A Novel Inhibitor of Methyltransferase SMYD2, AZ505 Protects Against Peritoneal Fibrosis in Mice
Funding: This study was supported by the grants from Wuxi Municipal Bureau on Science and Technology (Y20232014 to Taijing Xu), Key Discipline Construction Project of Pudong Health and Family Planning Commission of Shanghai (PWZxk2022-19 to Hualin Qi) and the Academic Leader Training Plan of Health System of Pudong New District of Shanghai, China (PWRd2019-13 to Feng Liu).
Taijing Xu, Binbin Cui and Feng Liu contributed equally to this study.
ABSTRACT
AZ505, a highly selective inhibitor of SMYD2, exhibits an antifibrotic effect in renal fibrosis. Its effect on peritoneal fibrosis remains unexplored. In this study, we investigated its effects on the development of peritoneal fibrosis induced by chlorhexidine gluconate (CG) in a murine model. We found that SMYD2 and trimethylated histone substrate H3K36 (H3K36me3) were highly expressed in the peritoneal tissue following CG injection, and administration of AZ505 remarkably inhibited their expression, along with attenuating CG–induced peritoneal fibrosis and expression of collagen I and fibronectin. Moreover, AZ505 also significantly reduced expression of CD31 (marker of angiogenesis) and CD68-positive macrophage infiltration in the CG-injured peritoneum. AZ505 further inhibited CG-induced epithelial-to-mesenchymal transition (EMT) of peritoneal mesothelial cells, manifested by decreasing expression of α-smooth muscle antigen (α-SMA) and Vimentin and restoring E-cadherin expression, accompanied by suppressing expression of two transcription factors, Snail and Twist. Finally, AZ505 inhibited CG-induced phosphorylation of AKT and increased expression of phosphatase and tensin (PTEN), a key phosphatase. These data suggest that AZ505 may protect against peritoneal fibrosis by inhibiting EMT, inflammation and angiogenesis, due to its blockade of methylation modification catalysed by SMYD2.
1 Introduction
Peritoneal dialysis (PD) is a cost-effective and better-adopted alternative treatment for patients with end stage renal disease (ESRD). However, with prolonged exposure of the peritoneum to dialysate, including hyperglycaemia, glucose degradation products (GDPs) and advanced glycosylation end products (AGEs), irreversible fibrosis occurs in the peritoneum, which is responsible for uraemic toxin transport and fluid removal, and problems with dialysis inadequacy and ultrafiltration failure inevitably arise [1]. Exploring the pathogenesis and reversible regulatory mechanism of peritoneal fibrosis is helpful in finding an effective treatment to prolong the continuity and effectiveness of PD.
In the initial stages of peritoneal fibrosis, mesothelial cells (MCs) undergo epithelial-to-mesenchymal transition (EMT) and transform into fibroblastoid cells, which continuously produce excessive extracellular matrix (ECM) components in submesothelial zones, leading to submesothelial thickening and peritoneal fibrosis [2]. In the pathophysiological mechanism of the EMT process, epigenetic modifications have garnered increasing recognition and several achievements, which are meaningful and encouraging [3]. Previous studies from our colleagues have demonstrated that histone acetylation catalysed by histone deacetylase 6 (HDAC6) and methylation mediated by enhancer of zeste homologue 2 (EZH2) play important reversible regulatory roles in peritoneal EMT, inflammation and angiogenesis [4, 5]. Hence, exploring a wider range of methylation modification by enzymatic catalysis may be beneficial to find more effective means to improve or even reverse peritoneal fibrosis.
One histone methyltransferase, SET and MYND domain protein 2 (SMYD2) exerts methylation modification mainly through methylation of histones (H3K36 and H3K27) and many non-histone proteins such as signal transducer and activator of transcription 3 (STAT3), nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), and phosphatase and tensin homologue deleted on chromosome 10 (PTEN) [6-8]. Our previous study has confirmed that pharmacological inhibition or gene silencing of SMYD2 can significantly improve acute kidney injury (AKI) induced by cisplatin, manifested by inhibiting cell apoptosis and inflammatory response, and blocking signalling pathway activation, such as STAT3 and NF-κB [9]. Furthermore, other researchers also found that SMYD2 plays a role in the renal fibrosis induced by unilateral ureteral ligation (UUO) [10] and diabetes nephropathy (DN) [11], with the mechanisms that SMYD2 may promote renal cell EMT and ECM deposition. Therefore, we speculate that SMYD2 may be involved in peritoneal fibrosis progression, and its role and mechanism need to be explored.
To confirm the hypothesis, we investigated the effect of AZ505, a substrate-competitive inhibitor that binds the peptide-binding groove of SMYD2, in a mouse model of peritoneal fibrosis induced by chlorhexidine gluconate (CG). Our results demonstrated that AZ505 significantly inhibited peritoneal fibrosis, manifested by attenuating peritoneal EMT, inflammation and angiogenesis.
2 Materials and Methods
2.1 Chemicals and Antibodies
Antibodies to SMYD2, H3K36me3, p-Akt, Akt, PTEN and Tubulin were purchased from Cell Signalling Technology (Danvers, MA). Antibodies to CD68, CD31, E-cadherin, Vimentin, Snail and Twist were purchased from Abcam Inc. (Cambridge, UK). Antibodies to α-SMA, Fibronectin and type I Collagen, CG and all other chemicals were purchased from Sigma (St. Louis, MO). AZ505 was purchased from MedChemExpress (New Jersey, USA).
2.2 Establishment of Mouse Peritoneal Fibrosis Models and AZ505 Administration
The male C57/BL6 mice weighing 24–28 g were selected to establish the peritoneal fibrosis model as described in our previous study [12]. In brief, the mouse peritoneal fibrosis model was conducted by intraperitoneal injection of 0.1% CG dissolved in 0.9% saline every other day for 21 days (named as CG groups), and the control mice (named as Sham groups) were injected with an equal volume of 0.9% saline. AZ505 (10 mg/kg) dissolved in 50 μL DMSO was given intraperitoneally immediately after CG injection or not and then administered daily (named as CG + AZ505 or Sham + AZ505 groups). On day 21 after CG injection, the whole parietal peritoneum apart from the injection point was harvested for histologic staining and immunoblot analysis. All the experimental procedures on animals were approved by the Institutional Animal Care and Use Committee at Tongji University, Shanghai, China.
2.3 Immunoblot Analysis
As described previously, peritoneum tissue specimens were used for immunoblot analysis [12]. Briefly, Peritoneum tissue stored in the refrigerator at −80°C was taken out and centrifuged at 12000 g at 4°C for 15 min. A BCA protein concentration assay kit was used to determine the protein concentration. The samples were separated by SDS-PAGE and transferred to the polyvinylidene difluoride membranes (PVDF, Millipore, MA, USA) by electric transmission. Membranes were incubated with 5% skim milk for 1 h at room temperature, then incubated with primary antibodies (Fibronectin, Collagen I, Tubulin, α-SMA, Vimentin, E-cadherin, Snail, Twist, phospho-AKT, AKT and PTEN), and then incubated overnight at 4°C. After washing the TBST membrane, secondary antibodies were labelled with horseradish peroxidase and incubated at room temperature for 2 h at room temperature. Bound antibodies were detected using chemiluminescence (Millipore, USA), then processed by densitometry analysis with ImageJ software (National Institutes of Health, USA). The quantification data is given as the ratio between the target proteins and loading control.
2.4 Histochemical Staining
Formalin-fixed peritoneum was used to be embedded in paraffin for sectioning (3-μm-thick sections), and then staining was conducted by the procedure described in our previous study [12]. According to the protocol provided by the manufacturer, Masson trichrome staining was conducted to evaluate peritoneal fibrosis. The collagen tissue area (blue colour) was quantitatively measured, and the average ratio to each microscopic field (×200) was calculated and graphed. We examined peritoneum tissues immunohistochemically for SMYD2, H3K36me3, CD31 and CD68 expression.
2.5 Statistical Analysis
All the experiments were conducted at least three times, and all the statistical analyses were conducted by SPSS 26.0. All data were expressed as means ± standard deviation. One-way Analysis of variance (ANOVA) was used to compare groups. Statistically significant differences between mean values were marked in each graph. p < 0.05 was considered statistically significant.
3 Results
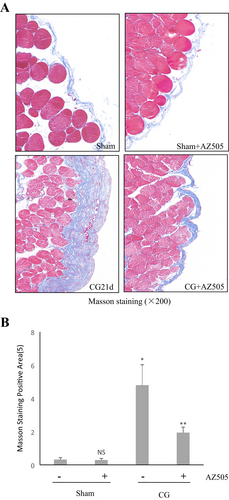
3.1 AZ505 Attenuates Peritoneal Fibrosis Induced by CG in Mice
As shown in Figure 1A,B, the Masson trichrome staining demonstrated that the thickness of the submesothelial zone and the area of collagen fibrils in CG + AZ505 groups (AZ505 was given by intraperitoneal injection immediately after CG injection and then daily for 21 days) was significantly reduced compared to that in CG groups (p < 0.05).
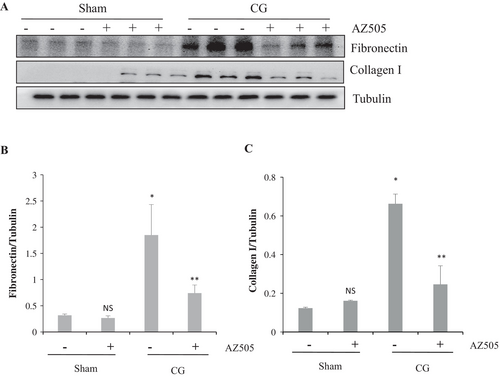
The results from immunoblot analysis further exhibited that the expression of collagen 1 and fibronectin, two major ECM proteins, increased in the peritoneum after CG injection (Figure 2A–C), which significantly decreased after AZ505 administration (p < 0.05, respectively). Therefore, we suggest that AZ505 may have a potential inhibiting effect on peritoneal fibrosis.
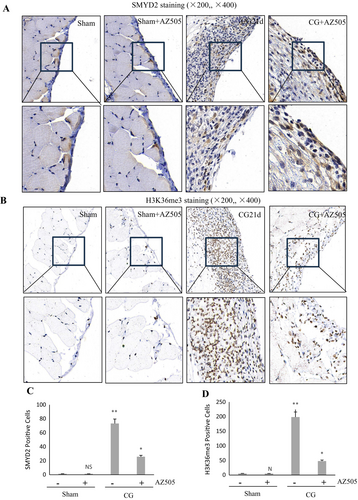
3.2 AZ505 Inhibits Expression of SMYD2 and H3K36me3 in Mice Following CG Injection
Histone H3K36 is the main methylated substrate of SMYD2, which may be trimethylated by SMYD2 [7]. To demonstrate the specificity of AZ505 on the activation of these targets in the peritoneum, we examined its effect on the expression of SMYD2 and H3K36me3 by immunohistochemical staining. The results demonstrated that SMYD2 and H3K36me3 were barely detected in the sham-operated peritoneum tissue, and their expression increased significantly after CG injection (p < 0.01, respectively); AZ505 was effective in suppressing SMYD2 and H3K36me3 expression (Figure 3A–D) (p < 0.05, respectively). Notably, SMYD2 was located in both the cytosol and nucleus of peritoneal mesothelial cells (PMCs), and H3K36me3 was located only in the cellular nucleus. Therefore, it indicated that CG injury enhances peritoneal SMYD2 expression and activation, which was sensitive to AZ505 treatment.
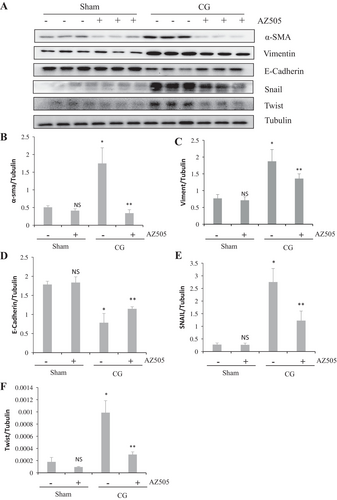
3.3 AZ505 Inhibits CG-Induced EMT of PMCs in the Peritoneum
It has proved that EMT plays an important role in peritoneal fibrosis [13]. The results from immunoblot analysis showed that CG resulted in decreasing expression of E-Cadherin (marker for epithelial cells) and increasing expression of α-SMA and Vimentin (markers for mesenchymal cells), as well as two key transcription factors, Snail and Twist (Figure 4A–F) (p < 0.05, respectively). Administration of AZ505 significantly promoted E-Cadherin expression, along with inhibiting expression of α-SMA, Vimentin, Snail and Twist (Figure 4A–D) (p < 0.05, respectively). These results demonstrated that AZ505 inhibited EMT of PMCs induced by CG injury.
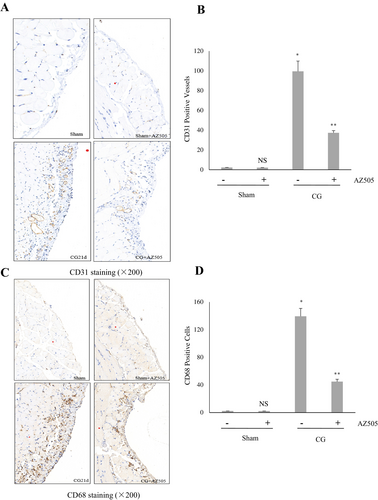
3.4 AZ505 Suppresses Angiogenesis and Infiltration of Macrophages in the Peritoneum After CG Injury
Long-term PD is frequently accompanied by angiogenesis and infiltration of inflammatory cells into the submesothelial compact zone in the fibrotic peritoneum. As shown in Figure 5A–D, immunohistochemistry staining demonstrated that CD31 (+) vessels and the number of CD68-positive macrophages increased in the submesothelial layer of the mouse peritoneum after CG injury (p < 0.01, respectively) and AZ505 treatment reduced their infiltration (p < 0.05, respectively). Collectively, we suggest that AZ505 is also effective in suppressing CG-induced angiogenesis and the inflammatory responses in the fibrotic peritoneum.
3.5 AZ505 Blocks Activation of AKT Signalling Induced by CG and Preserves PTEN Activation
The potential methylation non-histone substrates of SMYD2, PTEN has been proved to take a negative-regulated role in the progression of peritoneal fibrosis, which may be the principal negative regulator of AKT activation [14]. As shown in Figure 6A,B, a significant increase in AKT phosphorylation was observed after CG-induced injury (p < 0.05), and AZ505 significantly blocked its phosphorylation (p < 0.05). AZ505 did not affect the expression levels of total AKT (Figure 6A,C) (p > 0.05). We also found that PTEN was minimally expressed in the sham-operated peritoneum, and the expression of PTEN elevated after CG injection (p < 0.05). However, AZ505 administration significantly upregulated PTEN expression when compared with the sham peritoneum (p < 0.05). Our data indicated that AZ505 may block signalling pathways activation partly through its inhibitory effect on the methylation modification of SMYD2 upon PTEN, thereby preserving the activation of PTEN.

4 Discussion
Peritoneal fibrosis is one of the most common complications for patients on PD, due to prolonged exposure of the peritoneum to dialysate with high-dose glucose [1]. However, the pathogenesis of peritoneal fibrosis has not been fully elucidated. The role of methylation modification, as one of the main forms of epigenetic modification, in peritoneal fibrosis has garnered increasing recognition [3]. Here, we demonstrated that AZ505, a substrate-competitive inhibitor of SMYD2, one methyltransferase acting on histones and various non-histones, was effective in inhibiting peritoneal fibrosis in a mice model induced by CG. These results indicate that SMYD2 is a potent target for preventing peritoneal fibrosis.
Because of continual exposure to high-glucose dialysate, the peritoneal membrane may take the pathological change, such as progressive fibrosis, inflammation and angiogenesis [1]. In this study, we firstly found that AZ505 significantly alleviated the thickness of the submesothelial zone and inhibited ECM (fibronectin and collagen I) increasement induced by CG injection, suggesting a potent inhibitory effect of AZ505 on peritoneal fibrosis. Along with this observation, AZ505 was effective in suppressing SMYD2 and its main methylation histone substrate, H3K36me3 expression. It suggests that SMYD2 may be involved in the fibrosis process through its methylation modification, which has been proved in other tissue fibrosis models [10, 15, 16].
Factly, SMYD2 has been proved to play an important role in tissue fibrosis. In the previous study, we found that SMYD2 mediated the conversion of renal tubular epithelial cells to a profibrotic phenotype, renal fibroblast activation and renal fibrogenesis induced by UUO injury [10]. Recently, Li et al. found that SMYD2 not only participates in the growth of renal cysts in autosomal dominant polycystic kidney disease (ADPKD) [17] but also regulates fibrotic gene transcription and the activation of fibroblasts in cystic kidneys by the mechanism of a cross talk between TGF-β signalling and SMYD2 [15], in which TGF-β stimulates the expression of Smyd2 in a Smad3-dependent manner, and the upregulation of Smyd2 regulates the transcription of TGF-β and other fibrotic marker genes through direct binding to their promoters or methylating histone H3 indirectly to regulate the transcription of those genes. AZ505 can also protect against AKI induced by cisplatin [9] and inhibit the expression of EMT, fibrosis-related proteins and inflammatory cytokines induced by chronic cisplatin injury [16]. Our findings indicate that SMYD2 may be involved in the occurrence and development of peritoneal fibrosis, suggesting that it may be one prospective therapeutic target for tissue fibrosis.
EMT in PMCs is a complex biological process characterised by the gradual loss of PMCs epithelial-specific markers, such as E-Cadherin, and the acquisition of a fibroblast phenotype expressing α-SMA and Vimentin [13]. This process is accompanied by changes in cellular behaviour and the production of inflammatory cytokines and ECM proteins, which are regulated by the two major transcription factors, Twist and Snail, leading to the induction of E-Cadherin downregulation and drive EMT in PMCs [13]. In the present study, we found that AZ505 significantly inhibited the EMT of PMCs induced by CG injection. In fact, SMYD2 may take a key role in regulating EMT directly or indirectly in other cell types [18]. TGF-β/Smads and Wnt/β-catenin pathways are proved to be involved in EMT; Snail and Twist functions can be regulated by TGF-β downstream factors Smad2/3 and STAT3 [19, 20]. Meanwhile, Wnt has cross-talk with Smad3 and can inhibit Snail and Twist degradation, resulting in downregulation of Cadherin expression [19]. In 293T cells, SMYD2 can methylate H3K36 on the β-catenin promoter, induce β-catenin translocation into the nucleus, and upregulate the transcriptional activity of Wnt signalling [21]. A cross-talk between TGF-β/Smads signalling and SMYD2 has been identified in the epithelial cells of renal cysts [15], manifested by that TGF-β stimulating the expression of Smyd2 in a Smad3-dependent manner, and the upregulation of Smyd2 regulating the transcription of TGF-β. P53 can recruit mouse double minute 2 (MDM2) to form complexes that promote the degradation of Snail [22], whereas SMYD2 can methylate lysine at the 370 site of p53 and inhibit p53 function [23]. SMYD2 can also methylate PTEN, leading to the inhibition of its phosphatase activity, and promote the transcriptional expression of signal proteins AKT and STAT3 [14], which promote EMT of PMCs. Therefore, it suggests that SMYD2 may participate in regulating EMT of PMCs by methylation of multiple substrates, which may become an important target pathway for preventing peritoneal fibrosis from a mechanistic perspective.
Chronic hyperglycemia activates the mesothelial receptor of advanced glycation end products (AGEs), resulting in increased levels of vascular endothelial growth factor (VEGF), which promotes angiogenesis through binding and activating the VEGF receptor (VEGFR), referring to the formation of new blood vessels (CD31-positive) [24]. Angiogenesis is an important event in the process of peritoneal fibrosis [24]. In the present study, we found that AZ505 was effective in reducing the number of CD31-positive cells induced by CG injury. These results indicated that SMYD2 may be involved in angiogenesis. In the process of tumour metastasis, SMYD2 has been proven to promote angiogenesis and metastasis [18], but there is currently no evidence to identify that VEGFR is a directly methylated substrate of SMYD2. We speculated that SMYD2 may regulate angiogenesis through indirect pathways. EZH2 is another methylase that has been proven to promote angiogenesis and peritoneal fibrosis [5]. Meanwhile, it has been proved that EZH2 can methylate VEGFR [25], resulting in inducing tumour cell angiogenesis, and EZH2 is the methylation substrate of SMYD2 [26]. Therefore, we suggested that SMYD2 may indirectly promote angiogenesis mediated by VEGF/VEGFR and facilitate peritoneal fibrosis progression by methylation of EZH2, but the molecular mechanism remains to be further studied and validated.
Inflammation is another major manifestation of peritoneal fibrosis. AZ505 has been proved to inhibit inflammation in the cisplatin-induced AKI and UUO-induced renal fibrosis [9, 16]. In this study, we observed that AZ505 reduced elevated infiltration of CD68-positive macrophages in the peritoneum induced by CG injury. Previous studies have proved that SMYD2 can methylate STAT3 and NF-κB, two important inflammatory signalling pathways, thereby promoting their phosphorylation and activation [17, 27]. Thus, we suggest that SMYD2 plays a key role in regulating inflammation in peritoneal fibrosis. However, the role of SMYD2 in immune inflammation is inconsistent. In macrophages, Xu et al. proved that SMYD2 specifically facilitates H3K36 dimethylation at TNF and IL-6 promoters to suppress their transcription and inhibits NF-κB and ERK signalling [28]. Wu et al. reported that SMYD2 inhibits the expressions of IFN-I and proinflammatory cytokines in macrophages upon viral infections and identified SMYD2 as a negative regulator of IFN-I production against virus infection [29]. Therefore, it is necessary to further explore the mechanisms underlying the inconsistent roles of SMYD2 in inflammatory responses caused by different etiologies.
The pro-fibrotic signalling pathways, including AKT, are involved in the occurrence and development of peritoneal fibrosis [30]. PTEN is a phosphatase that acts on both polypeptide and phosphoinositide substrates, such as AKT, STAT3 and NF-κB, which can dephosphorylate and deactivate these signalling proteins [14]. In our study, we found that AZ505 significantly blocked AKT phosphorylation and upregulated PTEN expression. In breast cancer cells, Nakakido et al. demonstrated that SMYD2 can methylate PTEN at lysine 313 in vitro and in vivo, leading to the negative regulation of PTEN activity and resulting in activation of the AKT pathway [14]. Our results are consistent with it, and we have proved that AZ505 can inhibit the phosphorylation of STAT3 and NF-κB in the animal models of renal fibrosis and AKI [9, 10], suggesting that SMYD2 can participate in the activation of the fibrotic signalling pathway by methylation of PTEN.
In conclusion, our study provides evidence that AZ505 may protect against peritoneal fibrosis via its blockade of SMYD2. The underlying mechanism of its anti-peritoneal fibrotic effect is associated with preserving PTEN activation, as well as inhibiting ECM synthesis, EMT, inflammation and angiogenesis. Therefore, our results suggest that SMYD2 may be one of the important targets for preventing and treating peritoneal fibrosis.
Author Contributions
Hualin Qi, Feng Liu and Xuan Hong conceptualised the study. Taijing Xu, Binbin Cui, Mengjun Liu and Xiying Hou conducted the experiment. Taijing Xu, Mengjun Liu, Feng Liu, Xuan Hong and Hualin Qi analysed the data. Taijing Xu, Binbin Cui and Feng Liu wrote the manuscript. Hualin Qi, Feng Liu and Xuan Hong edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The sponsor and investigators thank the medical staff at the Department of Nephrology, Affiliated Huishan Hospital of Xinglin College, Nantong University, Wuxi City, Jiangsu Province; Department of Nephrology, Shanghai East Hospital, Tongji University School of Medicine; and Department of Nephrology, the People's Hospital of Pudong New District in Shanghai.
Disclosure
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




