Are psychiatric disorders associated with thyroid hormone therapy in adolescents and young adults with type 1 diabetes?
青少年和青壮年1型糖尿病患者的精神障碍与甲状腺激素治疗有关吗?
Funding information: Deutsche Diabetes Gesellschaft; Deutsches Robert Koch Insitut (RKI); Deutsches Zentrum für Diabetesforschung (DZD), Grant/Award Number: 82DZD14A02
Abstract
enBackground
To evaluate the association between thyroid autoimmunity and psychiatric disorders (depression, anxiety, eating disorder, schizophrenia or attention-deficit/hyperactivity disorder) among adolescents and young adults with type 1 diabetes (11-25 years).
Methods
We compared 9368 type 1 diabetes patients with thyroid autoimmunity (3789 of them treated with levothyroxine) with 62 438 type 1 diabetes patients without any thyroid disease from a multicentre diabetes patient follow-up registry (DPV) in terms of psychiatric disorders. Thyroid autoimmunity was defined as documented diagnosis of Hashimoto thyroiditis or positive antibodies against thyroid peroxidase or thyroglobulin. Multivariable logistic regression models were used to calculate odds ratios for the respective psychiatric disorders in type 1 diabetes patients with thyroid autoimmunity (overall and stratified by levothyroxine therapy) compared to type 1 diabetes patients without thyroid diseases (reference).
Results
Of the 9368 patients with thyroid autoimmunity, 62% were female with a median (Q1-Q3) age of 16.3 (14.2-17.6) years. Thyroid autoimmunity (with or without levothyroxine therapy) revealed a slight, but significant higher chance for depression (odds ratio [OR], 1.35, 95% confidence interval [CI], 1.19, 1.52), eating disorder (OR, 1.25, CI, 1.03, 1.51), attention-deficit/hyperactivity disorder (OR, 1.22, CI, 1.07, 1.39) and schizophrenia (OR, 1.63, CI, 1.04, 2.56). In individuals with prescribed levothyroxine therapy because of thyroid dysfunction significantly higher odds for depression (OR, 1.63, CI, 1.34, 1.99), anxiety (OR, 1.60, CI, 1.18, 2.18), and attention-deficit/hyperactivity disorder (OR, 1.71, CI, 1.38, 2.12) were observed compared to reference. Thyroid autoimmunity without required levothyroxine therapy revealed no differences to the reference group.
Conclusions
Patients on levothyroxine had significantly higher odds for psychiatric disorders, but thyroid autoimmunity in terms of high antibody levels only did not show higher odds for any psychiatric disorder.
摘要
zh背景
探讨青少年和青壮年(11~25岁)1型糖尿病患者甲状腺自身免疫功能与精神障碍(抑郁、焦虑、进食障碍、精神分裂症或注意力缺陷/多动障碍)的关系。
方法
我们比较了9368例有甲状腺自身免疫性疾病的1型糖尿病患者(其中3789例接受左旋甲状腺素治疗)和62438例来自多中心糖尿病患者随访登记(DPV)的无甲状腺疾病的1型糖尿病患者的精神障碍。甲状腺自身免疫疾病被定义为有记录的桥本甲状腺炎或甲状腺过氧化物酶或甲状腺球蛋白抗体阳性。用多变量logistic回归模型计算有甲状腺自身免疫性疾病的1型糖尿病患者与无甲状腺疾病的1型糖尿病患者各自精神障碍的优势比(OR)。
结果
9368例甲状腺自身免疫病患者中,62%为女性,中位年龄(Q1~Q3)为16.3(14.2~17.6)岁。甲状腺自身免疫性疾病(接受或不接受左旋甲状腺素治疗)显示抑郁(OR,1.35,95%置信区间[CI],1.19,1.52)、进食障碍(OR,1.25,CI,1.03,1.51)、注意力缺陷/多动障碍(OR,1.22,CI,1.07,1.39)和精神分裂症(OR,1.63,CI,1.04,2.56)发生的几率略高,且存在显著性差异。与对照组相比,因甲状腺功能障碍而接受左甲状腺素治疗的个体患抑郁症(OR,1.63,CI,1.34,1.99)、焦虑(OR,1.60,CI,1.18,2.18)和注意力缺陷/多动障碍(OR,1.71,CI,1.38,2.12)的几率显著增加。不需要左旋甲状腺素治疗的甲状腺自身免疫性疾病与对照组没有差异。
结论
服用左旋甲状腺素的患者患精神疾病的几率明显较高,但仅高抗体水平的甲状腺自身免疫疾病并不显示任何精神疾病的发病率增高。
1 INTRODUCTION
Hypothyroidism is one of the most common endocrine diseases with a prevalence of 3-10% in the general population in European countries.1 According to a meta-analysis from 2019 hypothyroidism shows a prevalence nearly twice as high (9.8% vs 4.6%) in individuals with type 1 diabetes (T1D, mean age 19.2 years) compared to the general population.2 It is characterized by undersupply of thyroid hormones such as triiodothyronine (T3) and thyroxine (T4) caused by hypofunction of the thyroid gland. Impaired thyroid function during childhood and adolescents can have a negative impact on growth and development and even on cardiovascular and neurological functioning.3
It is reasonable to discriminate between thyroid autoimmunity (TAI) and autoimmune thyroid disease. TAI is defined as positive thyroid antibodies; thyroid function may be normal or impaired. In autoimmune thyroid disease, autoimmunity has resulted in dysfunction of the thyroid gland and changes of gland morphology can be demonstrated by ultrasonography. Autoimmune thyroid disease may be associated with hypothyroidism (as in Hashimoto thyroiditis [HT]) or with hyperthyroidism (as in Graves' disease).4 Antibodies against thyroid peroxidase (TPO-ab) and thyroglobulin (TG-ab) are typical markers for HT, which is the leading cause for hypothyroidism. HT is more common in females than in males.5
Because cutoff points for positivity of TPO-ab and TG-ab differ widely,6, 7 antibody-based diagnosis is not trivial and the prevalence of TAI and autoimmune thyroid disease are dependent on these antibody cutoffs.
A recent meta-analysis reported that both autoimmune thyroid disease and hypothyroidism are associated with depression (odds ratio [OR] 3.3; 95% confidence interval [CI], 2.0-5.5) and anxiety disorders (OR 2.3; 95% CI, 1.4-3.9) in the general population.8 Other psychiatric disorders are suspected to be related to autoimmune thyroid disease as well. In a study from Danish national registers, schizophrenia was nonsignificantly related to patients with thyroiditis (adjusted incidence rate, 3.2; 95% CI, 0.7-14.3) and significantly associated to thyroiditis among subjects parents (adjusted incidence rate, 2.2; 95% CI, 1.4-3.9).9 Attention-deficit/hyperactivity disorder (ADHD) was associated with an OR of 2.2 (95% CI, 1.9-2.7) for hypothyroidism compared to individuals without ADHD.10 For further common psychiatric disorders such as eating disorders (ED), autism spectrum disorder (autism), or borderline personality disorder (BPD) we could not find clear evidences in relation to TAI/autoimmune thyroid disease in literature.
Autoimmune thyroid disease or TAI, respectively, is a particularly frequent comorbidity among children and adolescents with T1D11; the prevalence ranges from 19% to 35%.12-14 T1D is also known to enhance the risk for diverse psychiatric disorders such as depression or anxiety disorders15 as well as ED, especially in the first year after manifestation,16 but studies on the association between psychiatric disorders and TAI among adolescents and young adults with T1D are lacking. The current study tried to bridge that gap.
The aim of this study was to evaluate the rate of depression and anxiety disorders in adolescents and young adults with T1D and comorbid TAI compared to T1D patients without any thyroid disease. Additionally, we aimed at investigating whether further psychiatric disorders such as ADHD, ED, schizophrenia, autism, and BPD are associated with comorbid TAI in individuals with T1D.
2 METHODS
2.1 Participants and data collection
This study is based on data from the prospective, multicenter diabetes patient follow-up registry Diabetes-Patienten-Verlaufsdokumentation (DPV), which is a standardized electronic health record developed at the Institute of Epidemiology and Medical Biometry, ZIBMT, Ulm University, Germany.17 The initiative and analysis of anonymized data was approved by the Ethics Committee of Ulm University (approval number: 202/09) as well as by local review boards.
Four hundred and ninety-five diabetes centers from Germany, Austria, Switzerland, and Luxemburg provided anonymized data on diabetes treatment and outcome to the DPV registry until 1 January 2020. Twice a year participating centers report anonymized data for central analysis at Ulm University. The transferred data are checked for inconsistency or implausibility and reported back to the respective centers for correction, if necessary.
Individuals recorded in the DPV initiative were included if they had a clinical diagnosis of T1D and were between 11 and 25 years of age. For each subject the last documented year was analyzed. If a subject has been tested for TPO-ab or TG-ab, the data 1 year back from the most recent date of antibody testing were analyzed. The data were aggregated if the subject had more than one visit in the respective documentation period.
2.2 Definition of thyroid autoimmunity and group selection
TAI was defined as:
- A documented diagnosis of Hashimoto thyroiditis in the DPV registry or
- A conspicuous sonography finding in combination with a documented diagnosis of thyroiditis or
- Positive TPO-ab/TG-ab.
There is no overarching reference limit given for positivity of TPO-ab and TG-ab, which would allow diagnosis of TAI. Different cutoffs were used in previous studies and were given by assay manufacturers. These reference limits range from 6 to 36 U/mL (TPO-ab) and from 5 to 115 U/mL (TG-ab).6, 7, 18, 19 To ensure the best possible definition of TAI, we used very conservative (high) cutoff limits for TPO-ab (80 U/mL) and TG-ab (120 U/mL).
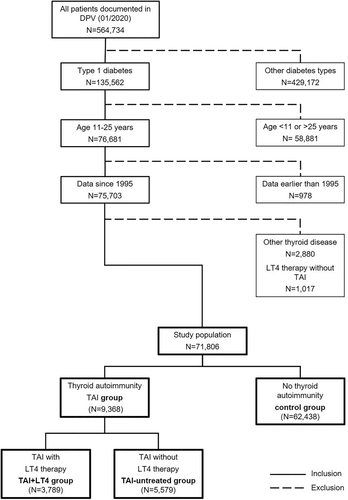
Patients who met at least one of these three criteria were allocated to the TAI group (thyroid autoimmunity). We want to point out that some patients in the TAI group had the clinical diagnosis autoimmune thyroid disease, whereas some patients revealed only positive TPO-ab or TG-ab, without evidence of thyroid disease.
Patients who met none of these criteria were allocated to the control group. Individuals with documented diagnosis of any other thyroid disease as well as patients who received levothyroxine (LT4) therapy without having TAI or autoimmune thyroid disease were excluded.
We further divided the TAI group into T1D patients with:
- LT4 therapy (TAI-LT4 group) and
- without LT4 therapy (TAI-untreated group) (Figure 1).
2.3 Definition of psychiatric disorders
The following psychiatric disorders were analyzed: depression, anxiety and obsessive-compulsory disorder (anxiety), ED, ADHD, autism, schizophrenia, and BPD. ED comprised anorexia, bulimia, binge eating, and eating disorders not otherwise specified (EDNOS). All of these psychiatric disorders were defined as documented diagnosis in DPV according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition manual and International Classification of Diseases, Tenth Revision codes. In terms of depression, anxiety, and ADHD, patients with a documented medication for these psychiatric disorders were also included.
2.4 Patient data
For descriptive comparison of demographics and diabetes-related outcomes between the TAI-LT4 group, the TAI-untreated group and the control group the parameters sex, migration background, age, height, weight, body mass index (BMI), diabetes duration, type of insulin treatment, daily insulin dose, thyrotropin (TSH), and hemoglobin A1c (HbA1c) (%; mmol/mol) were analyzed.
The variable migration background was defined as the patient or at least one of his/her parents born outside of Germany, Austria, Switzerland, or Luxemburg. Height, weight, and BMI were reported in SD score (SDS) according to the Kinder- und Jugendgesundheitssurvey (KiGGS) reference data using the lambda, mu and sigma (LMS) method.20 HbA1c values were standardized to the Diabetes Control and Complications Trial (DCCT) reference range of 4.05%-6.05% (20.7-42.6 mmol/mol) using the multiple of the mean transformation method to account for different laboratory methods.21 The type of insulin treatment was defined as injection therapy or pump therapy.
2.5 Statistical analysis
All statistical analyses were generated using SAS (Statistical Analysis Software, SAS Institute Inc., Cary, NC, USA) Version 9.4. Descriptive statistics were performed for baseline characteristics. The results were shown as median with quartiles for continuous variables using the Kruskal-Wallis test to compute unadjusted P values and as proportions for binary variables using the χ2 test.
We used multivariable logistic regression models to calculate the OR for the previous mentioned psychiatric disorders in T1D patients aged 11-25 years with TAI compared to T1D patients without TAI. All regression models were adjusted for age (in groups of 11-18 and >18-25 years), sex, diabetes duration (in groups of ≤5 and >5 years), and migration background. Identical regression models were used to analyze the OR for the TAI-LT4 group and the TAI-untreated group compared to the control group (reference). For some psychiatric disorders, the number of patients was too small to apply reliable regression models after stratification by sex and/or LT4 therapy. Therefore, autism, schizophrenia, and BPD were analyzed only for the whole cohort. ED was analyzed after stratification for sex or LT4 therapy, separately, but not after stratification for both parameters. Results from logistic regression models were presented as adjusted OR together with 95% CI. A two-sided P value <.05 indicated a significant difference.
3 RESULTS
3.1 Baseline characteristics
Of the 71 806 patients with T1D aged between 11 and 25 years, 9368 revealed a thyroid autoimmunity and 3789 of them were treated with LT4 (Figure 1).
Baseline characteristics of the study population are depicted in Table 1. Individuals in the TAI group were significantly more often female (63.2% vs 43.5% in the control group), had a longer diabetes duration, had a higher BMI-SDS, had higher TSH levels, and used pump therapy more often compared to T1D patients without TAI. No difference in HbA1c was apparent (Table 1). Similar results occurred after stratification by sex.
| Characteristic | All | Female | Male | |||
|---|---|---|---|---|---|---|
| Control group | TAI group | Control group | TAI group | Control group | TAI group | |
| N (%) | 62 438 (87.0) | 9368 (13.0) | 27 192 (82.1) | 5921 (17.9) | 35 246 (91.1) | 3447 (8.9) |
| Sex (% male) | 56.5 | 36.8** | ||||
| migration (%) | 15.1 | 18.3** | 15.1 | 18.3** | 15.2 | 18.4** |
| Age (y) | 16.3 (13.8; 17.8) | 16.3 (14.2; 17.6) | 16.2 (13.7; 17.8) | 16.3 (14.2; 17.6) | 16.4 (13.9; 17.8) | 16.3 (14.3; 17.5) |
| Diabetes duration (y) | 5.6 (2.3; 9.4) | 6.3 (3.0; 10.0)** | 5.9 (2.7; 9.6) | 6.6 (3.3; 10.3)** | 5.3 (2.1; 9.2) | 5.7 (2.6; 9.8)** |
| Height-SDS | 0.07 (−0.62; 0.77) | 0.07 (−0.63; 0.77) | 0.08 (−0.61; 0.77) | 0.08 (−0.62; 0.76) | 0.06 (−0.63; 0.76) | 0.06 (−0.64; 0.77) |
| Weight-SDS | 0.30 (−0.36; 0.90) | 0.38 (−0.27; 0.99)** | 0.44 (−0.20; 1.02) | 0.48 (−0.17; 1.06) | 0.17 (−0.47; 0.80) | 0.20 (−0.42; 0.82) |
| BMI-SDS | 0.28 (−0.35; 0.89) | 0.36 (−0.27; 0.98)** | 0.45 (−0.19; 1.03) | 0.47 (−0.16; 1.07) | 0.16 (−0.45; 0.76) | 0.16 (−0.41; 0.81) |
| HbA1c MOM-DCCT (%) | 8.0 (7.1; 9.4) | 8.0 (7.2; 9.2) | 8.1 (7.2; 9.4) | 8.0 (7.2; 9.3) | 8.0 (7.1; 9.4) | 8.0 (7.1; 9.2) |
| HbA1c MOM-DCCT (mmol/mol) | 64 (54; 79) | 64 (55; 78) | 65 (55; 79) | 64 (55; 78) | 64 (54; 79) | 64 (54; 77) |
| Daily insulin dose (U/kg) | 0.85 (0.67; 1.05) | 0.86 (0.69; 1.05) | 0.85 (0.67; 1.05) | 0.85 (0.69; 1.05) | 0.86 (0.68; 1.07) | 0.87 (0.69; 1.06) |
| TSH (μU/mL) | 1.8 (1.3; 2.5) | 2.2 (1.4; 3.5)** | 1.8 (1.3; 2.5) | 2.2 (1.4; 3.4)** | 1.9 (1.3; 2.6) | 2.4 (1.6; 3.7)** |
| Pump therapy (%) | 32.1 | 34.6** | 36.1 | 38.0 | 29.1 | 28.9 |
- Note: Data are presented in median (lower quartile; upper quartile) or %.
- Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; MOM-DCCT, multiple of the mean transformation method - Diabetes Control and Complications Trial; SDS, standard deviation score; TAI, thyroid autoimmunity; TSH, thyrotropin.
- ** Indicates a P value <.01 between TAI group and control group.
Comparing the TAI + LT4 with the TAI-untreated group, there was a higher percentage of female patients in the TAI + LT4 group (Table 2). Furthermore, individuals in the TAI + LT4 group had a longer diabetes duration, higher BMI-SDS, and higher TSH levels and used pump therapy less frequently than individuals in the TAI-untreated group.
| Characteristic | TAI + LT4 | TAI-untreated | P value |
|---|---|---|---|
| N (%) | 3789 (40.4) | 5579 (59.6) | |
| Sex (% male) | 32.2 | 39.9 | P < 0.001 |
| migration (%) | 18.1 | 18.4 | P = 1.000 |
| Age (y) | 16.5 (14.4; 17.6) | 16.2 (14.1; 17.5) | P = 0.002 |
| Diabetes duration (y) | 7.0 (3.8; 10.5) | 5.8 (2.5; 9.6) | P < 0.001 |
| Height-SDS | 0.06 (−0.66; 0.74) | 0.09 (−0.61; 0.79) | P = 0.872 |
| Weight-SDS | 0.46 (−0.23; 1.08) | 0.32 (−0.31; 0.92) | P < 0.001 |
| BMI-SDS | 0.45 (−0.19; 1.09) | 0.30 (−0.33; 0.90) | P < 0.001 |
| HbA1c MOM-DCCT (%) | 8.0 (7.2; 9.2) | 8.0 (7.1; 9.3) | P = 1.000 |
| HbA1c MOM-DCCT (mmol/mol) | 64 (55; 77) | 64 (54; 78) | P = 1.000 |
| Daily insulin dose (U/kg) | 0.86 (0.71; 1.06) | 0.85 (0.68; 1.04) | P = 0.031 |
| TSH (μU/mL) | 2.7 (1.6; 4.5) | 2.0 (1.4; 2.9) | P < 0.001 |
| Pump therapy (%) | 36.0 | 42.8 | P < 0.001 |
- Note: Data are presented in median (lower quartile; upper quartile) or %.
- Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; LT4, levothyroxine therapy; MOM-DCCT, multiple of the mean transformation method - Diabetes Control and Complications Trial; SDS, standard deviation score; TAI, thyroid autoimmunity; TSH, thyrotropin.
3.2 Association between psychiatric disorders and TAI
In the overall TAI group (with or without LT4-treatment), we observed a weak, but significant association of TAI with depression (OR, 1.35, 95% CI, 1.19, 1.52), ED (OR, 1.25, CI, 1.03, 1.51), ADHD (OR, 1.22, CI, 1.07, 1.39), and schizophrenia (OR, 1.63, CI, 1.04, 2.56) (Figure 2A).
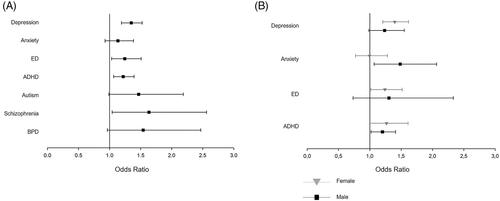
Females with TAI had significantly higher odds for depression (OR, 1.40; CI, 1.21; 1.62) and ED (OR, 1.24; CI, 1.02; 1.52), whereas males showed higher odds for anxiety (OR, 1.49; CI, 1.07; 2.07) and ADHD (OR, 1.20; CI, 1.02; 1.41), compared to the control group (Figure 2B).
3.3 Association between psychiatric disorders and TAI with LT4-therapy
Stratified by LT4 therapy, we found no significant difference for any psychiatric disorder in the TAI-untreated group (Figure 3). In contrast, the TAI + LT4 group displayed significantly higher odds for depression (OR, 1.63; CI, 1.34; 1.99), anxiety (OR, 1.60; CI, 1.18; 2.18), and ADHD (OR, 1.71; CI, 1.38; 2.12) compared to the control group (Figure 3A).
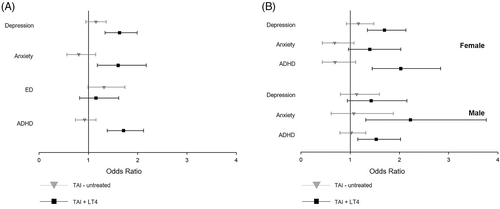
Female patients in the TAI + LT4 group showed higher odds for depression (OR, 1.70; CI, 1.35; 2.13) and ADHD (OR, 2.03; CI, 1.45; 2.83) (Figure 3B) whereas male patients in the TAI + LT4 group revealed a higher chance for anxiety (OR, 2.23; CI, 1.32; 3.76) and ADHD (OR, 1.53; CI, 1.15; 2.02) (Figure 3B).
4 DISCUSSION
In the present study on individuals with T1D aged 11-25 years, we detected a higher chance for depression, anxiety (only in males), ED, ADHD, and schizophrenia in patients that exhibited TAI compared to T1D patients without TAI. If LT4 therapy was required (because of thyroid dysfunction or goiter), the association with most of these psychiatric diseases was even higher, whereas no association could be found in individuals without LT4 therapy.
The relation between depression or anxiety disorders with TAI and hypothyroidism was recently described in a meta-analysis in the general population8 and several other studies reported similar findings. According to these studies, patients with hypothyroidism,22, 23 TAI24 or even with elevated TPO-ab only25 revealed an association to depression, anxiety, or bipolar disorders, respectively. We could confirm that this association between TAI and mood disorders is also observable in adolescents and young adults with T1D.
The higher chance for depression in females compared to males with TAI is in line with a previous study.26 Even though anxiety disorders in the general population are more prevalent in women than in men (13.5% vs 10.9%),27 we observed higher odds for anxiety disorders only in male but not in female T1D patients with TAI compared to individuals with T1D only. The reason for this gender difference remains unclear.
Findings from other studies on TPO-ab suggest that elevated thyroid antibodies, by activating autoimmune processes, may be a more important risk factor for mood disorders than thyroid dysfunction itself.28 However, in the present study, we found no significant differences in TAI patients without LT4 therapy compared to the control group, whereas the TAI + LT4 group revealed higher odds for depression and anxiety. This association could be owing to the difficulties in differentiation of depressive or anxious symptoms as well as symptoms of altered mood in patients with thyroid diseases. Another reason might be that elevated antibodies only do not increase odds for anxiety or depression, but thyroid dysfunction in terms of hypothyroidism that needs to be treated with LT4, seems to enhance the chance of developing depressive or anxiety symptoms. Elevated mean TSH as well as a higher proportion of individuals with documented TSH levels (94%) in the TAI + LT4 group compared to the control group (65%) suggest that LT4 therapy was generally prescribed to patients with at least the suspicion for hypothyroidism. In contrast, no evidence for overtreatment with LT4, which would have been confirmed by suppressed TSH levels in the TAI + LT4 group, could be detected. Otherwise, thyroid hormones are responsible for cognitive and mood changes29 and LT4 was successfully used as augmentation therapy in euthyroid people with bipolar depression.30 However, not only low levels, but also high levels of FT4 are associated with a higher risk for depression compared to normal levels31; therefore, the role of LT4 in depression and anxiety disorders remains unclear and there seem to be differences between euthyroid people and individuals with hypothyroidism.
Thyroid disorders such as hypothyroidism are more prevalent in individuals with schizophrenia compared to healthy people.32 Autoimmune disorders were also linked to schizophrenia. A study including data from Danish national registers found a higher prevalence of Graves' disease (autoimmune thyroid disease that causes hyperthyroidism) among people with schizophrenia, but no association with thyroiditis or T1D.9 Previous studies reported an inverse association between T1D and schizophrenia, but to date the reasons remain unclear.33 However, thyroid hormones seem to play a role in psychotic diseases. One study found higher free T4 levels in acute psychotic patients,34 and another study proposed T4 therapy to improve attention in individuals with schizophrenia.35 Higher T4 levels in schizophrenia would be in line with a higher number of patients with Graves' disease.
Because of the relatively low number of patients with schizophrenia in the different sub-groups (n < 30 in females and in individuals with TAI) in the present study, we could not evaluate the odds for schizophrenia related to LT4 therapy. But this is an important topic for further investigations.
In accordance to our findings on T1D patients, ADHD is related to both hypothyroidism and hyperthyroidism10 as well as to autoimmune thyroid disease36 in the general population. A study from India even suggested thyroid hormones as a putative biomarker for ADHD. They found that serum T4 level was lower in children and adolescents with ADHD compared to matched controls without ADHD (mean [SD] μg/dL): 7.2 (2.7) vs 9.3 (2.3), P = .002).37 However, a recent study reported that patients with resistance to thyroid hormones, who have ADHD more often, may be falsely diagnosed with Hashimoto thyroiditis and therefore maladjusted with LT4.38 On the other hand, no evidence for inadequate therapy with LT4 was observable in the present study as the TAI + LT4 group revealed the highest TSH levels compared to the TAI-untreated group and the control group. We assume similar mechanisms in ADHD as we mentioned before in depression and anxiety disorders. Therefore, thyroid dysfunction with the need of LT4 supplementation, in contrast to elevated antibodies only, might be associated with the higher odds for ADHD.
ED are related to T1D39 and to other endocrine diseases, but - to our knowledge - no association with TAI was observed so far. However, ED affect the hypothalamic-pituitary axis, which affects the thyroid gland. But TAI has not been mentioned as a comorbidity of ED.40 Our study is the first to demonstrate a correlation between TAI and ED in adolescents and young adults with T1D. The reason is not clear. ED was the only psychiatric disease in the current study that was not associated with LT4 therapy.
The strength of this study was the huge patient numbers, we were able to compare more than 60 000 adolescents and young adults with T1D with 9368 individuals with T1D and TAI. Additionally, we separated people with thyroid autoimmunity only (no LT4 treatment required) from individuals with prescribed LT4 therapy and therefore real implications such as hypothyroidism, an imminent thyroid dysfunction or goiter. To our knowledge this has not been investigated in a nationwide (diabetes patient) database so far. However, there are some limitations, first of all the difference in diagnosing of autoimmune thyroid disease in a multicenter database, as this is not even trivial in particular cases. For this reason, we decided to investigate TAI instead, so we could use TPO-ab and TG-ab as sole parameters for diagnosis. Because the cutoffs for these antibodies differ from manufacturer to manufacturer, we used cutoffs that were higher than the highest cutoffs we could find in literature or by manufacturers to avoid misdiagnoses. We cannot rule out that, because of these high cutoffs, some patients were allocated to the control group who should have been included in the TAI group. Therefore, we are not able to present a prevalence rate of thyroid autoimmunity or autoimmune thyroid disease among T1D patients. Another limitation was that we could not analyze the timeline of TAI diagnosis and the diagnosis of psychiatric disorders in this cross-sectional observation study. Furthermore, free T4 levels and dosage of LT4 treatment could not be presented as this was not sufficiently documented and free T4 levels were not reliably comparable because of the use of different analysis techniques among the participating centres.
In conclusion, the association between thyroid autoimmunity and psychiatric disorders was highly dependent on gender and whether levothyroxine therapy was needed (hypothyroidism) or not (euthyroid). Besides ED, all psychiatric disorders that were evaluated and stratified by LT4 therapy revealed a significant relation to the TAI + LT4 group, whereas no association could be observed with the TAI-untreated group. Females with thyroid autoimmunity showed higher odds for depression, ED and ADHD, whereas male patients displayed an association to anxiety disorders and ADHD. The highest association was found between female T1D patients in the TAI + LT4 group and ADHD and between male patients with T1D in the TAI + LT4 group and anxiety disorders. The striking relationship between LT4 therapy and several psychiatric disorders remains in large part unclear. A more severe thyroid disease with (subclinical) hypothyroidism in those with LT4 therapy might be the reason why only LT4 treated individuals had higher odds for psychiatric disorders. Additionally, LT4 seems to have a controversial relation to bipolar disorders that may depend on thyroid function (euthyroid vs hypothyroid). Although the impact of thyroid hormones on mental development is widely accepted, possible (negative) effects on mood, cognition, or anxiety of LT4 treatment in patients with autoimmune thyroid disease have not been systematically investigated so far. But this seems to be especially important in patients with T1D, who have a higher lifetime risk for numerous comorbidities.
ACKNOWLEDGEMENTS
We would like to thank all participating centers of the DPV initiative, especially the collaborating centers in this investigation. Special thanks to Reinhard W. Holl, who supervised the project, Andreas Hungele and Ramona Ranz for support and the development of the DPV documentation software, and Katharina Fink and Esther Bollow for the DPV data management (in each case clinical data manager, Ulm University). The list of all contributing centers is given in supplemental list 1. This study was supported through the German Federal Ministry for Education and Research within the German Centre for Diabetes Research (DZD, 82DZD0017G). Further financial support was received by the German Robert Koch Institute (RKI) and the German Diabetes Association (DDG). Sponsors were not involved in data acquisition or analysis. Open Access funding enabled and organized by ProjektDEAL. WOA Institution: Universitat Ulm. Blended DEAL: ProjektDEAL.
CONFLICT OF INTEREST
The authors declare that there is no duality of interest associated with this manuscript.




