frequency/rare variants (MAF <5%) in the gene in our in-home database of WES data consisting of 1271 young, severely obese cases (age, 23.8 ± 7.4 years; BMI, 35.2 ± 4.7 kg/m2) and 1455 matched nonobese controls (Table 1).16 Of 23 coding mutations detected, 22 were rare missense mutations with MAF <1% except for p.L212V (MAF = 3%). Of note, a heterozygous p.I337V substitution in TFRC tended to be enriched in severely obesity group (odds ratio, 2.30; 95% confidence interval, 0.98–5.38; p = .06) (Figure 3A). Interestingly, the amino-residue p.I337 is strictly conserved across different species with the exception of Pan troglodytes (Chimpanzee), which shows the same substitution with p.V337 as the rare mutation in carriers (Figure 3B). Of note, we overexpressed WT and Mut Tfrc in adipocytes and found decreased thermogenic and mitochondrial functions in mutant Tfrc overexpressed adipocytes, suggesting the impaired metabolic functionality of mutant Tfrc (Figure 3C,D). Indeed, Mut Tfrc overexpressed in mature beige adipocytes resulted in impaired mitochondrial quality and membrane integrity as revealed by MitoTracker staining when compared with WT Tfrc group (Figure 3E). We also compared the clinical features of obese carriers and noncarriers of the missense variant and found no substantial difference between the two groups in parameters related to metabolic disorders, including obesity traits, blood glucose, insulin sensitivity, islet β cell function, serum lipids, and liver enzymes (Supplementary Table S4).
| Positiona | rsID | Nucleotide changeb | Amino acid changeb | Cases | Controls | Case _freq | Control_freq | SIFT§ | PolyPhen2‡_HDIV | Mutation taster# |
|---|---|---|---|---|---|---|---|---|---|---|
| chr3:195800925 | rs201408488 | T > C | T104A | 1/1270 | 1/1454 | 0.0004 | 0.0003 | T | B | T |
| chr3:195800829 | - | C > T | D136N | 1/1270 | 0/1455 | 0.0004 | - | T | B | T |
| chr3:195798910 | - | C > A | R183L | 1/1270 | 0/1454 | 0.0004 | - | T | B | D |
| chr3:195798320 | rs41301381 | G > C | L212V | 1/77/1193 | 2/89/1364 | 0.0311 | 0.0320 | T | B | T |
| chr3:195796384 | - | G > A | T248I | 1/1270 | 4/1451 | 0.0004 | 0.0014 | T | B | T |
| chr3:195794955 | - | A > G | M283T | 0/1271 | 1/1454 | - | 0.0003 | T | P | D |
| chr3:195794438 | - | T > C | N331D | 1/1270 | 0/1455 | 0.0004 | - | T | B | T |
| chr3:195794420 | rs41295849 | T > C | I337V | 16/1255 | 8/1447 | 0.0063 | 0.0027 | D | D | D |
| chr3:195794416 | - | G > C | S338C | 1/1270 | 0/1455 | 0.0004 | - | D | D | D |
| chr3:195792457 | - | T > G | D352A | 0/1271 | 2/1453 | - | 0.0007 | T | B | D |
| chr3:195792370 | - | A > C | L381R | 1/1270 | 1/1454 | 0.0004 | 0.0003 | T | P | D |
| chr3:195791240 | rs41295879 | C > T | G420S | 1/1270 | 0/1455 | 0.0004 | - | T | B | D |
| chr3:195791192 | - | T > C | M436V | 1/1270 | 0/1455 | 0.0004 | - | D | P | D |
| chr3:195787103 | - | T > C | K495R | 1/1270 | 0/1455 | 0.0004 | - | T | B | D |
| chr3:195785491 | - | C > T | V517I | 0/1271 | 1/1454 | - | 0.0003 | T | B | T |
| chr3:195785467 | - | C > T | D525N | 1/1270 | 0/1455 | 0.0004 | - | T | P | D |
| chr3:195785461 | - | T > G | N527H | 0/1271 | 1/1454 | - | 0.0003 | D | D | D |
| chr3:195782151 | - | C > T | G567S | 0/1271 | 1/1454 | - | 0.0003 | T | P | D |
| chr3:195782058 | rs144131234 | C > T | V598M | 1/1270 | 0/1455 | 0.0004 | - | T | B | D |
| chr3:195782043 | - | G > A | H603Y | 1/1270 | 0/1455 | 0.0004 | - | T | B | D |
| chr3:195780362 | - | A > T | L656Q | 1/1270 | 0/1455 | 0.0004 | - | D | D | D |
| chr3:195778910 | rs202242239 | G > A | T729M | 3/1268 | 5/1450 | 0.0012 | 0.0017 | D | D | D |
| chr3:195778820 | - | T > G | E759A | 0/1271 | 1/1454 | - | 0.0003 | D | B | D |
- Note: Functional prediction is conducted by SIFT§ (https://sift.bii.a-star.edu.sg/) (T, Tolerated; D, Deleterious), PolyPhen2‡ (http://genetics.bwh.harvard.edu/pph2/) (B, Benign; D, damaging; P, Probably), and Mutation taster# (http://mutationtaster.org) (D, Damaging; T, Tolerated).
- a National Center for Biotechnology Information Build 37.
- b Variations are based on RefSeq records NM_001128148 and NP_001121620.
- Abbreviation: Freq, allele frequency.
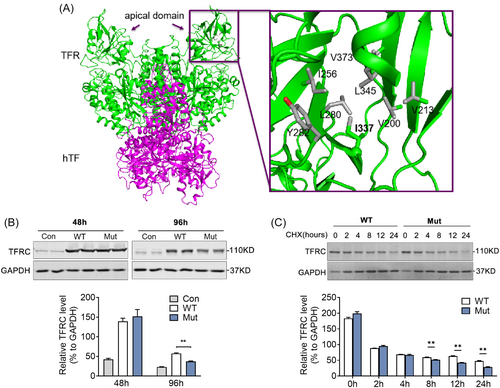
3.4 p.I337V substitution of TFRC shows impaired protein stability
We further analyzed the potential structural change (PDB 3S9M)23 of the missense variant p.I337V and found that in WT p.I337 residue was surrounded by a group of hydrophobic residues and these residues form extensive van der Waals interactions in the core of the apical domain, whereas removal of a key alkyl group (CH2-) as in the missense variant p.V337 may lead to instability of the hydrophobic core in the apical domain and compromise of TFRC's function (Figure 4A). Consistently, the missense variant was predicted to be functionally damaged by three types of predicting software (SIFT, PolyPhen2, and Mutation taster, Table 1). To verify this hypothesis, we constructed WT and Mut TFRC and transfected them in HEK293T cells to assess their protein stabilities. Indeed, we found that the TFRC Mut featured significantly reduced protein levels at 96 h, compared to WT TFRC (Figure 4B). The variants also showed decreased half-time of TFRC when treated with protein synthesis inhibitor CHX (Figure 4C). These findings suggested a potential association of rare TFRC missense variant with the instability of TFRC protein and development of obesity in humans.
4 DISCUSSION
In the present study, we reported that TFRC was strongly expressed in abdominal fat tissue from pheochromocytoma patients and decreased in overweight patients. Besides, the rare variant p.I337V in TFRC was marginally associated with human obesity and the I337V amino acid substitution led to TFRC protein instability.
Tfrc, also known as Tfr1, plays an important role for cellular iron uptake and homeostasis as receptors for transferrin. Apotransferrin (iron-free tranferrin) binds to iron atoms to form holotransferrin, which interacts with Tfrc and is internalized through receptor-mediated endocytosis into acidified endosomes. Ferrous iron is then released into cytosol for subsequent iron (Fe) metabolism.24 Iron metabolism has attracted great attention recently because it is recently reported to be involved in several physiological and pathological processes, including hematopoiesis, immunity responses, cancers, and metabolic homeostasis.25-27 It has been reported that cellular iron deficiency caused by knocking out ferritin heavy/heart chain component of ferritin led to marked mitochondria dysfunction and reduction in energy expenditure and adaptive thermogenesis, suggesting the critical role of Fe metabolism in thermogenic adipocytes.28 Of note, Tfrc fat-conditional knockout mice showed impaired thermogenesis, increased insulin resistance, and low-grade inflammation accompanied by iron deficiency and mitochondrial dysfunction,13 which highly support our clinical findings that missense variant p.I337V of TFRC may affect TFRC protein stability, as well as decreased thermogenic and mitochondrial gene levels, thus increase obesity risk. Because TFRC is indispensable for cellular iron uptake, and considering the essential role of Fe-S clusters for mitochondrial respiration, it is reasonable to postulate that an iron deficiency damaged mitochondrial thermogenesis function.29-31 Together, these studies and ours emphasized the critical role of intracellular iron balance in metabolic homeostasis in adipose tissues of rodents and humans.
It has been reported that browning of white adipose tissue occurs in pheochromocytoma patients due to the tumor-mediated release of catecholamines.7, 32, 33 Besides, we and others have identified that Tfrc is expressed predominantly in thermogenic adipocytes versus white adipocytes, and its expression levels are tightly correlated with browning status.13, 14 Thus, the increased TFRC levels in adipose tissues from pheochromocytoma patients are likely due to the high browning status. It would be interesting to further investigate the possible mechanism that TFRC was regulated by β-adrenergic signaling.
With genetic analysis of coding regions in TFRC gene, a rare missense variant p.I337V was identified to be enriched in obesity cases. The residue is evolutionally conserved across species from chicken to human only with the exception of chimpanzees, and the variant was predicted to be loss-of-function change by crystal structure analysis and predicting software. Whether the variant in those rare carriers was derived from chimpanzees or evolved separately remains to be explored in following studies.
In the present study, we have provided initial evidence supporting the association between TFRC levels and rare variants with human obesity. Future studies to validate the clinical relevance of missense variant p.I337V of TFRC with obesity need to be conducted in larger cohorts. Besides, it would be interesting to further explore the potential mechanisms underlying this relationship, as well as the detailed regulatory mechanism of TFRC protein instability. Further studies are warranted to potentially target TFRC signaling and iron metabolism in obesity treatment.
5 CONCLUSIONS
In summary, we identified that TFRC levels were increased in human browning adipose tissue and decreased in fat of obese patients. Besides, TFRC levels were positively correlated UCP1 levels in fat and negatively correlated with BMI in humans. Of note, TFRC rare missense variant p.I337V conservatively showed protein instability compared to WT TFRC in mice and humans. Overall, our study emphasized that TFRC rare variant may be associated with obesity development and TFRC gene may be a potential therapeutic target in treating human obesity.
AUTHOR CONTRIBUTIONS
Lingyan Xu, Jiqiu Wang, and Xuejiang Gu conceived the project and designed the experiments. Jin Qiu, Zhiyin Zhang, and Yepeng Hu carried out most of the experiments and collected human biopsies. Yuhan Guo, Yanru Chen, Junlei Su, and Caizhi Liu assisted in data analysis. Dongmei Wang, Sainan Wang, Sainan Xu, and Tianhui Hu provided rodent biological samples for association analysis. Gaojie Song assisted with TFRC structure analysis. Xinran Ma, Lingyan Xu, Jiqiu Wang, and Xuejiang Gu wrote and edited the paper.
ACKNOWLEDGEMENTS
This work was supported by funds from the National Key Research and Development Program of China (2019YFA0904500), the National Natural Science Foundation of China (32222024, 32071148, 32022034, 32271224), Science and Technology Commission of Shanghai Municipality (22ZR1421200, 21140904300), Natural Science Foundation of Chongqing, China (CSTB2022NSCQ-JQX0033), Key Research and Development Project of Zhejiang Province (2021C03069), Zhejiang Provincial Natural Science Foundation of China (LY20H070003), the Fundamental Research Funds for the Central Universities, ECNU public platform for Innovation (011) and the instruments sharing platform of School of Life Sciences.
CONFLICT OF INTEREST STATEMENT
No potential conflicts of interest relevant to this article were reported.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used during the current study are available from the corresponding authors upon reasonable request.




