Shifts in phytoplankton communities in inland waterways: Insights from the Beijing–Hangzhou Grand Canal, China
Abstract
Phytoplankton play a crucial role in maintaining the health of river ecosystems, and their communities are closely linked to river hydrodynamics. In inland waterways, disturbances generated by ship propellers alter flow dynamics and may affect phytoplankton communities. To clarify it, phytoplankton communities in the Zhenjiang section of the Beijing–Hangzhou Grand Canal (BHGC) in China, the world's longest canal, were studied and compared them with its undisturbed tributaries. The results revealed major alternations in seasonal patterns of phytoplankton communities in the BHGC, shifting the peak of phytoplankton density from spring to autumn and the lowest diversity from summer to autumn. Ship disturbances increased water turbidity and created optimal N/P ratios, which provided Cyanobacteria with a competitive advantage in autumn. The proliferation of Cyanobacteria resulted in a phytoplankton density in the BHGC, exceeding that in the tributaries by more than tenfold, accompanied by a decrease in diversity to its lowest level. Due to habitat alterations, functional groups emerged that are resilient to strong disturbances and high turbidity. The findings add to the understanding of the impact of ship traffic on river ecosystems.
1 INTRODUCTION
Phytoplankton serve as primary producers and play a significant role in food chains within aquatic ecosystems (González-Olalla et al., 2024; Hoppe et al., 2018; Kireta et al., 2012). Hébert et al. (2021) demonstrated that climate warming alters the seasonal dynamics of phytoplankton communities and affects food chains in polar freshwater lakes. Moreover, phytoplankton are highly sensitive to habitat changes in aquatic ecosystems, making them valuable indicators of ecosystem health. Therefore, understanding the characteristics of phytoplankton communities is essential for assessing the health of aquatic ecosystems.
Phytoplankton have different habitat preferences. For example, Cyanobacteria and Euglenophyta prefer lentic waters, while Bacillariophyta thrives in lotic waters (Zhao et al., 2024). River flow dynamics can directly shape these habitats, thereby affecting phytoplankton communities. Chen et al. (2008) identified 35 phytoplankton species in the Black Volta before impoundment, dominated by Chlorophyceae (43%) and Cyanophyceae (48.6%), and 18 species after impoundment, with a shift toward Cyanophyceae (71.7%). Similarly, Wang et al. (2017) observed increased phytoplankton abundance, a decline in Diatoms, and a rise in Pyrrophyta in the Three Gorges Reservoir, driven by changes in turbidity and light penetration influenced by river flow dynamics. These shifts in phytoplankton species led to corresponding changes in diversity. Al-Bader et al. (2011) also found that the diversity of Cyanobacteria increases with rising turbidity, with most Cyanobacteria in turbid waters belonging to Oscillatoriales.
Inland water transportation is a commonly used mode of cargo transport in flat regions due to its lower cost and higher transport capacity (Brand et al., 2012; Wang, Chen, et al., 2020; Zhou et al., 2023). For instance, the total mileage of inland waterways has exceeded 128,200 km in China, and is expected to continue increasing in the future (CEIC, 2023). In waterways, the high-speed rotation of ship propellers often generates intense waves (Wei et al., 2020). These waves not only convert lentic water into lotic conditions but also resuspend sediments, altering water turbidity and other environmental factors (Yan et al., 2023), which potentially impact phytoplankton communities. Although many studies have studied phytoplankton communities in waterways (Naskar et al., 2021; Wan et al., 2023), the effects of these ship-induced disturbances on these communities remain poorly understood. This gap limits a comprehensive assessment of the ecological impact of ship traffic on river ecosystems.
To clarify it, this study investigated and compared phytoplankton species, density, diversity, and functional groups in the Zhenjiang section of Beijing–Hangzhou Grand Canal (BHGC), China, with its undisturbed tributaries. The BHGC is an important man-made river, and exploring phytoplankton community seasonal dynamics in BHGC is crucial for aquatic environment management. Additionally, the characteristics of ship-induced waves and their effects on water quality were also analyzed to understand how these disturbances impact phytoplankton communities. These findings will help evaluate the effects of ship traffic on river ecosystems.
2 MATERIALS AND METHODS
2.1 Study area
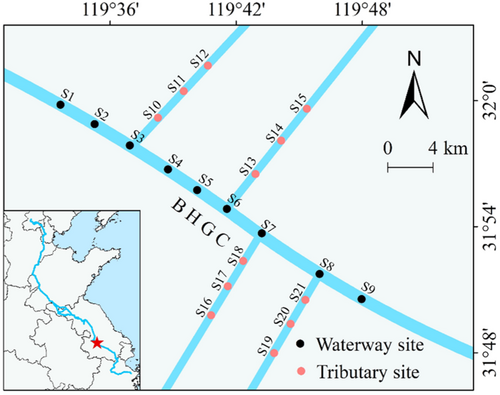
The BHGC is the longest artificial canal in the world, stretching from Beijing in the north to Hangzhou in the south of China. It has a width of 15–30 m and a length of 1794 km (Figure 1) (Liu et al., 2022). As one of the busiest canals, it had accommodated 1.4 million ships by 2014. The study area is located in the Zhenjiang section of the BHGC, an economically developed region, and a busy ship traffic section. Due to the busy ship traffic, the water surface in this area is persistently turbulent. The study area has a North Asian monsoon climate, characterized by hot, rainy summers and mild winters. The average annual temperature is 15.6°C, accompanied by an average annual rainfall of 1066 mm, with 60% of the rainfall occurring between May and September (Chen et al., 2008). Thus, high water levels typically occur from June to September, while low water levels prevail during the winter and spring seasons. Compared to the BHGC affected by ship-induced waves, the control tributaries are free from such waves.
2.2 Field surveys
Field surveys were conducted in spring (May 2022), summer (August 2022), autumn (November 2022), and winter (January 2023) at a total of 21 sites, including nine along the BHGC and three on each of its four tributaries, yielding a total of 84 samples. To minimize regional differences, sampling sites in the tributaries were positioned as close to the BHGC as possible while remaining unaffected by ship-induced waves from the canal. Water samples were collected at a depth of 20 cm below the water surface using a water sampler (WB-PM; Beijing, Beijing Pult Instrument Co., Ltd.). Then, 500 mL of each sample was stored in PET bottles for water quality analysis, and an additional 1000 mL was preserved for phytoplankton analysis after adding 15 mL of Lugol's solution. After standing for 48 h, the supernatant of phytoplankton sample was carefully removed using siphonage, and the concentrated phytoplankton was adjusted to 50 mL and stored in sample bottles for microscopic examination. The water turbidity, dissolved oxygen (DO), pH, and water temperature were measured in situ using a turbidity meter (WZB-170; Shanghai Yidi Science Instrument Co., Ltd.), DO meter (JPB-607A; Shanghai, Shanghai Yidi Science Instrument Co., Ltd.), and pH meter (PHB-4; Shanghai Yidi Science Instrument Co., Ltd.), respectively. Surface water waves were also assessed using automated wave recorders (YWS200-WXX; Chengdu Yufan Technology Ltd., Co.). Water wave data were collected every 1 s for 1 h.
2.3 Chemical analyses
The total nitrogen and total phosphorus in the water were analyzed using the alkaline potassium persulfate ultraviolet spectrophotometric method and the ammonium molybdate spectrophotometric method, respectively (Jin & Tu, 1990). According to the method proposed by Ringuet et al. (2011), the dissolved nutrients, such as ammonium, nitrate, and soluble reactive phosphorus, were determined using an enzyme-labeled microplate spectrophotometric method, which has been widely employed for analyzing soluble nutrients in inland waters due to the small sample requirement and high efficiency.
2.4 Functional analysis and classification of phytoplankton
The phytoplankton functional groups were classified based on their habitats according to S. Reynolds et al. (2002) and Padisák et al. (2009). All phytoplankton species and functional groups are listed in Supporting Information S1: Table S1.
2.5 Statistical analyses
Significant differences in environmental factors and phytoplankton density between the BHGC and its tributaries were assessed using a one-way analysis of variance, with a significance level of p < 0.05. Statistical analysis and graphical visualization were conducted using Origin software (Version 2023, OriginLab) and Canoco (Version 5, Microcomputer Power).
3 RESULTS
3.1 Water properties
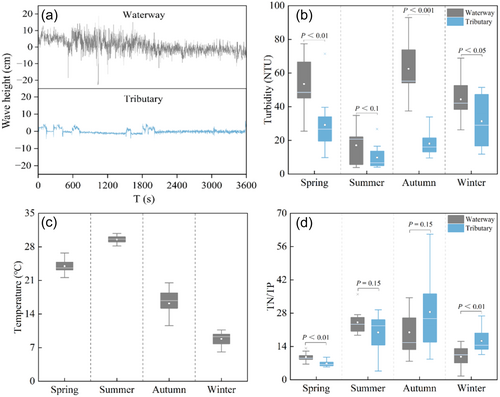
The water surface of the BHGC was lotic, with an average wave oscillation of 22 cm. In contrast, the adjacent tributaries maintained relatively lentic, with an average wave oscillation of only 4 cm (Figure 2a). Except for the summer, the water turbidity of the BHGC is significantly higher than that of the tributaries throughout the year (p < 0.05). In summer, both the BHGC and the tributaries displayed low turbidity levels, with the BHGC slightly higher than the tributaries, though the difference was not significant (p > 0.05). Specifically, water turbidity in the BHGC was 8.1, 17.1, 62.5, and 44.4 NTU in spring, summer, autumn, and winter, respectively, whereas in tributaries, it was 8.2, 9.8, 18.1, and 31.4 NTU, respectively (Figure 2b). Water temperature displayed distinct seasonal variations in both the BHGC and its tributaries, with the temperatures being 24.2°C, 29.6°C, 16.2°C, and 8.8°C in spring, summer, autumn, and winter, respectively (Figure 2c). Overall, the N/P ratio in both the BHGC and its tributaries showed an increasing trend, which was followed by a decrease. During spring and summer, the BHGC had a higher N/P ratio than the tributaries, which was significantly different in spring. Whereas during autumn and winter, the BHGC had a lower N/P ratio than its tributaries, which was significantly different in winter. Specifically, the N/P ratio in the BHGC was 9.6, 24.1, 20.0, and 9.7 in spring, summer, autumn, and winter, respectively, while that in the tributaries was 6.9, 19.9, 28.5, and 16.3, respectively (Figure 2d).
3.2 Relationship between phytoplankton density and environmental factors
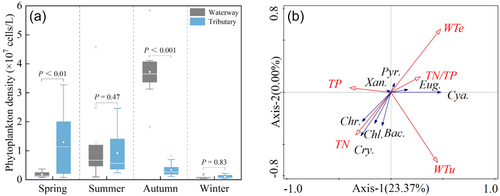
Generally, phytoplankton density in the BHGC initially increased and then decreased from spring to winter, reaching its peak in autumn, while the density in the tributaries gradually decreased. In spring, phytoplankton density in the BHGC was significantly higher than that in the tributaries, whereas in autumn, it was lower than that in the tributaries. However, there was no significant difference between the phytoplankton diversity in summer and winter (p > 0.05). The phytoplankton density in the BHGC was 1.9 × 106, 1.3 × 107, 3.7 × 107, and 9.7 × 105 cells L−1 in spring, summer, autumn, and winter, respectively, while that in the tributaries was 1.3 × 107, 9.2 × 106, 3.4 × 106, and 1.02 × 106 cells L−1, respectively (Figure 3a). Redundancy analysis indicated that water temperature, turbidity, and N/P ratio are key factors in regulating phytoplankton density (p < 0.05) (Figure 3b).
3.3 Phytoplankton species and diversity
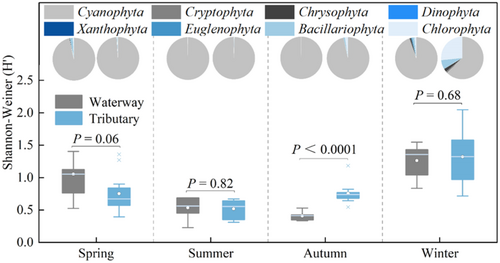
A total of eight phytoplankton phyla were identified, including Cyanobacteria, Cryptophyta, Chrysophyta, Bacillariophyta, Xanthophyta, Chlorophyta, Pyrrophyta, and Euglenophyta, of which Cyanobacteria are the dominant species. Its abundance was highest in the BHGC during autumn, reaching 99.9%, and lowest in the tributaries during winter, at 62.7%. From spring to winter, phytoplankton diversity generally exhibited a trend of initial decline followed by an increase. In spring, phytoplankton diversity in the BHGC was higher than in the tributaries, while in autumn it was lower. The Shannon index in the BHGC was 1.05, 0.53, 0.41, and 1.26 in spring, summer, autumn, and winter, respectively, while that in the tributaries was 0.75, 0.52, 0.75, and 1.32, respectively (Figure 4).
3.4 Phytoplankton functional groups
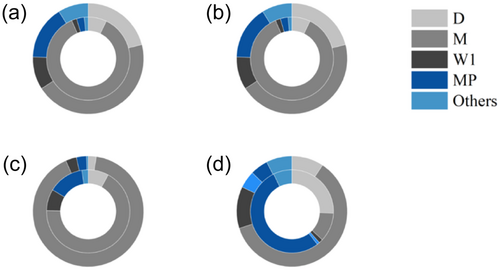
In the study area, the phytoplankton were categorized into 21 functional groups, including C, D, E, F, J, M, N, P, T, Y, X1, X2, X3, W1, W2, WO, LM, LO, MP, H1, and S2, with the M being the most dominant. The number of functional groups was 20, 17, 17, and 19 in spring, summer, autumn, and winter, respectively. The seasonal succession trends were as follows: M + D + MP in spring, M in summer, and M + MP + D + W1 in both autumn and winter. In spring, the main functional groups in the BHGC were M (45%), D (21%), and MP (16%), while that in the tributaries was M (86%). In summer, the main functional group in both the BHGC (85%) and tributaries (84%) was M. In autumn, the main functional group in the BHGC was M (91%), while those in the tributaries were M (67%), MP (14%), D (8%), and W1 (8%). In winter, the main functional groups in the BHGC were M (61%) and W1 (12%), while those in the tributaries were MP (53%), D (26%), and M (11%).
4 DISCUSSION
In aquatic ecosystems, phytoplankton density in regions with distinct seasonal climates often exhibits clear seasonal patterns due to their high sensitivity to temperature and light (Strock & Menden-Deuer, 2021). In the study area, phytoplankton density in the tributaries gradually decrease from spring to winter (Figure 3a). The lower water temperatures during autumn and winter (Figure 2c) may contribute to the lower densities in the tributaries during these seasons. However, in the BHGC, ship traffic shifted this seasonal pattern, displaying an initial increase followed by a decline, with a peak in autumn (Figure 3). In the BHGC, ship traffic (Figure 2a) caused the resuspension of bed sediments, resulting in higher turbidity in the water (Figure 2b). The higher turbidity in the BHGC during spring and the lower temperature during winter inhibited phytoplankton photosynthesis. The higher phytoplankton density during the autumn was primarily attributed to favorable N/P ratios, which conferred a competitive advantage to Cyanobacteria (as described below).
In the study area, although there were highly various phytoplankton species, Cyanobacteria overwhelmingly dominated, with their density accounting for up to 99.9% of phytoplankton communities (Figure 4). This could be attributed to the local climate and water quality. Taihu Lake Basin experiences a subtropical monsoon climate, which is characterized by high temperatures that favor Cyanobacteria (Van de Waal et al., 2024). Moreover, intense human activities have led to hyper-eutrophication in aquatic ecosystems, providing favorable conditions for Cyanobacteria (Bogard et al., 2020). Su et al. (2017) indicated that Cyanobacteria are the most dominant group in the Lake Taihu, accounting for over 92.6% of the total phytoplankton density. The favorable N/P ratios may contribute to the greater competitive advantage of Cyanobacteria during summer and autumn compared to other seasons. Tang (2002) observed that the N/P ratios in the lakes with cyanobacterial blooms varied in the range of 13–35, while the N/P ratios in the lakes without cyanobacterial blooms were less than 13. In this study, the N/P ratio ranged from 20.0 to 28.5 during summer and autumn and ranged from 6.9 to 16.3 during spring and winter (Figure 2d). Moreover, Cyanobacteria exhibit greater adaptability in the highly turbid environments. As a result, Cyanobacteria dominated the turbid BHGC during autumn, and their proportion was relatively lower in the tributaries with lower N/P ratios during the winter. The increase in N/P ratios during the summer and autumn is primarily attributed to these seasons being the rainy season, during which rising river water levels weaken the disturbances caused by ship traffic on the sediment, leading to decreased water turbidity (Figure 2b). In aquatic ecosystems, N and P exhibit distinct behaviors. P tends to adsorb onto suspended particles (Tang et al., 2018), leading to an increase in the N/P ratio as these particles settle.
Phytoplankton can be classified into different functional groups based on their morphology, physiological characteristics, and ecological functions, which are closely related to their habitats (Becker et al., 2010; Reynolds, 1998; Wang, Cai, et al., 2020). Xiao et al. (2011) demonstrated that the phytoplankton community in Liuxihe Reservoir was primarily composed of the A group. This composition was largely influenced by monsoon hydrology and water level regulation, which altered variables such as hydraulic retention time, mixing patterns, and thermal structure, ultimately affecting light and nutrient availability. Thus, understanding these functional groups is crucial for the conservation of aquatic ecosystems (Di Pane et al., 2022; Liu et al., 2022). In this study area, the M group was dominant (Figure 5), closely associated with the local climate and aquatic conditions, as M thrives in high-temperature and rich-nutrient environments (Cao et al., 2018). However, the BHGC exhibited different phytoplankton functional groups compared with its tributaries. In spring, the proportions of D and MP increased in the BHGC, as these groups are adapted to highly turbulent and turbid environments (Padisák et al., 2009; de Souza et al., 2016). In autumn, M dominated in the turbid BHGC because the Cyanobacteria-dominated M had a higher competitive advantage, whereas, in the less turbid tributaries, the proportions of MP, D, and W1 increased. In the cold winter, most of M remained dormant in the sediment, while D and MP, which are adapted to low temperatures, gradually became more dominant in both the BHGC and tributaries (Padisák et al., 2006; Reynolds, 2006). However, ship disturbances brought Cyanobacteria up from the sediment to the surface water, which possibly maintained the relative dominance of M in the BHGC. The phytoplankton functional groups not only reflect the ecological adaptation strategies of phytoplankton but also the corresponding environmental characteristics (McGill et al., 2006). Thus, the succession of these functional groups confirms the changes in the environment of river ecosystems under ship disturbances.
Inland waterways, characterized by their high transport capacity and low energy consumption, are indispensable as a major mode of transportation and play a significant role in regional economic development. In the Taihu Lake Basin, the waterway spans over 12,000 km and accommodates approximately 47,000 ships, with a potential cargo capacity of 13,400 tons. Our field observations indicate that the frequency of ship passages along the BHGC reached up to 45 ships per hour (Liu, Li, et al., 2023; Liu, Liu, et al., 2023). In recent years, the impact of ship traffic on river ecosystems has attracted widespread attention (Collas et al., 2018; Gabel et al., 2017). This study revealed that ship disturbances significantly influenced phytoplankton communities and their functional groups. These findings offer valuable insights for evaluating the ecological impact of ship traffic on river ecosystems. The phytoplankton communities may vary across different regions, and the impact of ship disturbances on them may also differ. Changes in phytoplankton communities are not solely attributable to shipping disturbances. Furthermore, ship disturbances may indirectly affect phytoplankton by factors such as zooplankton and fish predation. More data need to be collected for future studies to better understand these interactions.
5 CONCLUSIONS
In waterways, ships can alter river hydrodynamics and potentially affect phytoplankton communities. This study investigated phytoplankton communities in the BHGC in China and compared them with its undisturbed tributaries to identify the impacts of ship traffic on phytoplankton communities and the associated mechanisms. The results demonstrated that there were major alternations in seasonal patterns of phytoplankton communities in the BHGC. Ship disturbances shifted phytoplankton density from spring to autumn and the lowest diversity from summer to autumn, and regulated the seasonal succession of phytoplankton functional groups, leading to the emergence of functional groups resilient to strong disturbances and high turbidity. The findings add to the understanding of the impact of ship traffic on river ecosystems.
ACKNOWLEDGMENTS
The authors would like to thank Yajing Zhu from the Nanjing University of Information Science and Technology for identifying the phytoplankton species. This study was supported by Jiangsu Provincial Carbon-peak and Carbon-neutralization Technology Innovation Project (No. BK20220041), the National Natural Science Foundation of China (Nos. 42477073, 42277060).
ETHICS STATEMENT
None declared.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




