Massive Infiltrative Osteolysis of the Mandible in Oral Squamous Cell Carcinoma: A Case Report with a Review of the Literature
Abstract
Oral Squamous Cell Carcinoma (OSCC) is the most prevalent form of oral cancer, constituting over 90% of reported cases. This malignancy commonly infiltrates bone, making bone invasion a significant clinical issue. OSCC may invade bone via either an infiltrative or erosive pattern, with the pattern of invasion closely correlating with the clinical behavior of the disease and potentially holding prognostic value. Typically, OSCC spreads to the mandibular bone through direct infiltration of the alveolar ridge or lingual cortical plate. Interestingly, only 6% of OSCC cases initially present with a primary tumor, necessitating comprehensive whole-body imaging and clinical examinations to exclude other primary tumors. This report details a rare case of long-standing OSCC of the retromolar pad, which led to infiltrative osteolysis of the mandible, culminating in the near-total disappearance of the bone in a 47-year-old male patient.
1 INTRODUCTION
As described by Wills, a neoplasm is “an abnormal mass of tissue, the growth of which exceeds and is uncoordinated with that of normal tissues and persists in the same excessive manner after the cessation of the stimuli that evoked the change.” Squamous cell carcinoma (SCC) of the oral cavity accounts for 4% of cancer cases in men, 2% in women, and 3% of all cancer-related fatalities.1 Numerous factors, both internal and external, contribute to the multifactorial etiology of oral squamous cell carcinoma (OSCC). External and internal agents include tobacco, smoke, betelnut, alcohol, and systemic conditions such as iron deficiency anemia and malnutrition. An inadequate immune response also increases susceptibility to oral cancer.2 Intraorally, OSCC is most commonly found on the tongue and at the tip of the vermilion. Anatomical areas such as the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate are also affected, in decreasing order of frequency. Buccal mucosa and gingival lesions account for approximately 10% of all SCCs.3
Oral carcinoma affecting the mandibular region is defined as carcinoma of the mandibular retromolar trigone, lower buccal sulcus, sublingual sulcus, and mandibular alveolar ridge. The progression of the malignancy to directly infiltrate the mandible results in a poor prognosis. Direct tumor infiltration is the primary cause of mandibular involvement. If the tumor is positioned medially to the jaw, it typically enters within the alveolar crest and lingual cortex. Various routes of infiltration occur, with the inferior alveolar nerve canal being the most prevalent.4
The mandibular bone is invaded by OSCC in erosive, infiltrative, or mixed manners, correlating with clinical behavior. The erosive pattern is characterized by a wide, expansile tumor front with a distinct tumor-bone junction. Conversely, the infiltrative pattern consists of nests of tumor cells with finger-like extensions along an uneven front. Recent contrasts between these two histological patterns challenge the notion that mandibular invasion is always associated with a poor prognosis. The erosive type of bone invasion may extend more predictably compared to the infiltrative type. The recurrence rate for the erosive type of bone invasion is nearly 17%, while the infiltrative form has a higher rate of about 53%.5 This case report discusses the infiltrative type of bone invasion in a 47-year-old male patient, resulting in massive osteolysis and the near-total disappearance of the mandible.
2 CASE REPORT
A 47-year-old male patient was referred from the Department of Oncology to the Department of Oral Medicine and Radiology to assess unusual mandibular retrognathism. Two years prior, the patient presented with difficulty opening his mouth and experienced sudden weight loss. His habit history included 15 years of tobacco and ghutka consumption. A subsequent biopsy revealed dysplastic stratified squamous epithelium with invasive tumor nests greater than 1mm penetrating the subepithelium and scanty subepithelial tissue bits below the tumor nests. Signs of tumor invasion were evident in the skeletal muscle fibers, along with areas of hemorrhage and necrosis. Following this histopathological investigation, the patient was diagnosed with OSCC in 2022 but did not receive any treatment. Six months after the diagnosis, he experienced pain and observed a gradual recession of the lower jaw, leading to facial deformity and speech difficulties. The patient also presented bilaterally with draining sinuses discharging active pus and consulted an oncologist; incision and drainage were performed.
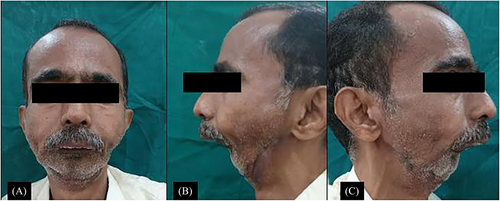
Extraorally, the patient exhibited complete retrusion of the mandible, resulting in a loss of facial contour and a sudden change in the angular configuration from both frontal and lateral views. Limited mouth opening was observed due to an ulceroproliferative lesion at the retromolar trigone bilaterally, accompanied by restricted tongue movements. An active draining sinus, along with a surgical scar extending from nearly 3mm away from the preauricular area to 2mm from the menton, was present with respect to the anatomical location of the antegonial notch bilaterally (Figure 1A–C).
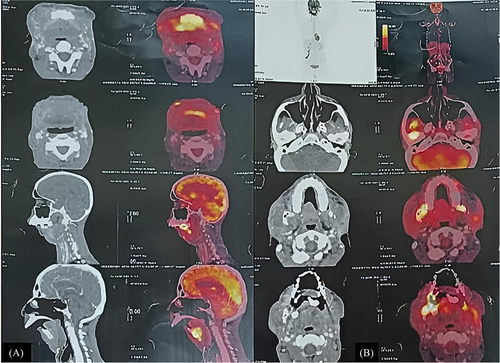
An 18F-FDG PET-CT scan revealed hypermetabolic heterogeneous thickening in the bilateral retromolar trigone, designating it as the multifocal malignant primary site. The scan also showed involvement of the central part of the mandible with extension into the tongue and lateral extension into the bilateral buccal mucosa and cheek region. Hypermetabolic nodular thickening was observed in the premaxillary region, along with multiple bilateral cervical lymph nodes displaying hypermetabolism, suggesting nodal metastatic disease. Mild diffuse FDG uptake was noted in the marrow of the visualized skeleton (Figure 2A–B).
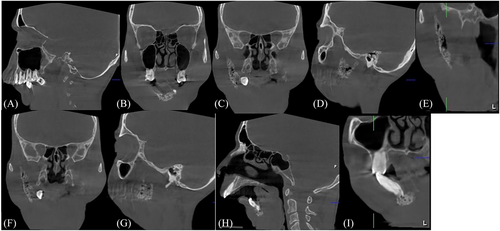
Cone beam computed tomography was performed to assess the extent of bone destruction and the amount of bone remaining in the mandible. Near-complete disappearance of the mandible was observed, with remaining bone only in the anterior region and right ascending ramus and condylar region (Figures 3A–C). The remaining bone showed multiple radiolucencies surrounded by hazy radiopacity, presenting a moth-eaten appearance indicative of tumor cells infiltrating the trabecular pattern, leading to its resorption (Figures 3D–G). The apical ends of the mandibular anterior teeth displayed bone infiltration by tumor cells, evident as radiolucencies surrounded by hazy radiopacities, with complete loss of the labial and lingual cortical plates (Figures 3H–I).
The infiltrative pattern of bone invasion by tumor cells resulted in the near-total disappearance of the mandible over the course of the disease, culminating in retrognathism with an altered bone pattern due to tumor infiltration in the mandibular anterior and right ascending ramus and condylar regions. Figures 4A–B show the 3D reconstructed image depicting this alteration.
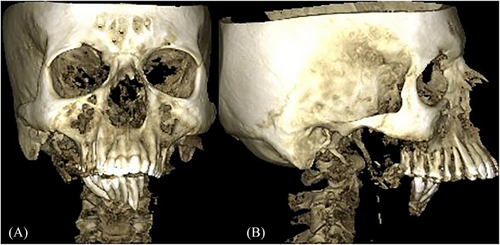
The radiological image suggested that the pattern of bone resorption might be attributed to Gorham's disease, also known as phantom bone disease or Gorham vanishing bone disease. This condition is typically monocentric and involves focally increased bone resorption caused by an increase in paracrine or autocrine-stimulated hyperactivated osteoclasts. There is no evidence to suggest the idiopathic genesis of this condition that leads to abnormal osteoclastic activity.6
Although the bone resorption pattern resembled that of vanishing bone disease, the diagnosis of Gorham's disease was ruled out since the patient had a histopathological diagnosis of OSCC and, contrary to Gorham's disease, the etiological factor for Gorham's is idiopathic. There is no literature evidence of OSCC contributing to vanishing bone disease. The bone resorption pattern in this patient only resembled vanishing bone disease radiologically. The definitive diagnosis of OSCC resulting in infiltrative bone invasion and near-total vanishing of the mandible was established by compiling both the clinical, histopathological, and radiological findings.
Chemotherapy and radiation therapy were not administered due to decreased bone density caused by tumor cell invasion of the bone, which increased the risk of pathological fractures and related infections. Therefore, the patient was maintained on palliative symptomatic treatment. Surgical procedures were not attempted due to metastatic nodal involvement, and the prognosis of the condition was deemed poor. The patient was kept on regular follow-up.
3 DISCUSSION
OSCC accounts for more than 90% of all oral cancer cases. The condition predominantly affects males in their fifth to eighth decades of life. Clinical presentations may range from exophytic, endophytic, leukoplakic, and erythroplakic lesions with characteristic observable surface alterations. Mandibular bone invasion associated with OSCC primarily occurs through direct extension and, at times, via alternative pathways. Various studies have reported that the lingual cortex and alveolar crest act as the main entry portals when the malignancy is situated medial to the mandible.7
When tumor cells multiply and undergo mitosis, they rupture the basement membrane, causing local tissue injury, evading the immune system, and releasing specific proteins and angiogenic factors that promote invasion of the lymphovascular system and result in local and distant metastases. OSCC is more likely to invade surrounding bones due to its proximity, resulting in higher bone invasion in areas where it comes into direct contact. The dimensions of the primary tumor, along with its proximity to the jawbones, determine the extent of invasion into the bone. The prognosis is influenced by the pattern of bone invasion, which may be erosive, infiltrative, or mixed.8
The erosive type of bone invasion is characterized by the absence of islands of bone within the tumor mass, osteoclastic resorption of bone, and fibrosis noted along the broad pushing front of the tumor, as well as a sharp demarcation between the tumor and bone. Conversely, the infiltrative pattern is defined by penetration of the Haversian system, bone islands remaining within the tumor, and nests and projections of tumor cells along an uneven front. In the case presented, near-total resorption of bone with infiltration by tumor cells was observed. Primary, regional, and distant recurrences are more likely to occur in infiltrative lesions. According to reports, the infiltrative pattern has a 30% three-year disease-free survival rate, compared to a 73% rate for the erosive pattern.9
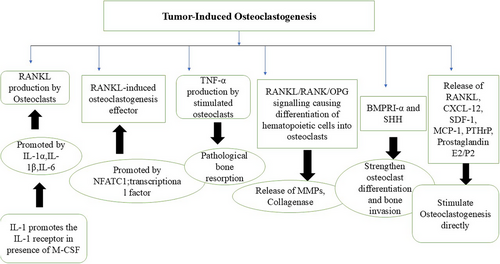
In OSCC, the tumor directly invades the bone through osteolytic reactions. The three main aspects of tumor-induced osteoclastogenesis include the production of the receptor activator of nuclear factor kappa-B ligand (RANKL) by malignant cells, the production of RANKL-induced osteoclastogenesis effectors, and Tumor Necrosis Factor (TNF)-α production, which itself stimulates osteoclasts. RANKL is expressed by tumor cells following stimulation.10
Numerous inflammatory cytokines are identified as key inducers of RANKL-induced osteoclastogenesis, including IL-1α, IL-1β, and IL-6. In inflammatory settings, these cytokines promote RANKL-induced osteoclastogenesis; however, they are also linked to tumor-induced osteoclastogenesis in both in vitro and in vivo conditions. It has been proposed that IL-1, expressed in carcinomas of the head and neck region, is essential for bone resorption caused by tumor invasion or metastasis. Moreover, in macrophages, IL-1 exhibits osteoclastogenic activity when there is synthetic overexpression of IL-1 receptor 1 in the presence of Macrophage Colony-Stimulating Factor (M-CSF). IL-1 secreted by OSCC cells can diffuse and affect osteoclast progenitor cells at the bone surface, stimulating both tumor-induced osteoclastogenesis and physiological RANKL-induced osteoclastogenesis. These results suggest that IL-1 inhibitors may be effective in treating OSCC-induced bone resorption, thus improving patient outcomes and quality of life.11
Inflammatory cytokines are recognized as soluble agents that promote osteoclastogenesis. TNF-α, expressed in various cancer cells, including OSCC cells, has been shown to stimulate osteoclastogenic activity. Various studies have proposed that TNF-α plays a role in the pathological resorption of bone.12 Osteoclastogenesis and osteoclastic activity are strongly stimulated by IL-1, which is thought to be due to elevated RANKL expression that, in turn, upregulates NFATc1, a key transcriptional factor for osteoclastogenesis. IL-6 and IL-8 also exhibit similar functions.13
Carter et al. observed in their studies that osteoclasts accumulate during the erosive phase, causing bone resorption, but decrease during the invasive phase, when the bone is destroyed by the tumor.14 When a tumor interacts with bone, it enhances RANKL expression, leading to osteoclastogenesis and osteolysis, resulting in an invasive tumor. The presence of increased RANK expression at the tumor front compared to the back end supports this notion. RANKL-expressing cells from myeloma, promyelocytic leukemia, and squamous cell carcinoma have been shown to stimulate osteoclastogenesis. The fact that OSCC can produce RANKL identifies it as a potential target for inhibiting bone invasion. RANKL inhibition may be an appealing option for palliative therapy of local recurrence or bone metastasis since bone-invading malignancies prefer nutrient-rich bone, which can encourage tumor growth. By limiting bone invasion, tumor growth can be prevented.15
Osteoclasts and proteolytic enzymes frequently cause osteolytic degradation when cancer cells invade bone. Tumorigenesis, progression, and response to treatment are all impacted by cell-cell contact in the tumor bone microenvironment (TBME), which is necessary for this process. During the process of bone resorption, osteoclasts, generated from hematopoietic stem cells, act as the primary effector cells. RANKL/RANK/OPG signaling triggers the differentiation of these hematopoietic stem cells into osteoclasts following their recruitment to the bone resorption site. These osteoclasts then release matrix metalloproteinases, collagenases, and other enzymes that break down and reabsorb bone mineral substances, ultimately leading to the loss of bone tissues.16
The Bone Morphogenetic Protein (BMP) family significantly influences various aspects of squamous cell carcinoma of the head and neck region (HNSCC), including development, growth, and metastasis. A key receptor mediating BMP signaling is Bone Morphogenetic Protein Receptor 1α (BMPR1α). Additionally, Sonic Hedgehog (SHH), a known modulator of the OSCC microenvironment, can stimulate cancer-associated fibroblasts and strengthen osteoclast differentiation and bone invasion. Tumor cell-released factors may alter the TBME by interfering with bone homeostasis. Previous research has indicated that SHH can facilitate osteoclast activation during malignant carcinogenesis. Inhibiting Hedgehog signaling may be an effective strategy to halt tumor growth and the subsequent bone defects caused by BMPR1α. The concept of “BMPR1α-SHH-associated bone remodeling” may pave the way for tailored treatments for OSCC that address severe bone invasion.17
Furthermore, the interaction between cancer cells and osteoblasts, as well as osteoclasts, promotes tumor cell proliferation in the bone environment and results in the suppression of resident bone pathways. This enables the growth and viability of tumor cells while also causing the destruction of bone structures. Osteoclastogenesis is driven by tumor-derived substances that induce osteoblasts to produce RANKL, such as IL-6 and Parathyroid Hormone-related Protein (PTHrP). Additionally, the presence of osteoblasts in the microenvironment enhances osteopontin expression in oral cancer malignant cells, thereby supporting the osteoclasts' ability to resorb bone. The release of RANKL, IL-6, CXCL12, SDF-1, CXCL13, TNF-α, Prostaglandin E2/F2, Monocyte Chemoattractant Protein-1 (MCP-1), and PTHrP by SCCs can directly stimulate osteoclastogenesis.18 The mechanism of bone invasion in OSCC is summarized in Figure 5.
Takeshi et al. used an animal model to better understand osteoclast-mediated bone invasion in OSCC. SCCVII cells were injected into the masseter regions of C3H/HeN mice. At the end of the third week, all surviving mice were analyzed using three-dimensional imaging with micro-computed tomography, histopathological observation with Hematoxylin-Eosin and Tartrate-Resistant Acid Phosphatase staining, and confirmation of mRNA expression of osteoclast-related cytokines IL-6, TNF-α, and PTHrP. The SCCVII cells were extremely invasive into the mandibular bone of C3H/HeN mice. This model closely mimicked the invasion of human oral cancer into maxillary and mandibular bones, highlighting the role of osteoclast activation factors IL-6, TNF-α, and PTHrP in the process of bone invasion.19
In a cell line-derived xenograft model, Quisheng et al. examined the impact of gingival and periodontal ligament tissue-derived stromal cells (G-SCs and P-SCs, respectively) and human dermal fibroblasts (HDFs) on bone resorption and osteoclast activation using Hematoxylin and Eosin and Tartrate-Resistant Acid Phosphatase staining. Interaction with the human OSCC HSC-3 cell line resulted in G-SCs promoting bone invasion and osteoclast activation while inhibiting osteoclast proliferation. P-SCs inhibited bone resorption and promoted osteoclast proliferation in vitro but had minimal effect on osteoclast activation both in vitro and in vivo. The findings from this study suggested that P-SCs had an inhibitory effect on bone invasion, whereas G-SCs stimulated osteoclasts on the bone surface to promote bone invasion in OSCC. These results pointed to a possible regulatory mechanism for bone invasion observed in OSCC.20
OSCC patients are treated with surgery, chemotherapy, radiation, or a combination of these modalities. A low disease-free and overall survival rate is associated with OSCC patients exhibiting bone infiltration. Bone invasion is clinically significant because it often necessitates partial mandibular resection, significantly affecting function, aesthetic appearance, and quality of life. Postoperative dysfunctions in OSCC patients are influenced by the primary tumor site, the area of surgical resection, and the severity of chemotherapy or radiotherapy. In terms of OSCC treatment techniques, patient characteristics such as age, general health, and postoperative loss of function all impact postoperative quality of life (QoL), especially in older patients. With these factors in mind, extensive research in the field of oncology has culminated in the development of Denosumab (DMB), a human monoclonal antibody that efficiently inhibits osteoclast function. DMB differs from bisphosphonates in that it is designed to suppress RANKL, a protein that serves as the primary signal for bone resorption. However, understanding the process of bone invasion observed in OSCC patients is critical for exploring additional implementation possibilities of medications like DMB, along with other therapeutic modalities, thereby enhancing the associated survival rate.21
4 CONCLUSION
OSCC is the most common malignant epithelial tumor, exhibiting a wide range of presentations. The infiltrative pattern displayed by tumor cells indicates aggressiveness, making surgical resection challenging. This invasion pattern provides valuable prognostic information. Novel technologies have been proposed and developed to explore the cellular and molecular mechanisms of OSCC invading the bone. Blocking RANKL and RANK with inhibitory antibodies curtails osteoclast differentiation and function, laying the groundwork for targeted therapy.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflict of interest.
ETHICS STATEMENT
This study was approved by the institutional research ethics committee. An informed written consent was provided for the report publication.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study