Mitochondria ATP Pro-Ferroptosis by Adjusting the Conversion of PUFA to PUFA-PLs
ABSTRACT
Ferroptosis is a form of iron-dependent regulated necrosis characterized by the abnormal accumulation of peroxidized phospholipids containing polyunsaturated fatty acids (PUFA-PLs). The conversion of PUFA to PUFA-CoA is critical for the synthesis of PUFA-PLs and is reliant on ATP, yet the role of mitochondrial ATP production in regulating ferroptosis remains unclear. In this study, we employed a metabolite deprivation and replenishment system coupled with flow cytometry to investigate the interplay between glutamine metabolism, mitochondrial ATP, and ferroptosis. We demonstrated that depriving cells of glutamine increases intracellular levels of reactive oxygen species (ROS), while also unexpectedly inhibiting ferroptosis induced by cystine deprivation. Mechanistically, glutamine deficiency impaired mitochondrial ATP production, and pharmacological inhibition of mitochondrial ATP export to the cytosol effectively blocked ferroptosis. Further analysis revealed that mitochondrial ATP depletion under glutamine-deficient conditions hindered the conversion of PUFAs to PUFA-CoA, thereby limiting PUFA-PL synthesis and ferroptosis execution. Notably, although glutamine deprivation alone did not directly trigger ferroptosis, it promoted PUFA oxidation and prostaglandin-endoperoxide synthase 2 (PTGS2) expression via ROS accumulation. Together, our findings highlight the critical role of mitochondrial ATP in ferroptosis regulation and provide new insights into the metabolic control of cell death pathways.
Graphical Abstract
1 Introduction
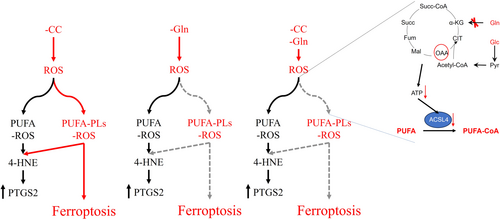
Ferroptosis, a form of regulated necrosis that is driven by the abnormal buildup of peroxidized phospholipids [1]. Studies have shown that ferroptosis plays a role in various pathological conditions, such as ischemia-reperfusion (I/R) injury, neurodegeneration, and cancer [2-7]. Not limited to mammals, ferroptosis is also observed in trypanosomes and higher plants [2], indicating its prevalence as a mechanism of cell death across different organisms.
Ferroptosis is the result of excessive accumulation of phospholipid hydroperoxides that exceed the clearance capacity of the defense system. Studies have shown that lipid metabolism, iron metabolism, and redox balance are three key factors in regulating the level of intracellular lipid peroxides [8-10]. Phospholipids are the substrates of peroxidized phospholipids that generally contain one polyunsaturated fatty acyl (PUFA) chains at the sn2 position, although there are also phospholipids that have two polyunsaturated fatty acyl tails [11]. Free PUFA needs to be activated in the form of PUFA-CoA to be esterified into PLs, with ACSL4 being the key enzyme catalyzing this reaction [12]. Research indicates that knocking out ACSL4 can significantly reduce PLs containing arachidonic and inhibit ferroptosis [13, 14]. It is important to note that both PUFA and PUFA-PLs are susceptible to peroxidation, with peroxidation of PUFA-PLs being essential for ferroptosis [11]. Reactive oxygen species (ROS), which are byproducts of metabolic processes, serve as the catalyst for lipid peroxidation. Numerous studies have highlighted the significance of either eliminating ROS or regulating ROS production as effective strategies in preventing ferroptosis [15-17]. Glutathione (GSH) is identified as the predominant nonenzymatic scavenger of ROS [18]. The tripeptide GSH is synthesized from glutamate, cysteine, and glycine [19], although glutamine is not a direct precursor for GSH synthesis, it plays a crucial role in the process. It is precisely because glutamate is primarily derived from glutamine, and the main precursor of cysteine is cystine, which is transported via the cystine/glutamate transporter xCT [20, 21]. In addition, as a nonessential amino acid, the de novo synthesis pathway of glycine also depends on the nitrogen source provided by glutamine through the transamination reaction mediated by glutamate-phosphoserine aminotransferase 1 (PSAT1) [18, 22]. However, the reason why glutamine fails to prevent ferroptosis remains unknown.
In this study, we identified that glutamine deficiency can lead to increased intracellular reactive oxygen species (ROS) levels and an upregulation of PTGS2 expression, without inducing ferroptosis. Unexpectedly, the absence of glutamine suppressed ferroptosis induced by cystine deprivation. Additionally, we observed that glutamine deprivation can result in a depletion of mitochondrial ATP, and that inhibiting ATP production or transport within the mitochondria could prevent ferroptosis. This study underscores the critical role of ATP in the conversion of polyunsaturated fatty acids (PUFA) to PUFA-phospholipids (PUFA-PLs) and in the process of ferroptosis. Furthermore, the study suggests that PTGS2 may have limitations as a biomarker for ferroptosis.
2 Results
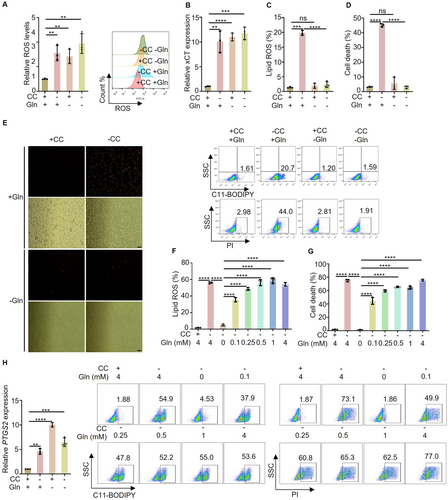
2.1 Glutamine Deficiency Induced ROS But Did Not Induce Ferroptosis
Glutathione (GSH) is the most abundant antioxidant in cells and is the core of the cyst(e)ine/GSH/GPX4 system, playing a critical role in defending against ferroptosis [23]. GSH is a tripeptide formed from glutamate, cysteine, and glycine. Given that glutamine and cystine serve as precursor substrates of glutamate and cysteine, respectively, they should play important roles in combating oxidative stress. As expected, we found that deprivation of glutamine and cystine respectively, can upregulate intracellular ROS levels (Figure 1A) and activate the oxidative stress system, leading to an increase in the expression of SLC7A11 (also known as xCT), a crucial indicator of intracellular ROS levels (Figure 1B). However, unexpectedly, glutamine deprivation did not induce lipid ROS, it inhibited lipid ROS and cell death triggered by cystine deprivation (Figure 1C,D). To further confirm our findings, we determined the growth and death of cells through fluorescence microscopy combined with PI staining (Figure 1E). In addition, we introduced a glutamine gradient to the culture medium devoid of cystine and glutamine. Subsequently, we assessed lipid ROS levels and cell death. Our results demonstrated a gradual increase in cystine-induced ferroptosis with the rise in glutamine concentration (Figure 1F,G). Notably, the transcriptional upregulation of PTGS2 (Prostaglandin endoperoxide synthase2) is a crucial indicator of ferroptosis [24]. Meanwhile, we also investigated the impact of glutamine and cystine deprivation on the expression of the PTGS2 gene. The results indicated that glutamine deprivation increases the expression of PTGS2 similarly to cystine deprivation. Despite this similarity, glutamine deprivation does not trigger ferroptosis (Figure 1H). The implication of this unexpected finding on the mechanism of glutamine-dependent ferroptosis induced by cystine deprivation remains unclear. Togther, our study revealed that glutamine deprivation elevates intracellular ROS levels akin to cystine deprivation. Interestingly, while glutamine deprivation does not induce ferroptosis, it can inhibit ferroptosis induced by cystine deprivation.
2.2 α-Ketoglutarate Can Relieve the Inhibitory Effect of Glutamine Deprivation on Ferroptosis, But Pyruvate Cannot
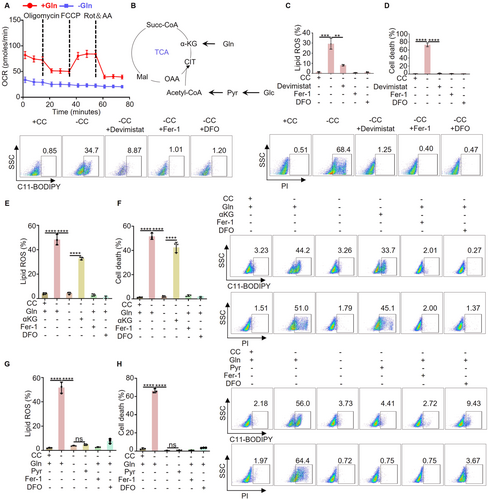
The atypical regulatory impact of glutamine on PTGS2 indicates the necessity for additional research into how glutamine influences ferroptosis. Glutamine acts as a carbon provider for mitochondrial energy metabolism, a process crucial in ferroptosis [25]. To investigate this further, we utilized the seahorse XFp analyzer to assess the effect of glutamine on mitochondrial energy metabolism. Our findings demonstrated that depriving cells of glutamine led to a near-complete inhibition of mitochondrial oxygen consumption (Figure 2A). The tricarboxylic acid (TCA) cycle is the center of mitochondrial energy metabolism. Previous studies have indicated that pyruvate derived from glucose and αKG obtained from glutamine are the primary carbon sources for the TCA cycle (Figure 2B). To investigate whether glutamine influences ferroptosis by modulating the TCA cycle within mitochondria, we initially exposed the cells to devimistat (an antagonist of lipoic acid that inhibits α ketoglutarate dehydrogenase and pyruvate dehydrogenase), Fer-1 (the inhibitor of ferroptosis), DFO (the inhibitor of ferroptosis), and a medium lacking cystine. Subsequently, we assessed lipid ROS levels and cell death. The results showed that devimistat can inhibit cystine deprivation induced ferroptosis, similar to the inhibitor of ferroptosis (Figure 2C,D). Furthermore, under the conditions of cystine and glutamine deprivation, we separately replenished α-Ketoglutarate (αKG) and pyruvate, then measured lipid ROS and cell death. Our results indicate that supplementing with αKG can counteract the inhibitory effect of glutamine restriction on ferroptosis, (Figure 2E,F), whereas supplementing with pyruvate does not have the same effect (Figure 2G,H). This suggests that the TCA cycle driven by glutamine is not reliant on glucose. Taken together, our findings demonstrate that the positive regulation of ferroptosis by glutamine is dependent on αKG rather than pyruvate.
2.3 Blocking Mitochondrial ATP Production Can Inhibit Ferroptosis
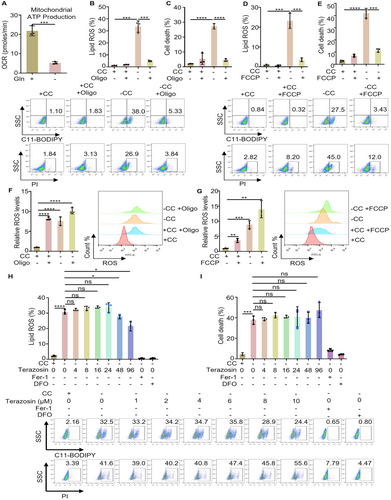
One of the main products of mitochondrial energy metabolism is the generation of ATP, which serves as the energy currency for cells. Research indicates that ATP produced by mitochondria plays a role in regulating the enzyme activity of long-chain-fatty-acid-CoA ligase 4 (ACSL4) situated in the mitochondrial associated endoplasmic reticulum membrane, thereby influencing the uptake of fatty acids [26]. Moreover, it is important to highlight that ACSL4 can impact ferroptosis sensitivity through lipid composition modulation, and the deletion of ACSL4 has been shown to impede ferroptosis [13, 27]. Therefore, we speculate that a key point in which glutamine deprivation increases intracellular ROS levels without triggering ferroptosis may be that glutamine deprivation exhausts mitochondrial ATP. To this end, we used the seahorse XFp analyzer to evaluate the ability of mitochondrial ATP production after glutamine deprivation, and the results showed that glutamine deprivation can limit the ability of mitochondrial ATP production (Figure 3A). Furthermore, we treated cystine deprived cells with inhibitor of mitochondrial ATP synthase (as known as FOF1-ATPase) and then examined their effects on ferroptosis. The results showed that blocking mitochondrial ATP synthesis can effectively prevent lipid ROS generation and cell death induced by cystine deprivation (Figure 3B,C). In addition, we treated cystine deprived cells with the uncoupling agent FCCP of the mitochondrial respiratory chain, and the results showed that lipid ROS and cell death were significantly inhibited (Figure 3D,E). At the same time, both the mitochondrial ATP synthase inhibitor (oligomycin) and the respiratory chain uncoupling agent (FCCP) were found to increase intracellular ROS levels, further enhancing the ROS accumulation triggered by cystine deprivation (Figure 3F,G). Meanwhile, cells were treated with terazosin, an activator of the key enzyme PGK in glycolysis [28], to enhance ATP production in glycolysis. The results showed that terazosin did not significantly impact cystine deprivation-induced lipid ROS and cell death, but Fer-1 and DFO are completely feasible (Figure 3H,I). In summary, our study confirms blocking mitochondrial ATP generation, while upregulating intracellular ROS levels, can specifically inhibit ferroptosis.
2.4 Blocking the Transport of ATP From Mitochondria to Cytoplasm Can Inhibit Ferroptosis
ACSL4 is mainly localized on the endoplasmic reticulum membrane associated with mitochondria, while ATP is mainly produced in mitochondria. Therefore, the transport of ATP from mitochondria to the cytoplasm is a critical process. The voltage-dependent anion-selective channel protein (VDAC) and adenine nucleotide (ADP/ATP) transposase (ANT) act as gated channels for mitochondrial material transport and are essential for ATP efflux [29, 30]. VDAC consists of VDAC1, VDAC2, and VDAC3, with similar expression levels observed in MEFs cells (Figure 4A). We speculate that inhibiting the transport activity of VDAC may inhibit cystine deprivation induced lipid ROS and cell death. As expected, the results showed that the inhibitor of VDAC (VBIT-12) can inhibit the lipid ROS and cell death induced by cystine deprivation (Figure 4B,C). Furthermore, when cystine-deprived cells were treated with inhibitors targeting mitochondrial ANT (bongkrekic acid or SLV319), a reduction in lipid ROS and cell death was observed, suggesting that inhibiting ANT can mitigate ferroptosis (Figure 4D,E). Although VBIT-12 was found to inhibit ferroptosis by targeting VDAC, it did not impact ROS levels or oxidative stress response signals (Figure 4F,G). Together, we found that inhibition of mitochondrial ATP transport can effectively impede cystine deprivation-induced ferroptosis.
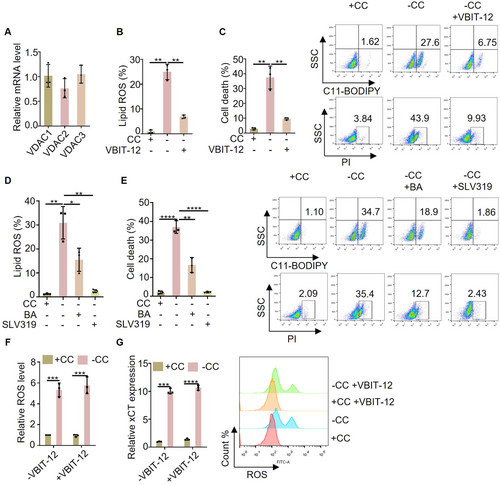
2.5 The Upregulation of PTGS2 Expression Is Not Solely Dependent on PUFA-PLs Peroxidation
Although inhibiting mitochondrial ATP synthesis and transport can inhibit ferroptosis, it does not decrease intracellular ROS levels. Furthermore, we investigated the effect of inhibiting mitochondrial ATP synthesis and transport on the expression of PTGS2 gene. The results showed that the expression of PTGS2 induced by cystine deprivation was not inhibited by oligomycin, FCCP or VBIT-12 (Figure 5A,B). Our findings suggest that while cystine deprivation can induce both ferroptosis and an increase in PTGS2 expression, glutamine deprivation only leads to an upregulation of PTGS2 expression without triggering ferroptosis. Moreover, blocking the synthesis and transport of mitochondrial ATP can effectively inhibit ferroptosis without affecting the expression of PTGS2. We speculate the reason is that glutamine deprivation or inhibit mitochondrial ATP synthesis and transport blocks the conversion of PUFA to PUFA-PLs, because both PUFA and PUFA-PLs can undergo peroxidation and induce the expression of PTGS2. But only the peroxidation of PUFA PLs is capable of inducing ferroptosis. Current understanding suggests that 4-hydroxylinoleic acid (4-HNE) produced by PUFA-PLs after peroxidation directly contributes to the upregulation of PTGS2 expression (Figure 5C). It is worth noting that PUFA is an essential fatty acid for mammals that must be obtained from external sources, primarily from serum. To test this hypothesis, we examined the impact of cystine deprivation and glutamine deprivation on intracellular ROS levels in the absence of serum. The results showed that regardless of the presence or absence of serum, it did not affect the upregulation of intracellular ROS levels by cystine deprivation and glutamine deprivation (Figure 5D,E). At the same time, the expression of xCT, a key indicator of ROS levels, was consistent across conditions (Figure 5F,G). Furthermore, in an experimental system lacking serum, the study investigated the effects of cystine deprivation and glutamine deprivation on PTGS2 expression. It was observed that serum deficiency could suppress the elevation of PTGS2 expression induced by cystine deprivation and glutamine deprivation (Figure 5H,I). In addition, the impact of serum deficiency on cystine deprivation-induced lipid ROS was examined, revealing that serum deficiency could hinder the generation of lipid ROS induced by cystine deprivation (Figure 5J). Taken together, the study highlights the crucial role of PUFA and PUFA-PLs peroxidation in the upregulation of PTGS2 expression, with only the peroxidation of PUFA-PLs being able to trigger ferroptosis.
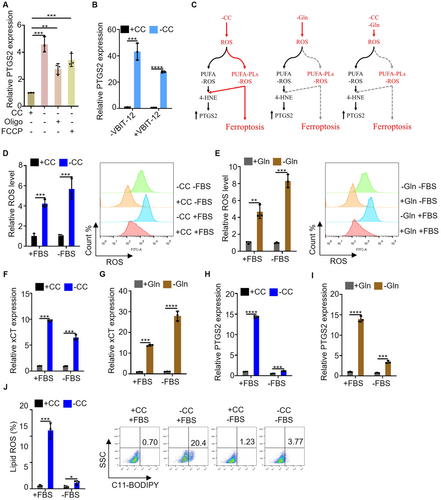
2.6 Glutamine Deprivation Can Inhibit the Uptake of Fatty Acids
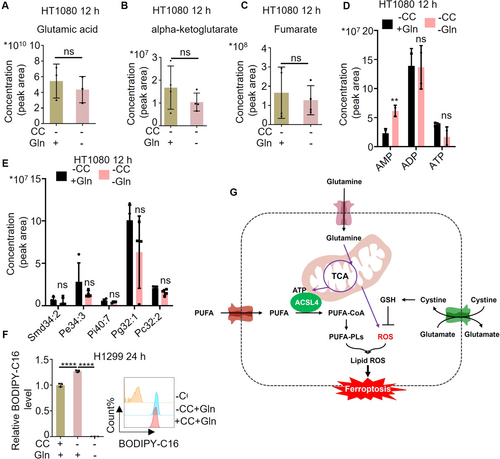
One key point of glutamine deprivation in inhibiting ferroptosis is its ability to deplete ATP within mitochondria. In the system of cystine deprivation, the impact of glutamine deficiency on key metabolites and energy metabolites in the TCA cycle was explored through metabolomics analysis. The findings revealed that glutamine deprivation leads to a decrease in glutamate, αKG, and fumarate levels, while simultaneously reducing ATP levels and increasing AMP levels (Figure 6A–D). Furthermore, it was observed that glutamine deprivation results in a downregulation of intracellular phospholipids (Figure 6E). The findings from this study once again support the notion that glutamine deprivation leads to a decrease in mitochondrial ATP levels, subsequently inhibiting the conversion of PUFA to PUFA-PLs. In addition, we treated cells with culture media deprived of glutamine, cysteine, and co-deprived of both, and then detected the uptake capacity of long-chain fatty acids using the BODIPY-C16 probe. The results indicated a significant decrease in fatty acid uptake under glutamine deprivation conditions (Figure 6F). Together, our study demonstrates that glutamine deprivation can reduce the ability of cells to uptake fatty acids.
3 Discussion
Ferroptosis, a regulatory cell death mode induced by aberrant iron-dependent peroxidation of polyunsaturated phospholipids on cellular membranes [1], plays an important role in organ ischemia-reperfusion injury, neurodegenerative diseases, and the occurrence and development of tumors [16, 31]. As the classical antioxidant system, cyst(e)ine/GSH/GPX4 axis plays a critical role in preventing ferroptosis via reducing phospholipid hydroperoxides to phospholipid alcohol [24]. The GSH is the core of cyst(e)ine/GSH/GPX4 axis and synthesized from glutamate, cysteine, and glycine. Because glutamate is mainly converted from glutamine, and glutamate efflux mediated cystine is the main precursor of cysteine via the cystine/glutamate transporter xCT, although glutamine is not a precursor for the synthesis of GSH, it is still playing an important role in the synthesis of GSH. However, it is puzzling why glutamine does not have a defensive effect against ferroptosis. In this study, we found that glutamine deprivation can upregulate intracellular ROS levels, but it does not induce ferroptosis. Instead, it can inhibit ferroptosis induced by cystine deprivation. The research further demonstrates that glutamine deprivation hinders the conversion of PUFA to PUFA-PLs by depleting mitochondrial ATP, thereby inhibiting ferroptosis. In addition, the study highlights the limitations of using PTGS2 as a gene marker for ferroptosis, as both PUFA peroxidation and PUFA-PL peroxidation can upregulate PTGS2 expression, but only PUFA-PL peroxidation leads to ferroptosis.
The cell membrane is primarily composed of phospholipid bilayers, with each phospholipid containing one saturated fatty acid chain and one unsaturated fatty acid chain, which may be either monounsaturated or polyunsaturated [32, 33]. For mammals, polyunsaturated fatty acids are classified as essential fatty acids, as they cannot be synthesized by the body and must be obtained through dietary sources. Lipid peroxidation underlies the mechanism of ferroptosis [32], polyunsaturated fatty acids (PUFAs) are particularly good substrates for peroxidation because the C–H bonds of the methylene groups flanked by C–C double bonds are among the weakest C–H bonds known [34]. Although both PUFA and PUFA-PLs can undergo peroxidation, it is only when the PUFA in PUFA-PLs undergoes peroxidation that ferroptosis is induced [35]. Based on this, numerous studies have shown that blocking the conversion of PUFA to PUFA-PLs can effectively inhibit the onset of ferroptosis, with ACSL4 playing a crucial role in this process [13, 27, 36-39]. Our study indicates that glutamine deprivation impedes the conversion of PUFAs to PUFA-PLs by depleting mitochondrial ATP, thereby preventing ferroptosis. The critical factor in this mechanism is the activity of ACSL4, which is dependent on mitochondrial function.
Glutamine, the most abundant amino acid in cells, plays a crucial role in providing glutamate for the synthesis of glutathione, serving as a carbon source for the tricarboxylic acid cycle, and acting as a nitrogen source for cells [40]. However, existing studies have shown that glutamine, which has antioxidant potential, does not participate in the defense against ferroptosis, but is necessary for ferroptosis [25, 41]. Jiang et al. research reveals that glutamine may enhance ferroptosis by supplying a carbon source for mitochondrial energy metabolism. However, mitochondrial energy metabolism also produces ROS while supplying ATP. It is unclear whether glutamine regulates ferroptosis by regulating mitochondrial ATP production or by regulating mitochondrial ROS production. In this study, it was discovered that depriving cells of glutamine not only hinders mitochondrial energy production and decreases ATP levels, but also increases cellular levels of ROS. Meanwhile, it was shown that inhibiting the generation and transportation of ATP in mitochondria can prevent ferroptosis. In summary, we confirm that ATP is the key factor in the positive regulation of ferroptosis by glutamine.
Adenosine triphosphate (ATP), a pivotal energy currency in cellular metabolism, has long been the focus of energy regulation studies. However, its role in the regulation of ferroptosis remains ambiguous. Our study reveals that ATP generated by mitochondria plays a crucial role in modulating the conversion of polyunsaturated fatty acids (PUFAs) to PUFA-phospholipids (PUFA-PLs) by regulating the activity of acyl-CoA synthetase long-chain family member 4 (ACSL4) on the mitochondria-associated endoplasmic reticulum membrane, ultimately influencing ferroptosis. Furthermore, research indicates that energy metabolic stress induced by glucose deficiency can trigger AMP-activated protein kinase (AMPK) activation and inhibit acetyl-CoA carboxylase (ACC) activity, consequently impeding de novo fatty acid synthesis and suppressing ferroptosis [42]. However, it is still unclear whether the activation of AMPK is caused by the depletion of mitochondrial ATP, as some studies suggest that glucose deficiency activation of AMPK does not depend on ATP depletion [43]. These findings underscore the need for further elucidation of the mechanisms through which ATP regulates ferroptosis.
This study demonstrates that mitochondria and mitochondrial-derived ATP are critical components in glutamine's promotion of ferroptosis. However, we were unable to elucidate how the tricarboxylic acid cycle operates effectively when relying solely on glutamine. Furthermore, the involvement of other ATP production pathways in regulating ferroptosis remains inadequately explored. Finally, this study does not address whether other amino acids capable of undergoing oxidative degradation within mitochondria can also participate in the regulation of ferroptosis by modulating mitochondrial ATP.
4 Materials and Methods
4.1 Cell Culture
MEFs, HT1080, and H1299 cell lines were acquired from the American Tissue Culture Collection (ATCC) and cultured following ATCC's guidelines. These cell lines are sustained in DMEM with high glucose, sodium pyruvate (1 mM), glutamine (2 mM), penicillin (100 U/mL), streptomycin (0.1 mg/mL), and 10% (v/v) FBS at 37°C and 5% CO2.
4.2 Reagents
The reagents used are Ferrostain-1 (S7243, Selleck), DFO (Y0001937, Sigma), Propidium iodide (P4170, Sigma), BODIPY581/591 C11 (D3861, ThermoFisher), DCFH-DA (35845, Sigma), Cystine (1161586, Sigma), Methionine (M0960000, Sigma), Glutamine (1294808, Sigma), αKG (349631, Sigma), Pyruvate (P2256, MCE), Devimistat (HY-15453, MCE), Terazosin (HY-B0371, MCE), oligomycin (HY-N6782, MCE), FCCP (HY-100410, MCE), rotenone (557368, Sigma), antimycin A (1397-94-0, MK), Bongkrekic acid (HY-136406, MCE), SLV319 (HY-121616, MCE), VBIT-12 (S8936, Selleck), BODIPY-C16 (D3821, ThermoFisher), Dulbecco's Modified Eagle's Medium high glucose (D0422-100 mL, Sigma). All the other reagents used were from Sigma-Aldrich.
4.3 Lipid ROS Detection
Lipid ROS levels were assessed using flow cytometry: Cells were seeded at a density of 2 × 105 per well in a 12-well dish and cultured overnight in DMEM. Following treatment, 4 μM BODIPY581/591 C11 was added into cell culture medium and incubated for 30 min. After incubation, excess BODIPY581/591 C11 was removed by washing the cells with PBS twice. The labeled cells were then trypsinized, resuspended in PBS containing 5% FBS, and analyzed using a flow cytometer.
4.4 Cell Death Detection
Cell death was assessed using flow cytometry: Cells were seeded at a density of 2 × 105 per well in a 12-well dish for 12 h in fresh medium. After indicated treatment, 1 μg/mL propidium iodide (PI) was added into cell culture medium and incubated for 30 min. Then cells were trypsinized, washed and resuspended in 0.3 ml of PBS with 5% fetal bovine serum for flow cytometry analysis of the cell death level.
4.5 ROS Detection
ROS was assessed using flow cytometry: Cells were seeded at a density of 2.5 × 105 per well in a six-well dish and grown overnight in DMEM. 10 μM DCFH-DA was added into cell culture medium and incubated for 30 min after indicated treatment. Excess DCFH-DA was then removed by washing the cells with PBS twice. Labeled cells were trypsinized and resuspended in PBS plus 5% FBS for flow cytometry analysis. A minimum of 10,000 cells were analyzed per condition.
4.6 Oxygen Consumption Rate (OCR) Assay
For the OCR assay, the Seahorse XFp Analyzer (Agilent) and Mito Stress Test Kits (Agilent) were used to assess the OCR of cells followed the instructions provided with the kit. In brief, cells were collected XFp plates(1 × 104/well) via centrifugation in serum-free XF Base Medium (Agilent). OCR was measured under basal state and in response to 1 μM oligomycin, 2 μM fluorocarbonyl cyanide phenylhydrazone (FCCP), and 0.5 μM rotenone plus 0.5 μM antimycin A (Rot/Ant A) employing the Seahorse XFp Analyzer using Wave software, version 2.3.0 (Agilent).
4.7 Cellular Metabolites Level Assessment
Cells were seeded at a density of 2 × 106 per well in a 10 cm Glass Bottom Cell Culture Dish and grown for 12 h in DMEM supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin, cells were treated as indicated for 10 h. Cells were then trypsinized, washed and resuspended in methanol: acetonitrile: ddH2O2 (2:2:1, v/v) after indicated treatment. Then perform mass spectrometry testing.
4.8 Quantitative RT-PCR
Total RNA was extracted from cells using TRNzol. Total RNA was then reverse-transcribed using the PrimeScript TM RT reagent Kit with gDNA Eraser and subjected to real-time PCR using the TB Green Premix Ex Taq (Tli RNase H Plus). The sequences of primers are shown in Supporting Information S1: Table S1.
4.9 Fatty Acid Uptake Detection
Fatty acid uptake was measured by flow cytometry: Cells were seeded at a density of 5 × 104 per well in a 24-well dish and grown 12 h in DMEM supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin. 2 μM BODIPY-C16 was added into cell culture medium and incubated for 10 h along with indicated treatment. Cells were then trypsinized, washed and resuspended in 0.3 ml of PBS with 5% fetal bovine serum for flow cytometry analysis. A minimum of 10,000 cells were analyzed per condition.
4.10 Statistical Analysis
A two-tailed unpaired Student t test, one-way or two-way ANOVA test were used for comparisons between groups. All data represent-ted in the figures with error of mean (means ± SD). p < 0.05 was considered statistically significant between groups. Data were graphed using GraphPad Prism 9.
Author Contributions
Chaoyi Xia: conceptualization (lead), data curation (lead), formal analysis (lead), funding acquisition (equal), investigation (equal), supervision (lead), writing – original draft (equal), writing – review and editing (equal). Lianchao Gao: investigation (equal). Jingsu Min: investigation (equal). Caiyun Fu: funding acquisition (equal), writing – original draft (equal), writing – review and editing (equal). All authors have read and approved the final manuscript.
Acknowledgments
Figure 6G was created using BioGDP, https://biogdp.com/. This work was supported by the Zhejiang Provincial Natural and Science Foundation of China under Grant No. LD22H310004.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data associated with this study are present in the paper or the Supporting Information.