Pre-existing type 2 diabetes is an adverse prognostic factor in patients with renal cell carcinoma
2型糖尿病是肾细胞癌患者的不良预后因素
Funding information No funding received.
Abstract
enBackground
Diabetes is a risk factor for various cancers, but its prognostic role in renal cell carcinoma (RCC) is controversial and understudied. This study investigated the prognostic value of type 2 diabetes (T2D) in RCC patients.
Methods
The clinicopathological and follow-up data of 451 RCC patients undergoing radical or partial nephrectomy at the First Hospital of Shanxi Medical University from 2013 to 2018 were reviewed. Associations of T2D with clinicopathological parameters of RCC were evaluated using the Kaplan-Meier method for survival estimates and Cox regression univariate and multivariate analyses.
Results
Of 451 patients, 74 (16.4%) had T2D. These patients were older, had a higher body mass index, higher incidence rates of hypertension and cardiovascular morbidity, a higher rate of laparoscopic surgery, and smaller neoplasms (all P < .05). Patients with T2D exhibited shorter overall survival (OS; P = .009), cancer-specific survival (CSS; P = .043), and recurrence-free survival (RFS; P = .008) than patients without T2D. Fuhrman grade (hazard ratio [HR] 2.542, 95% confidence interval [CI] 1.115-5.795, P = .026) and T2D (HR 3.391, CI 1.458-7.886, P = .005) were independent predictors of OS; T2D was an independent predictor of CSS (HR = 4.637, 95% CI 1.420-15.139, P = .011) and RFS (HR 3.492, 95% CI 1.516-8.044, P = .003).
Conclusions
Renal cell carcinoma patients with T2D have a shorter OS and higher recurrence rate and mortality risk than those without T2D.
摘要
zh背景
糖尿病是多种癌症的危险因素, 但其在肾细胞癌预后中的作用一直存在争议以及研究不足。本研究旨在探讨2型糖尿病对肾细胞癌患者的预后价值。
方法
回顾性分析2013年至2018年山西医科大学第一医院行根治性或部分肾切除术治疗的451例肾细胞癌患者的临床病理资料和随访数据。评估2型糖尿病与肾细胞癌各种临床和病理参数的潜在关联。应用Kaplan-Meier法估算生存率, 应用COX回归模型进行单因素和多因素分析。
结果
纳入研究的451例患者中, 共有74例患者(16.4%)术前患有2型糖尿病。这些患者的年龄更大, 具有更高的体质指数, 更高的高血压发病率和心血管疾病发病率, 更高的腹腔镜手术率, 以及更小的肿瘤直径(P值均<0.05)。Kaplan-Meier曲线显示2型糖尿病患者的总生存率(OS;P =0.009)、癌症特异性生存率(CSS;P =0.043)和无复发生存率(RFS;P =0.008)均低于非2型糖尿病患者。多因素分析发现, Fuhrman分级(风险比[HR] 2.542, 95%置信区间[CI] 1.115-5.795, P=0.026)和2型糖尿病(HR 3.391, CI 1.458-7.886, P=0.005)是肾细胞癌患者术后总生存率的独立危险因素, 2型糖尿病是肾细胞癌患者术后癌症特异性生存率(HR 4.637, 95% CI 1.420-15.139, P=0.011)和无复发生存率(HR 3.492, 95% CI 1.516- 8.044, P=0.003)的独立危险因素。
结论
与非2型糖尿病患者相比, 合并2型糖尿病的肾细胞癌患者总生存期更短, 肾癌死亡风险更高, 复发风险增加。
1 INTRODUCTION
Renal cell carcinoma (RCC) is the most frequent and fatal malignant tumor of the kidney in adults, accounting for 3% to 5% of all malignant tumors in adults and 85% to 93% of all malignant tumors of the kidney.1 The incidence of RCC has been steadily increasing worldwide and varies geographically, with the highest incidence in developed countries (eg, Western Europe and the US).2 The incidence of RCC in China is also increasing, with 66 800 new cases and accounting for 23 400 deaths in 2015.3 The occurrence and development of RCC are related to many factors, such as heredity, environment, age, lifestyle-related chronic diseases (eg, smoking, obesity, hypertension, and diabetes mellitus). However, no studies have been conducted to assess the effect of surgical approach on RCC.4, 5
The incidence of type 2 diabetes (T2D), a metabolic disease, has also increased markedly worldwide in recentdecades.6-8 China is the most populous country in the world, and with the development of the social economy and the aging of the population, the number of people suffering from diabetes is increasing. Recently, T2D has been shown to increase the risk of breast,9 endometrial,10 liver,11 pancreatic,12 colorectal,13 and bladder cancer.14 Moreover, T2D also increases the risk of kidney cancer.15 Type 2 diabetes has negative effects on the prognosis of certain tumors, such as breast, liver, endometrial, and colorectal tumors, in which a significant reduction in cancer-specific survival (CSS) was observed in patients with T2D.16, 17 Therefore, researchers have questioned whether T2D may exert adverse effects on the prognosis of RCC. Although a few studies have investigated the effects of T2D on the prognosis of RCC, the results have been controversial.18-20
The present study used a single-center surgical series with a retrospective design to analyze the clinicopathological features of patients with T2D and RCC and to explore the effects of T2D on the prognosis of patients with RCC who received surgical treatment.
2 METHODS
After obtaining approval from the Ethics Committee of the First Hospital of Shanxi Medical University, data were collected from 471 patients with sporadic, unilateral RCC who underwent a radical or partial nephrectomy at the First Hospital of Shanxi Medical University between 2013 and 2018. After excluding patients who only underwent a renal biopsy, patients with inadequate follow-up, and patients with missing data, 451 patients were enrolled in the study. All patients underwent a preoperative auxiliary examination that included chest computed tomography (CT) or X-ray, a urological ultrasound and CT, and a laboratory examination. The histopathological subtypes, pathological grades, and stages were determined after surgery. The histological subtype was determined according to the World Health Organization (WHO) 2004 classification criteria,21 tumor staging was determined according to the guidelines from the American Joint Committee on Cancer from 2010,22 and grading was determined according to the 1997 WHO-recommended Fuhrman nuclear grading system.23
In the present study, a preoperative diagnosis of T2D was made if the patient was using oral hypoglycemic drugs or the subcutaneous injection of insulin to control blood glucose levels. The following clinicopathological variables were evaluated: age, gender, body mass index (BMI), history of hypertension and cardiovascular disease, history of T2D, history of smoking, laterality, type of surgery, surgical approach, tumor size, histological subtype, TNM stage, and Fuhrman nuclear grade. The tumor size that was recorded was the largest diameter (cm) described in the pathology report. All patients were followed-up regularly after surgery: once every 3 months for the first 2 years, once every 6 months for the next 2 years, and annually thereafter. The follow-up visits included a physical examination, laboratory tests, ultrasonography, X-rays, and CT scans; bone scintigraphy was also required in some cases. Recurrence was defined as local relapse, lymph node metastasis, or distant metastasis and was primarily determined by a CT scan of the chest and abdomen and a bone scan.
2.1 Statistical analysis
Patients were divided into T2D and non-T2D groups according to their history of T2D. Student's t test was used to analyze and compare continuous variables in the patients' clinicopathological data, whereas categorical variables were analyzed using the Chi-squared test. Overall survival (OS), CSS, and recurrence-free survival (RFS) were estimated using Kaplan-Meier analysis, and differences between groups were assessed using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard regression model to identify prognostic factors, and hazard ratios (HRs) and 95% confidence intervals (CIs) were computed. Only variables showing a statistically significant difference in the univariate analysis were analyzed using the Cox regression model. The effects of different surgical approaches on the postoperative recurrence rate in patients with T2D were also evaluated. Statistical analyses were performed using SPSS 22.0 (IBM Corp., Armonk, New York). All P-values are two-tailed, and P < .05 was considered statistically significant.
3 RESULTS
3.1 Clinical characteristics
For all 451 patients analyzed in this study, the mean age at the time of diagnosis was 57.6 years (range 21-80 years). Of these 451 patients, 74 (16.4%) had a history of T2D; 133 (29.5%) had undergone partial nephrectomy and 318 (70.5%) had undergone radical nephrectomy. Table 1 compares the clinicopathological features of patients with and without T2D. Compared with non-T2D patients, the mean age of patients with T2D was significantly higher (61.1 vs 56.9 years; P = .001), mean BMI was higher (25.3 vs 24.4 kg/m2; P = .037), and a greater percentage underwent laparoscopic surgery (P = .04). Furthermore, T2D was more common in patients with a history of hypertension (P = .007) and cardiovascular disease (P = .002). However, the non-T2D group had a larger tumor diameter than the T2D group (4.6 vs 4.1 cm, P = .016). No significant differences in sex, smoking status, laterality, type of surgery, histological subtype, pathological T stage, or grading were observed between the T2D and non-T2D groups (all P > .05).
| All patients | T2D | Non-T2D | P-value | |
|---|---|---|---|---|
| No. patients | 451 | 74 (16.4) | 377 (83.6) | |
| Age (y) | 57.6 ± 10.3 | 61.1 ± 9.0 | 56.9 ± 10.4 | .001 |
| Sex | .642 | |||
| Male | 297 (65.9) | 47 (63.5) | 250 (66.3) | |
| Female | 154 (34.1) | 27 (36.5) | 127 (33.7) | |
| BMI (kg/m2) | 24.6 ± 3.2 | 25.3 ± 2.9 | 24.4 ± 3.3 | .037 |
| Hypertension | 180 (39.9) | 40 (54.1) | 140 (37.1) | .007 |
| Cardiovascular disease | 46 (10.2) | 15 (20.3) | 31 (8.2) | .002 |
| Smoking status | .638 | |||
| Never smoked | 327 (72.5) | 52 (70.3) | 275 (72.9) | |
| Current or former smoker | 124 (27.5) | 22 (29.7) | 102 (27.1) | |
| Laterality | .789 | |||
| Right | 238 (52.8) | 38 (51.4) | 200 (53.1) | |
| Left | 213 (47.2) | 36 (48.6) | 177 (46.9) | |
| Type of surgery | .149 | |||
| Radical nephrectomy | 318 (70.5) | 47 (63.5) | 271 (71.9) | |
| Partial nephrectomy | 133 (29.5) | 27 (36.5) | 106 (28.1) | |
| Surgical approach | .040 | |||
| ON | 95 (21.1) | 9 (12.2) | 86 (22.8) | |
| LN | 356 (78.9) | 65 (87.8) | 291 (77.2) | |
| Tumor diameter (cm) | 4.5 ± 2.3 | 4.1 ± 1.6 | 4.6 ± 2.4 | .016 |
| Histological subtype | .794 | |||
| Clear | 406 (90.0) | 66 (89.2) | 340 (90.2) | |
| Unclear | 45 (10.0) | 8 (10.8) | 37 (9.8) | |
| Pathological T stage | .074 | |||
| T1a | 253 (56.1) | 47 (63.5) | 206 (54.7) | |
| T1b | 135 (29.9) | 23 (31.1) | 112 (29.7) | |
| T2 | 40 (8.9) | 3 (4.1) | 37 (9.8) | |
| T3-4 | 23 (5.1) | 1 (1.3) | 22 (5.8) | |
| Fuhrman grade | .152 | |||
| G1-2 | 384 (85.4) | 59 (79.7) | 325 (86.2) | |
| G3-4 | 67 (14.6) | 15 (20.3) | 52 (13.8) |
- Note: Unless indicated otherwise, data are given as the mean ± SD or as n (%).
- Abbreviations: BMI, body mass index; LN, laparoscopic nephrectomy; ON, open nephrectomy; T2D, type 2 diabetes.
3.2 Survival analysis and prognostic factors
The median follow-up time was 24 months (range 2-64 months). At the time of analysis, 28 patients (6.2%) had died, and 28 patients (6.2%) had relapsed at the time of the most recent follow-up. Specifically, 15 patients (3.3%) died of RCC, 13 patients (2.9%) died of other causes, five patients (1.1%) had local recurrence, and 23 patients (5.1%) presented with lymph node metastasis or distant metastasis.
Regarding OS, CSS, and RFS, the Kaplan-Meier survival curve showed a significantly higher survival rate for patients without T2D than for patients with T2D (log-rank test, P = .009, P = .043, and P = .008, respectively; Figure 1). The OS, CSS, and RFS at 5 years after surgery was 79.3%, 89.1%, and 80.5%, respectively, for patients with T2D and 90.8%, 94.9%, and 90.0%, respectively, for patients without T2D.
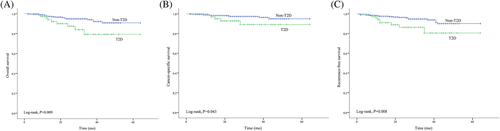
Table 2 lists results of the univariate and multivariate analyses of factors affecting OS in patients with RCC. According to the univariate analysis, sex, smoking status, tumor diameter, pathological T stage, Fuhrman nuclear grade, and T2D were significant predictors of OS. The variables that exhibited significant differences in the univariate analysis were introduced into the Cox multivariate regression analysis. The multivariate analysis revealed that T2D (HR = 3.391, 95% CI 1.458-7.886, P = .005) and Fuhrman nuclear grade (HR = 2.542, 95% CI 1.115-5.795, P = .026) were independent prognostic factors for OS. The results of the Cox survival analysis for predicting CSS and RFS are presented in Tables 3 and 4, respectively. Type 2 diabetes was an independent risk factor for CSS (HR = 4.637, 95% CI 1.420-15.139, P = .011) and RFS (HR = 3.492, 95% CI 1.516-8.044, P = .003).
| Variables | Univariate Cox regression | Multivariate Cox regression | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.031 (0.994-1.070) | .103 | ||
| Sex (male vs female) | 0.325 (0.113-0.937) | .038 | 0.594 (0.184-1.916) | .384 |
| BMI | 1.019 (0.916-1.134) | .726 | ||
| T2D (yes vs no) | 2.757 (1.244-6.108) | .012 | 3.391 (1.458-7.886) | .005 |
| Hypertension (yes vs no) | 1.307 (0.622-2.747) | .481 | ||
| Cardiovascular disease (yes vs no) | 0.682 (0.162-2.873) | .602 | ||
| Smoker (yes vs no) | 2.857 (1.357-6.015) | .006 | 2.111 (0.917-4.858) | .079 |
| Laterality (right vs left) | 1.047 (0.498-2.202) | .903 | ||
| Type of surgery (PN vs RN) | 2.284 (0.791-6.591) | .127 | ||
| Surgical approach (ON vs LN) | 1.452 (0.639-3.298) | .373 | ||
| Tumor diameter | 1.336 (1.201-1.486) | <.001 | 1.231 (0.961-1.577) | .100 |
| Histological subtype (clear vs unclear) | 1.784 (0.677-4.704) | .242 | ||
| Pathological T stage | <.001 | .879 | ||
| T1a | Reference | Reference | ||
| T1b | 2.046 (0.766-5.463) | .153 | 1.192 (0.374-3.799) | .767 |
| T2 | 8.865 (3.290-23.887) | <.001 | 2.240 (0.283-17.696) | .445 |
| T3-4 | 5.571 (1.676-18.516) | .005 | 1.412 (0.279-7.149) | .677 |
| Fuhrman grade (G1-2 vs G3-4) | 5.340 (2.540-11.225) | <.001 | 2.542 (1.115-5.795) | .026 |
- Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; LN, laparoscopic nephrectomy; ON, open nephrectomy; PN, partial nephrectomy; RN, radical nephrectomy; T2D, type 2 diabetes.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.023 (0.973-1.075) | .378 | ||
| Sex (male vs female) | 0.487 (0.137-1.725) | .265 | ||
| BMI | 0.981 (0.838-1.148) | .808 | ||
| T2DM (yes vs no) | 2.893 (0.985-8.494) | .053 | 4.637 (1.420-15.139) | .011 |
| Hypertension (yes vs no) | 0.760 (0.260-2.224) | .616 | ||
| Cardiovascular disease (yes vs no) | 0.626 (0.082-4.763) | .651 | ||
| Smoker (yes vs no) | 2.169 (0.784-6.001) | .136 | ||
| Laterality (right vs left) | 1.828 (0.625-5.350) | .271 | ||
| Type of surgery (PN vs RN) | 2.486 (0.560-11.036) | .231 | ||
| Surgical approach (ON vs LN) | 3.226 (1.169-8.903) | .024 | 2.098 (0.648-6.797) | .217 |
| Tumor diameter | 1.383 (1.200-1.593) | <.001 | 1.191 (0.877-1.617) | .263 |
| Histological subtype (clear vs unclear) | 2.119 (0.596-7.530) | .246 | ||
| Pathological T stage | .001 | .683 | ||
| T1a | Reference | Reference | ||
| T1b | 1.536 (0.343-6.884) | .575 | 1.016 (0.194-5.337) | .985 |
| T2 | 10.815 (2.859-40.904) | <.001 | 2.570 (0.195-33.829) | .473 |
| T3-4 | 8.174 (1.827-36.561) | .006 | 2.841 (0.386-20.924) | .305 |
| Fuhrman grade (G1-2 vs G3-4) | 5.381 (1.950-14.844) | .001 | 2.937 (0.975-8.845) | .056 |
- Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; LN, laparoscopic nephrectomy; ON, open nephrectomy; PN, partial nephrectomy; RN, radical nephrectomy; T2D, type 2 diabetes.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.028 (0.991-1.067) | .134 | ||
| Sex (male vs female) | 0.927 (0.419-2.049) | .851 | ||
| BMI | 1.001 (0.895-1.120) | .989 | ||
| T2D (yes vs no) | 2.803 (1.265-6.213) | .011 | 3.492 (1.516-8.044) | .003 |
| Hypertension (yes vs no) | 1.106 (0.523-2.339) | .791 | ||
| Cardiovascular disease (yes vs no) | 0.676 (0.160-2.850) | .594 | ||
| Smoker (yes vs no) | 1.565 (0.730-3.352) | .249 | ||
| Laterality (right vs left) | 0.897 (0.428-1.883) | .774 | ||
| Type of surgery (PN vs RN) | 1.739 (0.660-4.586) | .263 | ||
| Surgical approach (ON vs LN) | 1.762 (0.797-3.898) | .162 | ||
| Tumor diameter | 1.258 (1.118-1.415) | <.001 | 1.262 (0.972-1.638) | .081 |
| Histological subtype (clear vs unclear) | 2.354 (0.954-5.809) | .063 | ||
| Pathological T stage | .016 | .978 | ||
| T1a | Reference | Reference | ||
| T1b | 1.482 (0.595-3.690) | .398 | 0.858 (0.285-2.583) | .786 |
| T2 | 4.753 (1.738-13.000) | .002 | 1.111 (0.125-9.859) | .924 |
| T3-4 | 2.974 (0.829-10.663) | .094 | 1.027 (0.177-5.972) | .976 |
| Fuhrman grade (G1-2 vs G3-4) | 2.425 (1.068-5.507) | .034 | 1.493 (0.614-3.629) | .376 |
- Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; LN, laparoscopic nephrectomy; ON, open nephrectomy; PN, partial nephrectomy; RN, radical nephrectomy; T2D, type 2 diabetes.
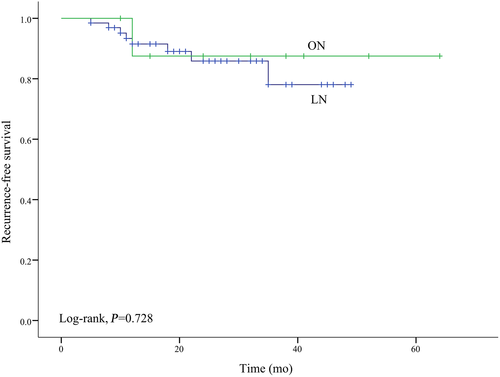
3.3 Effects of different surgical approaches on recurrence in patients with T2D
To assess the relationship between laparoscopic and open surgery and RCC recurrence, a Kaplan-Meier analysis was conducted in the subgroup of 74 patients with T2D to assess the relationships between laparoscopic and open surgery with RCC recurrence. No significant difference was observed in RFS between the two surgical approaches (log-rank test, P = .728; Figure 2).
4 DISCUSSION
Type 2 diabetes is a systemic endocrine and metabolic disease with a rapidly increasing incidence worldwide, particularly in developing countries. The International Diabetes Federation has estimated that 629 million patients will have T2D in 2045, compared with 151 million in 2000.8 Similarly, as a metabolic disease, the incidence of RCC is also increasing annually.24 Some studies have reported that, compared with the general population, patients with T2D have a significantly higher incidence of malignant neoplasms, such as breast, endometrial, liver, pancreatic, colorectal, and bladder cancer, as well as kidney cancer.9-15 In particular, patients with T2D have a 42% higher risk of developing RCC.15 However, Zucchetto et al25 did not observe a significantly increased risk of RCC in patients with T2D. Although a conclusive consensus on the relationship between T2D and RCC has not been reported, the relationship deserves further study. In particular, the effects of T2D on the long-term prognosis of patients with RCC remains to be elucidated.
In the present study, a preoperative diagnosis of T2D was a significant predictor of OS, CSS, and RFS, and T2D remained an independent risk factor for OS, CSS, and RFS in the Cox multivariate analysis of variables that exhibited significant differences in the univariate analysis. Ha et al19 evaluated the effect of pre-existing T2D on the prognosis of patients after nephrectomy and drew similar conclusions to those reported in the present study. Ha et al19 concluded that pre-existing T2D is an unfavorable factor for postoperative survival in patients with RCC. Based on these findings, there is an association between pre-existing T2D and RCC prognosis.
Several mechanisms have been proposed to explain the association between cancer and T2D. One of these mechanisms is hyperinsulinemia associated with insulin resistance and the secretion of insulin-like growth factor (IGF)-1 to stimulate cell proliferation. High levels of insulin increase levels of IGF-1 to promote tumor cell proliferation, differentiation, and inhibition of apoptosis, and these effects may promote the development of cancer. In addition, high levels of insulin and IGF-1 also increase the secretion and upregulate the expression of vascular endothelial growth factor (VEGF), which induces tumor angiogenesis, leading to tumorigenesis and metastasis.26 Other possible mechanisms are related to hyperglycemia, inflammatory cytokines, and oxidative stress.27 Some of the aforementioned mechanisms may also explain the potential association between RCC and T2D. For example, the relationship between IGF-1 and kidney cancer has been confirmed in animal experiments,28 and the abnormal glycolysis process induced by the Von Hippel Lindau (VHL) gene mutation can promote the overexpression of hypoxia-inducible factor α in RCC cells and promote the angiogenesis, chemoresistance, and mitophagy of tumor cells.24 These previous studies partially supported the findings of the present study, namely that T2D is associated with a poor prognosis in patients with RCC. Therefore, we questioned whether the prognosis of patients with RCC presenting with T2D differs from patients without T2D.
In the present study, patients who were diagnosed with both T2D and RCC were significantly older, had a higher incidence of hypertension and cardiovascular disease, and had a higher BMI than patients without T2D, similar to previous reports.29 Previously, other researchers reported that BMI is an independent risk factor for the prognosis of patients with RCC,30 but the prognostic value of BMI was not observed in the present study. The present study did not find that hypertension and cardiovascular disease are independent prognostic factors for patients with RCC, consistent with previous studies.4, 31 This finding may be attributed to the significant aggravation of the occurrence and development of atherosclerosis and cardiovascular disease by T2D, but the prognosis is significantly improved by active treatment, particularly strategies that lower low-density lipoprotein cholesterol concentrations and control blood pressure and blood sugar levels.32 The prevalence of in the present study T2D was 16.4%, consistent with a previous study that reported a prevalence of T2D of 16.5% in patients with RCC.18
Compared with previous studies,4, 18-20, 31, 33 we analyzed the surgical approach for the first time and found that a greater proportion of patients with both T2D and RCC underwent laparoscopic than open surgery, but no significant difference in the recurrence rate was observed between the two surgical approaches. A significant difference in prognosis between patients with localized RCC who undergo laparoscopic surgery and patients who undergo open surgery has not been observed,34 but laparoscopic surgery can result in a quicker recovery and reduce the incidence of complications.35 The meta-analysis by Zheng et al36 confirmed a lack of significant differences in OS, CSS, or RFS after 5 years between patients who underwent laparoscopic surgery and those who underwent laparotomy. The most recent research of Vartolomei et al37 also found that robot-assisted laparoscopic partial nephrectomy has a good prognosis and fewer complications in 52 elderly patients. In the present study, 95 patients underwent laparotomy and 356 patients underwent laparoscopic surgery. No significant differences in OS, CSS, and RFS were observed between the two groups, consistent with the findings of a previous study.36 Based on these results, laparoscopic surgery is as safe as open surgery in terms of the prognosis of RCC and has certain advantages in postoperative rehabilitation. These advantages are also why laparoscopic surgery is more frequently used.
According to Habib et al38 and Antonelli et al,20 tumor diameters are smaller in patients with than without T2D, which was confirmed in the present study. However, Hofner et al33 did not detect significant differences in tumor size between patients with RCC who had pre-existing T2D and those who did not. We speculate that this difference may be related to ethnic, genetic, and environmental differences in the study cohorts.
One of the major complications of T2D is microangiopathy, and the resulting development of diabetic nephropathy renders the patient susceptible to renal insufficiency.39 In this case, patients with diabetes should be treated with nephron-sparing surgery whenever possible. In the present study, patients with T2D were more commonly treated with nephron-sparing surgery (36.5% vs 28.1%). More international research centers have also recognized that the optimal preservation of renal function is important for improving the long-term prognosis of patients with both diabetes and kidney cancer.40
As a complicated disease, T2D is characterized by hyperglycemia and other metabolic disorders (eg, irregular levels of testosterone, insulin or IGFs). The prognosis of patients may be affected by various metabolic factors. In addition, the duration and severity of diabetes, different drug types, and timing of administration are important aspects to consider when assessing postoperative survival and recurrence in patients with RCC. Finally, we did not analyze lifestyle variables related to glucose metabolism, such as physical activity and diet. Future investigations will require more in-depth studies of the aforementioned factors.
In conclusion, T2D is an independent predictor of survival and recurrence after surgery in patients with RCC. This finding confirms the importance of T2D in determining the prognosis of patients with RCC. The present study is preliminary in nature, and prospective cohort studies that include patients of various ethnicities are needed to confirm these results. Based on the results of the present study, we postulate that clinicians should monitor and actively treat T2D to improve the survival of and reduce the recurrence rate in patients with RCC.
ACKNOWLEDGEMENTS
The authors thank all those who participated in the study.
DISCLOSURE
None declared.




