Targeting Immune Checkpoints: Basic Signaling Pathways and Clinical Translation in Cancer Therapeutics
ABSTRACT
Immune checkpoints, the key gatekeepers of immune homeostasis, have become the central targets of modern cancer immunotherapy. These regulatory pathways, composed of co-suppressive and co-stimulatory molecules, enable the immune system to distinguish between self and non-self while preventing excessive tissue damage. However, tumor cells strategically block these protective mechanisms through aberrant expression of checkpoint ligands, creating an immunosuppressive microenvironment that promotes tumor immune evasion and metastatic progression. Yet, immune checkpoint therapy is not universally applied due to its specific mechanisms. This review systematically describes the immune checkpoints that function on various types of immune cells, as well as their molecular structure and functional diversity, and elucidates their role in achieving tumor immune escape. We analyze the clinical translation of immune checkpoint inhibitors (ICIs) and their combination therapies. In addition, we combine preclinical findings with clinical trial data to provide a comprehensive framework for understanding the mechanisms of action and clinical applications of immune checkpoints, as well as to present the challenges in terms of immune-related adverse events of ICIs. This review provides a valuable perspective for developing next-generation immunotherapies and optimizing personalized treatment strategies.
Graphical Abstract
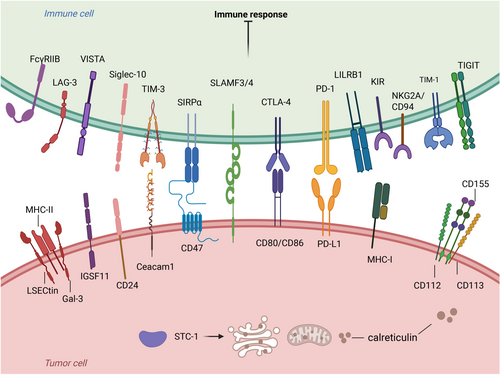
Immune checkpoints in tumor immunotherapy. Ligand binding to the receptor inhibits immune cell function, promotes tumor cell immune escape, and suppresses the immune response. The pathways of action are VISTA-IGSF11, TIM-3-Ceacam1, CD47-SIRPα, CTLA-4-CD80/CD86, PD-L1-PD-1, MHC-1-LILRB, and CD24 - Siglec -10. There are also other immune checkpoints such as FcγRIIB, LAG-3, SLAMF3/SLAMF4, KIR, NKG2A/CD94, TIGIT, and STC-1.
1 Introduction
Tumor immunotherapy is widely regarded as one of the most successful approaches in the field of cancer treatment in recent years [1, 2]. Currently, emerging cancer treatment methods mainly include immune checkpoint inhibitors (ICIs), cancer vaccines, covalent inhibitors, microtubule-targeting agents, and lysosomal viruses [3, 4]. ICIs, in particular, can stimulate a durable immune response, activate immune memory, and can be widely used in the treatment of many types of cancer [5]. The successful development of ICIs has demonstrated significant efficacy in a variety of solid tumors and hematologic malignancies, providing survival benefits to cancer patients [6]. As research progresses, new immune checkpoints are being discovered to provide effective treatments for more cancer patients.
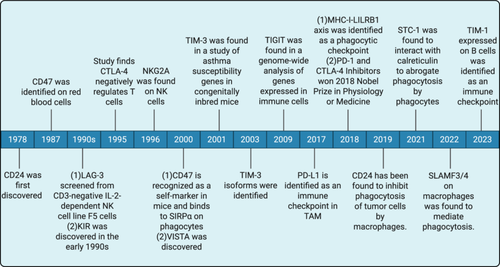
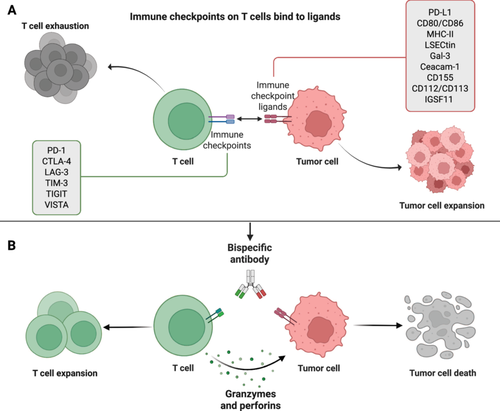
Immune checkpoints are essential for immune system regulation. Stimulatory and inhibitory checkpoint molecules are the two kinds of checkpoint molecules linked to immune regulation [7]. Stimulatory immune checkpoint molecules are a class of molecules that promote immune cell activation [8]. They give the signal required to support immune cell activation and proliferation by binding to receptors on the surface of immune cells [8, 9]. For example, CD28 attaches to molecules of the B7 family, such as CD80 and CD86, and sends out co-stimulatory signals that activate T cells [10, 11]. To prevent excessive immune responses and autoimmune diseases, inhibitory immune checkpoint molecules are immune system regulatory mechanisms that bind to their corresponding ligands and prevent T cell activation and proliferation [12]. Then, tumor cells frequently employ these inhibitory mechanisms to avoid immune monitoring and assault. [13]. These inhibitory immune checkpoint molecules are targets for the action of ICIs [14]. Currently, ICIs have shown remarkable results in clinical applications for antitumor treatment. The 2018 Nobel laureates in medical science revolutionized immuno-oncology through their independent identification of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1) as therapeutic targets [15, 16]. It may be surprising to many that immunotherapy, as a “trendy” and “advanced” approach to treating cancer, has actually been around for more than a century [17]. As far back as the late 19th century, American surgeon William Coley pioneered tumor immunotherapy through his use of Coley's toxin. Immune checkpoints have made significant contributions to the treatment of tumors since their discovery to the present day (Figure 1).
Within the immune system, a diverse set of checkpoint mechanisms is expressed on T cells, B cells, and antigen-presenting cells (APCs), such as macrophages, dendritic cells (DCs), natural killer (NK) cells, monocytes, granulocytes, and mast cells [12]. These checkpoints contribute to the suppression of immune reactions, thereby creating an environment conducive to tumor growth and survival [18, 19]. Tumor cells exploit this characteristic by overexpressing anti-phagocytic membrane proteins, known as “don't eat me” signals, and binding to these checkpoints to evade phagocytosis [20]. Furthermore, boosting the adaptive immune system to fight cancer, especially with T-cell treatment, has gained attention in the field of cancer immunotherapy [21]. And ICIs targeting the PD-1/PD-L1 axis and CTLA-4 have demonstrated clinical effectiveness by blocking the inhibitory effects of immune checkpoints on T cells, thereby activating antitumor immune responses. The PD-1/PD-L1 axis and CTLA-4 are key regulators in the immune system that regulate T cell activity through different mechanisms and stages. In this process, by inhibiting these checkpoints, the ability of T cells to attack tumor cells can be enhanced, which is the basis for many novel cancer immunotherapies [22, 23]. In the immune system, different types of immune cells also play different functions because they have different surface receptors, differentiation pathways, activation states, and roles in the immune response [24, 25].
The purpose of this review is to provide an overview of immune checkpoints expressed on immune cells and to comprehensively elucidate their mechanisms of action and targeted therapeutic approaches. In this review, in addition to systematically discussing the classical immune checkpoint molecules (PD-1/PD-L1, CTLA-4), we also reviewed the newly emerging immune checkpoint molecules, and further discussed the classification of immune checkpoints that function on different immune cells, and introduce the structure and mechanism of each immune checkpoint. In addition, we explore clinical trial breakthroughs in the combination of ICIs with traditional therapies such as radiotherapy and targeted therapy, as well as immune-related adverse reactions (irAEs). Through multi-dimensional analysis, it provides a comprehensive perspective of both theoretical innovation and transformation value for the field of immune checkpoint therapy.
2 Molecular Mechanisms and Signaling Pathways of Immune Checkpoints
The study of immune checkpoints expressed on immune cells represents an emerging and effective strategy in the field of cancer therapy. This is particularly true for B cells, macrophages, and DCs, which play crucial roles in antigen presentation, as well as for NK cells and T cells, which are responsible for cytotoxic functions. Investigating the immune checkpoints expressed on these cells holds significant promise for improving the tumor microenvironment (TME) and reversing immunosuppressive states. For macrophages, the polarization of macrophages into M1 (pro-inflammatory) and M2 (anti-inflammatory) phenotypes represents distinct activation states elicited by various stimuli [26-28]. Secretion of pro-inflammatory cytokines (IFN-γ, IL-6, and TNF-α) is a characteristic of M1 macrophages, which promote immune activation, inflammation, and antitumor immunity [29]. These cells exhibit robust phagocytic and cytotoxic activities, enabling them to directly target tumor cells and pathogens [30]. In contrast, M2 macrophages secrete anti-inflammatory cytokines such as IL-10 and TGF-β, which suppress inflammatory responses and facilitate tissue repair and immune tolerance [31, 32]. M2 macrophages are often induced within TME and contribute to tumor growth, angiogenesis, and metastasis [33]. The discovery and investigation of immune checkpoints, such as CD47 and the PD-1/PD-L1 axis, have profoundly enhanced our comprehension of immunological processes in oncology and hold substantial opportunities for the development of novel therapeutic strategies targeting tumor-associated macrophages (TAMs) [34]. DCs serve as pivotal APCs, initiating acquired immune responses and activating T cells to combat pathogens and tumors [35]. The immune checkpoint molecules expressed on their surface can restrain the overactivation of T cells, thereby maintaining immune homeostasis [35]. Targeting these checkpoints has the potential to augment the functionality of DCs and enhance antitumor immune efficacy. In addition, NK cells are key effector cells of the innate immune system, capable of directly killing tumor cells and infected cells without prior sensitization [36]. The interplay between HLA class I molecules (classified into polymorphic Ia [HLA-A/B/C] and conserved Ib [HLA-E/F/G] subgroups) and NK cells serves as a critical regulator of immune checkpoint responses [37, 38]. While HLA-Ia presents antigenic peptides to cytotoxic T cells, HLA-Ib directly engages NK receptors through noncanonical mechanisms, modulating inhibitory/excitatory signals in immune surveillance [38]. The interaction between NK cells and immune checkpoints affects their cytotoxic activity and persistence in TME, making them a potential target for cancer immunotherapy. Furthermore, B cells and T cells constitute the core components of the adaptive immune system, mediating immune responses through highly specific antigen receptors. B cells produce antibodies to neutralize pathogens and toxins, while T cells are divided into helper T cells (Th cells) and cytotoxic T lymphocytes (CTLs), which can mediate immune responses and directly kill infected cells or tumor cells, respectively [39, 40].
The core of the molecular mechanism and signaling pathway of the immune checkpoint is to dynamically regulate the activation of the immune response through inhibitory receptor-ligand interactions. These molecules usually carry immunoreceptor tyrosine inhibitory motifs (ITIM), when bound to ligands, recruit phosphatases such as SHP-1/2 to inhibit the phosphoinositide 3-kinase-Akt (PI3K/AKT), nuclear factor-κB (NF-κB), and mitogen-activated protein kinase (MAPK) pathways downstream of T cell receptor (TCR) or co-stimulatory signals such as CD28, blocking the proliferation, activation, and effect of immune cells. At the same time, some checkpoints indirectly weaken immune cell activation signals by competitively binding co-stimulating molecular ligands (such as the B7 family). In the microenvironment, immune checkpoints can induce the expansion of immunosuppressive cells (such as Treg and M2 macrophages), promote the accumulation of metabolites such as adenosine and lactic acid, and inhibit the function of effector cells. In addition, certain checkpoints directly block immune cells' recognition and clearance of targets by regulating “self-identifying” molecules on the cell surface (such as the “don't eat me” signal) or by interfering with immune synapse formation. The synergistic action of these multilevel inhibitory signals constitutes the core molecular basis of tumor immune escape. In this section, we will introduce a variety of widely studied immune checkpoint molecules.
2.1 Immune Checkpoints That Act on a Wide Range of Immune Cells
A variety of immune checkpoint molecules, such as PD-1/PD-L1, TIM-3 and VISTA, can be widely expressed and play a regulatory role in a variety of immune cells; they are the key research targets of tumor immunotherapy.
2.1.1 PD-1/PD-L1: Molecular Mechanisms and Therapeutic Potential in Cancer Immunotherapy
PD-1, also known as CD279, is an immune checkpoint receptor belonging to the B7-CD28 family [41]. It shares a 15% similarity in amino acid sequences to CD28 [42]. The discovery of PD-1 occurred in 1992, in a mouse T-cell hybridoma (2B4-11) cell line and in mouse hematopoietic progenitor cells (LyD9) lacking IL-3 [42, 43]. PD-1 can phosphorylate these two motifs, and they can also recruit the Src homology region 2 structural domain containing SHP-1 and SHP-2 [44]. PD-1 plays an essential function in both innate and adaptive immune reactions and is expressed on a wide range of immune cells within the TME [45], including activated T cells, B cells, NK cells, macrophages, DCs, and monocytes [43]. It is particularly strongly expressed on tumor-specific T cells [46]. PD-1 is the most extensively studied immunosuppressive factor on the surface of T cells [47].
PD-L1 is a ligand for PD-1, also known as CD274 and B7-H1, which is a member of the B7 family [42]. In 2002, research first showed that antibodies against PD-L1 could inhibit tumor growth and that PD-L1 could suppress T-cell proliferation, thereby confirming PD-L1's role in tumor immune evasion [48]. Its extracellular portion is made up of 290 amino acids [49]. PD-L1 is expressed on the surface of many immune cells [50, 51]. It is also expressed on the surface of nonhematopoietic cells, such as vascular endothelial cells, pancreatic islet cells, and placental cells [52]. Studies have confirmed that PD-L1 can also be expressed on the surface of various tumor cells, such as hematological malignancies and non-small cell lung cancer (NSCLC) [53, 54]. In addition, PD-L1 expression can be regulated by a variety of factors. For instance, For example, PD-L1 can be upregulated by TNF-α, vascular endothelial growth factor (VEGF) [55], type I and type II interferons, as well as IFN-α and IFN-β can all upregulate PD-L1 [53, 56]. TNF-α also upregulates PD-L1 expression through activation of the NF-κB pathway [57]. In addition, the JAK-STAT1-IRF1 signaling pathway, JAK-STAT3, or MEK/ERK signaling pathway all upregulated PD-L1 expression [57].
The interaction between PD-1 and PD-L1 can inhibit TCR signaling, suppress the immune response of T cells to tumors, and mediate tumor immune evasion (Figure 2A) [58, 59]. After binding to PD-1 on the surface of T cells, PD-L1 induces the phosphorylation of tyrosine in the tyrosine switch motif of PD-1's immune receptor [60]. This subsequently recruits the SHP-2 [61], causing the dephosphorylation of downstream kinases, including splenic tyrosine kinase (Syk) and PI3K [62, 63]. This inhibition affects signaling pathways such as downstream protein kinase B (PKB, also known as AKT) and extracellular regulated protein kinase (ERK) [64], ultimately inhibiting T cell activation. Furthermore, it inhibits transcription of genes and cytokines required for T-cell activation, serving a detrimental function in modulating T-cell activity [65]. Tumor cells that highly express PD-L1 bind to PD-1 on tumor-specific T cells and inhibit their activation, proliferation, and cytokine production [66, 67]. Therefore, blocking the PD-1/PD-L1 signaling pathway can restore the T cell immune cytotoxicity [68], and activate the endogenous antitumor immune response, thus exerting antitumor effects (Figure 2B).
Not only does the PD-1/PD-L1 axis have an immunosuppressive effect on T cells, but it is also essential for TAMs. PD-1 is highly expressed on TAMs, and its expression is positively correlated with tumor volume [69, 70]. Results from bone marrow transplantation experiments have shown that circulating leukocytes are the main source of PD-1 on TAMs [71, 72]. Moreover, the phagocytosis of TAMs was found to be reduced as PD-1 expression increased [73, 74]. The findings indicate that PD-1 significantly hinders the phagocytosis of TAMs [71]. Furthermore, the PD-1/PD-L1 axis suppresses macrophage activation and proliferation by blocking the mechanism of mTOR signaling in macrophages [69]. Through the Erk/Akt/mTOR regulation pathways, PD-L1 stimulates M2-type macrophage polarization and inhibits the release of pro-inflammatory cytokines and co-stimulatory molecules (CD86, MHC-II) [75]. It is noteworthy that the absence of PD-L1 has a significant impact on phagocytosis in PD-1-containing TAMs [75, 76]. PD-L1 membrane expression in these cellular populations enables engagement with macrophage-resident PD-1 receptors, thereby suppressing phagocytic functionality [77, 78]. However, it has been observed that blocking PD-L1 on the surface of extracellular cells secreted by TP53 mutant tumor cells can mitigate this inhibitory effect [69]. All of these results highlight the PD-1/PD-L1 axis's crucial function in macrophage phagocytosis. PD-L1 is expressed on DCs and can bind to PD-1 to inhibit T cell activation. It has been shown that DCs in human ovarian cancer can also express PD-1, which inhibits NF-κB activation through a SHP-2-dependent mechanism, leading to immunosuppression [79]. The combination of PD-1 and PD-L1 inhibits CD8+ T-cell activity and infiltration, reduces MHC I expression, and decreases cytokine secretion (e.g., IL-12, TNF-α, and IL-1), resulting in impairments of T-cell expansion and cytotoxic responses [80, 81]. In addition, PD-1 is expressed in peripheral B-cell subsets [81]. The presence of PD-1 can regulate B cell stimulation, expansion, and immune tolerance. Upon binding to PD-L1, SHP-2 is recruited and causes dephosphorylation of the BCR signaling molecule, which inhibits B cell activation, proliferation, and cytokine production [81]. Targeting the PD-1/PD-L1 axis can help restore immune cell function.
2.1.2 TIM-3: Surface Molecule on Immune Cells
T cell immunoglobulin (Ig) and mucin domain 3 (TIM-3) is a member of the TIM gene family [82], characterized by its presence as a cell surface molecule containing Ig and mucin domain, a type I transmembrane protein [83]. TIM-3 was mainly expressed on CD8+ T cells and Th1 cells [84]. In TME, TIM-3 is highly expressed on CD8+ tumor-infiltrating lymphocytes and CD4+ regulatory T cells. At the same time, TIM-3 is also expressed on innate immune cells such as DCs, macrophages, monocytes, and mast cells, and its expression is higher in M2 macrophages [85]. Functional fatigue is linked to elevated TIM-3 expression in NK cells [86]. The initial discovery of TIM-3 was made in 2001 in the course of examining genes related to asthma susceptibility in inbred mice with a congenital background. Its isoforms were later identified in 2003 [87], and since then, many studies have been conducted on TIM-3's function as an immunological checkpoint [88]. Its extracellular structural domains consist of a membrane-distal N-terminal Ig domain [89], a membrane-proximal mucin domain (which contains a site for O-linked glycosylation), and a peptide linker in the mucin and transmembrane structural domains that contains a site for N-linked sugars [84, 90]. It also has a cytoplasmic tail and a transmembrane structural domain [91]. T Five tyrosine residues are found in the cytoplasmic tail; Y256 and Y263 are essential for TIM-3 signaling because of their association with HLA-B-associated transcript 3 (Bat3) [92]. When there is no ligand binding, Bat3 can bind to the cytoplasmic tail and catalytically activate lymphocyte-specific protein tyrosine kinase (Lck), leading to the phosphorylation of the CD3ζ subunit of the TCR [93, 94]. This process promotes T cell proliferation and activation. When TIM-3 is bound to its ligand, Bat3 dissociates from the cytoplasmic tail, resulting in the phosphorylation of Y256 and Y263, Lck inactivation, and subsequent inhibition of T cell proliferation and activation [95]. TIM-3 is predominantly expressed on T helper 1 (Th1) cells rather than T helper 2 (Th2) cells among T cells [96]. Research has indicated that TIM-3 is also present on various tumor cells, including melanoma, NSCLC, hepatocellular carcinoma (HCC), and prostate cancer [97].
Current research has revealed the identification of four ligands for TIM-3. The primary ligand is galectin-9 (Gal-9) [98], which is widely expressed and considered the most significant ligand for TIM-3. High-mobility group protein B1 (HMGB1) includes carcinoembryonic antigen cell adhesion molecule 1 (Ceacam-1) and phosphatidylserine (PtdSer) is the second ligand [99, 100]. TIM-3 binds to these ligands, thereby suppressing the expansion and stimulation of adaptive immune cells (Figure 2A). In addition to these ligands, retinoic acid-inducible gene I (RIG-I) can also interact with TIM-3 [101]. Furthermore, TIM-3 attenuates RIG-I expression on macrophages in response to signal transducer and activator of transcription 1 (STAT1) [102]. It encourages RIG-I ubiquitination and destruction through RNF-122, an E3 ubiquitin ligase, leading to reduced IFN production and inhibition of antiviral activity [101, 103].
Gal-9 is a soluble protein with two tandem carbohydrate-recognition domains that recognize N-linked sugar chains of the TIM-3 immunoglobulin variable (IgV) structural domain and bind specifically to TIM-3 [83]. The activity of TIM-3-Gal-9 promotes the death of effector Th1 cells, thereby suppressing tissue inflammation and the autoimmune disease multiple sclerosis (EAE) [104]. Furthermore, the binding of TIM-3 and Gal-9 alters the production of cytokines (IL-12 and IL-23) [105], which in turn affects the Th1 response. Additionally, TIM-3 mediates T cell dysfunction and depletion by binding to Gal-9 [106]. In order for nucleic acids to translocate to endosomal cells, activated DCs release HMGB1, a damage-associated molecular pattern (DAMP) protein [107]. The interaction between TIM-3 and HMGB1 prevents nucleic acid translocation to nuclear endosomes, leading to suppression of immune responses mediated by pattern recognition receptors (PRRs) to nucleic acids from tumors. [99]. Therefore, blocking the inhibition of the nucleic acid sensing system induced by the binding of TIM-3 and HMGB1 contributes to DNA vaccine research and cytotoxic chemotherapy [99]. PtdSer functions as a non-protein ligand for TIM-3, and its binding to TIM-3 is linked to the uptake of apoptotic cells [100]. Furthermore, the co-expression of the cytokine IL-10 with T cells that express TIM-3 results in the induction of IL-10 in T cells when combined with PtdSer [108]. There are structural similarities between the membrane-distal IgV domains of TIM-3 and Ceacam-1 [109]. Co-expression of Ceacam-1 is crucial for the glycosylation and protein stability of TIM-3. Defective expression of Ceacam-1 impedes the inhibitory function of TIM-3 [100, 109]. Additionally, Ceacam1-TIM-3 transregulatory inhibition of effector T cell function plays a role in maintaining T cell tolerance.
2.1.3 V-Domain Immunoglobulin Suppressor of T Cell Activation (VISTA): A Checkpoint With Emerging Roles in Antigen Presentation and Tumor Immune Evasion
VISTA is currently a focal point in immune checkpoint research. VISTA [110], additionally referred to as VSIR, SISP1, B7H5, PD-1H, DD1α, Gi24, and Dies1, is a member of the B7 family and exhibits strong similarities to PD-L1 [111, 112]. Unlike other members, VISTA proteins contain only one IgV-like structural domain [113]. In contrast to this singular domain structure of VISTA proteins, other B7 family members possess an additional IgC-like structural domain alongside the IgV-like structural domain [114, 115]. This unique feature renders VISTA more receptor-like in nature compared to other molecular structures within the B7 family, which generally act as ligands [116]. The IgV structural domain of VISTA is characterized by a canonical disulfide bond between the putative B and F domains [117, 118]. In contrast to the B7 family, VISTA does not contain the ITIM/ITAM motifs typical of the B7 family in its cytoplasmic structural domains [119]. Instead, it contains potential latent protein kinase C-binding sites and proline-rich motifs that serve as platforms for interactions with other complexes [112, 117]. Within leukocytes [120], monocytes, macrophages, DCs, and bone marrow cells (neutrophils and microglia) all express the VISTA protein [117, 121]. In the T lymphocyte compartment, VISTA protein expression levels were highest on naive CD4+ and Foxp3+ regulatory T cells [121].
VISTA has a complex function in immunomodulation, with studies demonstrating that it functions not only as a co-inhibitory receptor for T cells but also as a ligand expressed on APCs [111, 122]. The majority of current research has focused on VISTA's function as an inhibitory receptor on T cells, where it serves to negatively modulate the immune response [123]. The recombinant VISTA Ig fusion protein was shown to suppress CD4+ T cell proliferation and the cytokine production of IL-2 and IFN-γ in vitro [124]. Additionally, it promoted the conversion of naive T cells into FoxP3+ regulatory T cells [125]. Furthermore, elevated expression of VISTA on DCs from bone marrow led to decreased cell proliferation and cytokine production [111, 112, 123]. Additionally, the treatment of CD4+ T cells with VISTA-specific agonistic antibodies has been shown to inhibit CD4+ T cell activation, both in vitro and in vivo [126]. This demonstrates that VISTA functions as a CD4+ T cell inhibitory receptor [127, 128]. The ligand for VISTA, immunoglobulin superfamily (IgSF) member 11 (IGSF11), can also be expressed on tumor cells [129]. The interaction between VISTA and IGSF11 inhibits human T cell proliferation and cytokine production [130, 131]. Under acidic conditions, VISTA expressed on TAMs, MDSC, or tumor epithelial cells may interact with T cell-expressed PSGL-1 (P-selectin glycoprotein ligand-1) [132]. This interaction enables VISTA to specifically inhibit immune activation in acidic microenvironments (Figure 2A) [133, 134].
2.2 Other Immune Checkpoints Acting on APCs
DCs, macrophages, and B cells make up the majority of APCs. These cells have the capacity to constitutively produce costimulatory and MHC-class II molecules, as well as significant antigen absorption, processing, and presentation capacities [135]. These cells are vital for the immune response because they can trigger adaptive immunological responses by activating naive T cells. In addition to antigen presentation capacity, macrophages and B cells have other important functions. Macrophages possess the capacity to identify, engulf, and eliminate tumor cells and pathogens, and their own senescent and abnormal cells [72]. They fuse with lysosomes through phagosomes and use enzymes in lysosomes to digest and break down the phagocytosed foreign bodies [72]. In addition, macrophages can also process the phagocytosed antigens and present them to CD4+ T cells in the form of antigen peptide-MHC-II complexes, thereby activating specific immune responses [136]. The main function of B cells is to produce antibodies, participate in the humoral immune response, neutralize pathogens with antibodies, activate the complement system, or eliminate pathogens by antibody-dependent cell-mediated cytotoxicity (ADCC) mechanisms [137]. In addition, B cells can secrete cytokines that regulate immune responses. Below, we provide an overview of important checkpoints expressed on these cells.
2.2.1 MHC-I: From Classic Antigen Presentation to Novel Regulatory Functions in Immune Homeostasis
The MHC-I plays a crucial role in antigen presentation and the regulation of macrophage phagocytosis [138]. MHC is a term used to describe a group of highly polymorphic gene clusters that encode major histocompatibility antigens in animals. In humans, the MHC is referred to as human leukocyte antigen (HLA) [139], which includes classical HLA-I, HLA-II, and nonclassical HLA-III molecules [140, 141]. In mice, the MHC is known as H-2 [142]. The human HLA is located on the short arm of chromosome 6, while the mouse H-2 is located on chromosome 17. MHC-I is found on the surface of APCs and represents the first human leukocyte antigenic product involved in tumor immune response [143, 144]. It consists of a heterodimer containing two structural domains: an alpha heavy chain and a fully extracellular light chain β2m [145]. The α heavy chain consists of three extracellular structural domains (α1, α2, and α3) as well as one transmembrane structural domain [146]. The α3 structural region is located on the surface of T cells and is responsible for binding to CD8 [147]. The antigen-binding site for MHC-I is formed by the interaction of the α1 and α2 structural domains [147, 148]. β2m is a soluble protein that does not cross cell membranes but instead binds noncovalently to the extracellular portion of the α heavy chain [149].
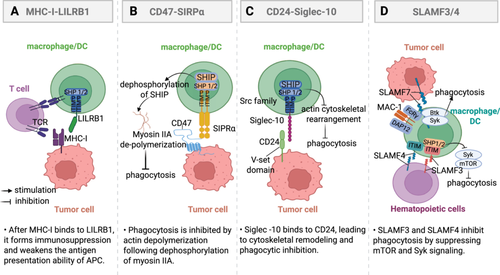
Binding of MHC-I to leukocyte immunoglobulin-like receptor b1 (LILRB1) isoforms promotes immune evasion, serving as a phagocytic checkpoint in cancer immunotherapy [150-152]. LILRB1 belongs to the class B LIR receptor subfamily and contains multiple cytoplasmic ITIMs [153]. It is also known as CD85J and LIR-1 [154]. LILRB1 is widely distributed and expressed on macrophages, NK cells, DCs, B cells, and T cells [155-157]. LILRB1 is composed of an extracellular Ig-like domain, a transmembrane domain, and a cytoplasmic domain containing ITIM sequences [158]. It triggers inhibitory signals primarily through the ITIM sequences in the cytoplasmic domain, which can also recruit SHIP [154]. The binding of LILRB1 to the α3 structural domain and β2m subunit of MHC-I results in ITIM phosphorylation [159]. Phosphorylated ITIM is activated by Src-family protein tyrosine kinases, which phosphorylate tyrosine residues and then recruit the phosphatase SHIP [160]. The recruitment of SHIP inhibits ITAM tyrosine kinase activity, prompting the engagement of ITAM from the Syk/ZAP70 kinase family [161]. Consequently, this triggers the PI3K/AKT pathway to promote tumor cell proliferation and suppress immune cell function [162]. Compared to CD8+ T cells, MHC-I exhibits a higher affinity for LILRB1. The binding of MHC-I to LILRB1 not only triggers inhibitory signals but also hinders MHC-I from presenting antigens to CD8+ T cells and stimulates adaptive immune responses (Figure 3A) [163, 164], thereby negatively regulating immune function and promoting tumor immune escape.
2.2.2 CD47-SIRPα: The “Don't Eat Me” Signaling Pathway in Immune Regulation
CD47, commonly referred to as the integrin-associated protein, is a highly glycosylated integral membrane protein with an approximate molecular weight of 50 kDa [165]. It is a member of the Ig family and is expressed across various tissues. CD47 contains a single IgV-like domain at its N-terminus [166]. Within the immune system, CD47 stands out as the sole 5-transmembrane (5-TM) receptor [167]. In 1987, CD47 was initially identified on the surface of red blood cells [168]. Until 2000, CD47 was considered a “self-marker” on murine red blood cells, which interacts with signal regulatory protein alpha (SIRPα) on phagocytes [169]. In 2019, it was confirmed that CD47 acts as a tumor phagocytosis checkpoint by delivering a “don't eat me” signal [170]. In cancer immunotherapy, research on CD47 has primarily focused on the inhibitory role of the CD47-SIRPα axis in phagocytosis [165]. SIRPα was initially identified as a receptor tyrosine kinase that is associated with the inhibitory phosphatases SHP-1 and SHP-2. It consists of three extracellular IgSF structural domains, with the furthest domain being the Ig-variable structural domain [167, 171, 172]. The intracellular structural domains of SIRPα contain immunoreceptor tyrosine motifs, which confer inhibitory receptor properties upon it. The n-terminus of SIRPα and the single lg-V structural domain of CD47 are the key mediators of the mutual engagement of SIRPα and CD47 [169, 173]. Ligation of CD47 to SIRPα promotes the phosphorylation of intracellular ITIM and activates the inhibitory phosphatases SHP-1 and SHP-2 [174]. The dephosphorylation of proteins with tyrosine-activating motifs present on immune receptors is one of the many ways that SHP phosphatases mediate the suppression of immune cell activation [169]. In contrast to the T cell-associated immune checkpoint pathway, which is targeted in cancer immunotherapy, the CD47/SIRP pathway serves as a natural immune checkpoint that inhibits the phagocytic activity and antigen presentation ability of macrophages [169, 175]. Macrophages operate as key effectors in innate immunity as specialized phagocytes that engulf senescent and dead cells within the body [176]. Within tumor tissues, macrophages have the ability to remove tumor cells through phagocytosis. When CD47 binds to SIRPα, it induces phosphorylation of the ITIM tyrosine [177, 178]. Activation of the ITIM through phosphorylation leads to the recruitment and stimulation of SHP-1 and SHP-2, which in turn dephosphorylates several intracellular proteins, leading to the loss of their biological functions [174]. In the end, this results in the suppression of macrophage phagocytosis and antigen presentation functions (Figure 3B) [179]. This function can be utilized in the normal human environment to distinguish between “self” and “non-self” to prevent unintentional injury [180]. When tumor cells that have been phagocytosed bind to IgG through in vivo conditioning, the Fc domain of IgG attaches to Fc receptor (FcR) on the surface of the macrophage, thereby activating the macrophage [181, 182]. Activation of the FcR signaling pathway leads to the formation of a “phagocytic synapse” between tumor cells and phagocytes. This process involves three sequential events: initial contact between tumor cells and phagocytes [183], followed by encirclement of tumor cells by macrophage pseudopods, and ultimately the phagocytosis of tumor cells. The current findings suggest that CD47-SIRPα functions as an immune checkpoint by inhibiting the formation of macrophage pseudopods [178, 184].
In addition to SIRPα, CD47 also binds with significantly lower affinity to SIRPγ. SIRPγ is expressed on human T cells and is believed to play a role in transendothelial migration [185, 186]. Other proteins that interact with CD47 include extracellular matrix proteins, platelet reactive protein-1, and various integrins. These integrins include integrin αVβ3, integrin α2bβ3, integrin α2β1, and integrin α4β1, with which CD47 binds and interacts in cis [187]. CD47 is frequently overexpressed in various hematological and solid tumors, and it is often associated with a poor prognosis [188]. This suggests that tumor cells can avoid being destroyed by phagocytes because of the CD47-SIRPα pathway [189, 190]. Studies in mouse tumor models have suggested that the blockade of SIRPα or CD47, in combination with other ICIs targeting PD-1 or CTLA-4, may potentially have synergistic antitumor effects [191].
2.2.3 Cluster of Differentiation 24 (CD24)-Siglec-10: A Key Player in Immune Regulation and Tumor Escape Mechanisms
In 1978, scientists first discovered CD24 [192], a short peptide containing only 31 amino acids [192, 193]. CD24 has been found to be strongly expressed in specific types of tumor cells, such as those found in ovarian cancer and triple-negative breast cancer [194]. This suggests a potential role for CD24 in the development and progression of these malignancies [195]. A 2019 study revealed CD24's role in suppressing innate immune phagocytic activity, functioning as a tumor-protective signaling marker through macrophage evasion mechanisms. CD24 acts as a mediator of intermolecular interactions at cellular junction sites, facilitating intercellular and matrix adhesion. It is essential for mediating cellular identification, triggering activation pathways, transducing signals, and regulating both multiplication and specialization processes in cells, as well as cellular stretching and motility [196, 197]. CD24, a GPI-anchored surface protein, chiefly resides within plasma membranes across healthy and malignant cellular populations [198]. The function of CD24 in the cell membrane is dependent on its binding protein. Siglec-10, which serves as the ligand for CD24, is a sialic acid-binding immunoglobulin-type lectin (Siglecs) [197]. CD24 evades macrophage phagocytosis by engaging with siglec-10. Siglec-10 is a lectin that specifically binds to α-2, 3- or α-2, 6-linked sialic acid [197]. It is a member of the Siglecs family that functions to inhibit the activation of immune cells. This protein is typically expressed on cells of the innate immune system. Its main function is to regulate the signaling of immune cells [17, 199]. Siglec-10 features a singular transmembrane segment and consists of an extracellular region with a V-set Ig-like domain accompanied by three C2-set domains, along with an intracellular region that possesses an ITIM [200]. Following tyrosine residue modification, the structural motif engages Src homology 2 (SH2)-containing phosphatase effectors, including SHP-1/PTPN6, effectively terminating intracellular communication cascades [201, 202]. CD24, CD52 (target of the therapeutic monoclonal antibody alemtuzumab), and vascular adhesion protein-1 have been described as ligands for Siglec-10 [197, 203]. Studies demonstrate that CD24 engagement with Siglec-10 on innate immune effector cells inhibits detrimental inflammatory activation during pathogenic challenges, including infection, sepsis, hepatic damage, and chronic graft-versus-host disease [204]. Many researchers have characterized CD24-Siglec-10 interaction as a natural immune checkpoint crucial for mediating antitumor immunity and as a potential target for future immunotherapy [203, 205].
CD24 is significantly overexpressed in various tumors compared to normal tissue, while Siglec-10 shows higher expression in TAMs [203]. The CD24-Siglec-10 axis functions by releasing a “don't-eat-me” signal that helps the tumor cells evade the immune system and avoid phagocytosis [206]. When CD24 binds to Siglec-10, it triggers a suppressive signaling pathway that involves the activation of phosphatases SHP-1 and SHP-2 through the Src homology region 2 domain (Figure 3C) [207]. Siglec-10 recruits dual ITIM-bound phosphatases that suppress TLR-induced inflammatory signaling and disrupt actin-dependent phagocytic machinery in macrophages. [208]. Phosphorylation of Src kinases is triggered through ITIM-related signaling modules following ligand engagement between Siglec-10's Ig variable region and CD24's ectodomain-bound sialic acid residues [209, 210]. Subsequently, the phosphorylated ITIM tyrosine in the cytoplasm recruit's tyrosine phosphatases, leading to a reduction in signaling within the CD24-Siglec-10 axis [207, 210]. This process suppresses the immunoreactivity of macrophages, diminishes their phagocytic activity, and weakens their ability to surveil tumor cells.
2.2.4 SLAMF 3/4: Key Inhibitory Receptors in Immune Regulation
The signaling lymphocyte activation molecule (SLAM) family of immune cell surface receptors belongs to the CD2 subfamily of the IgSF and is comprised of nine members, namely SLAMF1-9 [211]. SLAMs play a crucial role as immunomodulatory receptors [69]. SLAM family receptors (SFRs) belong to the IgSF and are widely expressed on hematopoietic cells, including macrophages [212]. They play a crucial role in regulating the activation and cytotoxicity of these cells [22, 69]. LAM family receptors serve as critical regulators orchestrating immunoregulatory functions across both innate and adaptive immune cellular networks [213]. They typically recruit downstream SAP family proteins to deliver activation signals that regulate a variety of immune responses, such as NK cell activation, NK-T cell development, and antibody production [214]. The SLAM family of receptors, often utilized as markers to distinguish hematopoietic stem cells from other hematopoietic precursor cells, has been demonstrated to bear similarity to CD47 and may serve as a novel “self” molecule that specifically inhibits macrophage phagocytosis of its own blood and maintains macrophage tolerance [215]. Therefore, the SLAM family of receptors may serve as blood tissue-specific “do not eat me” receptors [216]. As a result, SFRs are anticipated to function as novel immune checkpoint molecules, offering new targets for tumor immunotherapy [217]. Specifically, SFR members, particularly SLAMF3 and SLAMF4 (receptors for SLAMF2), work together to inhibit the phagocytosis of hematopoietic cells by macrophages (Figure 3D). They achieve this by suppressing “eat-me” signaling in macrophages through SH2 domain-containing phosphatases [211, 218].
2.2.5 Stanniocalcin 1 (STC-1): Key Regulators of Tumor Invasion, Metastasis, and Immune Escape
STC-1 is a homodimeric glycoprotein initially discovered in the corpuscles of Stannius in teleost fish [219]. Functions as a pivotal regulator maintaining calcium-phosphate equilibrium while exhibiting multifaceted roles spanning reproductive biology through gestation and lactation, developmental processes including vascular patterning and tissue morphogenesis, cellular fate determination via proliferation-apoptosis balance, and pathological pathways such as ischemic injury and oncogenic progression [220, 221]. There is emerging evidence suggesting that STC1 is present in various human cancer cells [69]. It can promote the invasiveness of breast cancer by activating the JNK/c-Jun pathway. STC1 induces activation of the JNK pathway and modulates the expression of key molecular targets, such as p-JNK and p-c-Jun [222], thereby enhancing the metastatic potential of HCC [223]. STC-1 orchestrates pro-tumorigenic vascularization and metastatic dissemination through VEGF transactivation mediated by PKCβII-dependent ERK1/2 phosphorylation cascades in neoplastic cells [69]. STC1 can also upregulate its expression through the PI-3K/Akt/NF-κB-dependent signaling pathway to promote TNBC cell metastasis. Abnormal STC1 expression has been observed to be associated with metastasis or advanced tumor stage in tumor tissues compared with corresponding normal tissue controls, suggesting that STC1 may play a role in promoting tumor progression [224].
STC-1 has been shown to be an intracellular “eat-me” signaling inhibitor and an immune checkpoint. Mechanistically, tumor STC-1 interacts with calreticulin as an “eat-me” signal to block targeted phagocytosis of membrane calreticulin by APCs (Figure 4A) [225]. Such disruption compromises the antigen presentation process between APCs and T lymphocytes while suppressing macrophage-mediated clearance of malignant cellular targets [226]. Targeting the interaction between STC-1 and calmodulin represents a promising approach to enhance the efficacy of cancer immunotherapy in patients. Tumor-derived STC-1 plays a critical role in evading immune responses within the TME, exerting suppressive effects on anticancer immunity [227]. STC1 suppresses intracellular second messenger signaling through calcium, leading to a reduction in macrophage mobility [228]. Specifically, it inhibits the expression of uncoupling protein-2 (UCP2) in response to chemical inducers, resulting in decreased mitochondrial membrane potential and superoxide production in macrophages [229]. This ultimately contributes to the modulation of cellular functions and inflammatory responses [229, 230]. UCP2 plays a crucial role in regulating the production of superoxide in macrophages, which is a key factor in controlling macrophage function and viability [231]. Therefore, STC-1 reduces superoxide production in macrophages by promoting UCP2 expression, thereby establishing its significance in tumor immunotherapy [226, 230]. The targeting of STC-1-expressing tumor cells demonstrates potent antitumor effects.
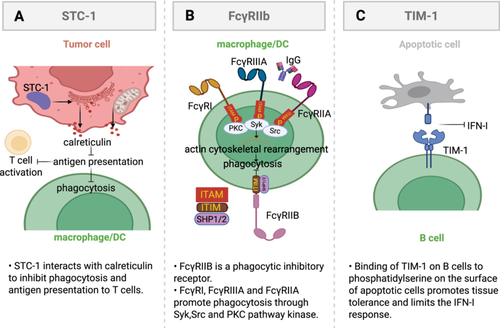
2.2.6 Fc Gamma Receptors: FcγRIIB Was Used as a Key Inhibitory Regulator
FcRs are specific receptors for Igs that bind the Fc portion of antibodies [232]. They are predominantly expressed on innate immune cells and serve as receptors for antigen-antibody immune complexes, thereby providing a cellular effector arm of the humoral immune system that links the adaptive and innate immune systems [233, 234]. The cross-linking of IgG immune complexes by FcγRs induces phosphorylation of their ITAMs, leading to the activation of SYK, SRC, and PKC pathway kinases (Figure 4B) [235]. This kinase activation subsequently triggers actin remodeling, which is crucial for the phagocytosis of IgG immune complexes [236]. FcRs have the ability to induce a variety of biological functions, including the modulation of macrophage phagocytosis, cytolysis, and transcriptional activation of cytokine genes. This can trigger an inflammatory cascade response [237]. FcRs consist of FcγRIIB (CD32B), FcγRI, FcγRIIIA (CD16), and FcγRIIA (CD32A), all of which are expressed on macrophages [238]. However, FcγRIIB stands out as the sole inhibitor of phagocytosis among the human Fcγ receptor family, while the remaining members serve to enhance this process [69]. Activated FcRs related to FcR common γ-chain all contain an ITAM [239]. In contrast, the FcγRIIB, a single-chain molecule belonging to the inhibitory FcγR subclass, serves as a potent negative regulator by containing an ITIM in its cytoplasmic structural domain [232]. Activation of ITIM through phosphorylation brings in the SHP1 and SHP2 phosphatases that work to suppress the subsequent phagocytosis process [234, 237]. FcγRIIB is commonly expressed in various types of immune cells, such as B cells, mast cells, DCs, neutrophils, and macrophages [240]. It plays a vital role in negatively regulating immune cell functions, including the proliferation of B cells and DCs, as well as the degranulation of mast cells [241]. Additionally, FcγRIIB has been implicated in the development of numerous autoimmune and neoplastic diseases, such as arthritis, systemic lupus erythematosus, inflammatory bowel disease, colorectal cancer, and melanoma [242]. Autoimmune symptoms are exacerbated in FcγRIIB-deficient mice, and normal expression of FcγRIIB prevents mice from developing an SLE-like phenotype [243]. Currently, commercially available monoclonal antibodies specific for FcγRIIB, such as mAb2B6, have been humanized and are capable of directly inducing myeloid cytotoxicity in B cells [244].
2.2.7 TIM-1: Mediates B-Cell Immunosuppression
T cell Ig and mucin domain 1 (TIM-1) encoded by Havcr1 gene is an important immune checkpoint molecule on B cells [39]. Its expression and action mechanism on B cells are of great significance for the regulation of immune response [39]. In B cells, TIM-1 is mainly expressed in specific subsets of B cells, especially the subsets of B cells that expand in TME [40]. The mechanism of TIM-1 on B cells mainly includes the following aspects: first, TIM-1 binds to phosphatidylserine on the surface of apoptotic cells to enhance tissue tolerance [245]. Second, TIM-1 expression limits the type I interferon (IFN-I) response of B cells, thereby inhibiting B cell activation and antigen presentation ability(Figure 4C) [40]. The suppressive impact not only hinders the growth of T cells but also undermines the body's ability to combat tumors through immune mechanisms. In addition, TIM-1 interacts with a variety of co-inhibitory molecules, such as PD-1, TIM-3, to form an immunosuppressive microenvironment and promote tumor immune escape [40].
TIM-1 expression is regulated by a variety of factors. Both IFN-I and IFN-II regulate TIM-1 expression, and the loss of TIM-1 enhances the ability of B cells to respond to these interferons [246]. Because of the immunosuppressive effect of TIM-1 on B cells, therapeutic strategies targeting TIM-1 have shown great potential. For example, the use of a high-affinity anti-TIM-1 antibody can significantly inhibit tumor growth and enhance the antitumor immune response. Furthermore, PD-1 blockade and anti-TIM-1 antibodies together can enhance the therapeutic outcome. These discoveries offer fresh concepts and targets for immunotherapy against cancer.
2.3 Other Immune Checkpoints Acting on Killer Cells
Killer cells within the immune system primarily encompass CD8+ T cells, NK cells, and macrophages [247]. Each of these cells plays a key role in immune defense by directly killing pathogens or infected cells through different mechanisms. CD8+ T cells induce apoptosis of target cells by recognizing and binding to antigenic peptide-MHC class I complexes on infected cells, releasing perforin and granzyme [248, 249]. In addition, by secreting cytokines, Th cells help activate other immune cells and enhance the overall immune response [248]. Furthermore, T cells can develop memory cells that offer sustained defense against tumor recurrence in the future. NK cells recognize abnormal signals on the surface of stressed cells, such as lack of MHC class I expression, release cytotoxic particles or directly kill target cells through the ADCC mechanism [250]. Moreover, FcγRIIIA on the surface of NK cells binds to specific IgG antibodies and mediates the killing of corresponding target cells [250]. The phagocytosis of macrophages has been described above. Next, we mainly introduce the inhibitory immune checkpoints expressed on NK cells and T cells.
2.3.1 Role of CTLA-4 in Immune Regulation
CTLA-4, also known as CD152, is a leukocyte differentiation antigen [251]. It is classified under the IgSF of functionally important Igs and serves as a transmembrane receptor on T-cells [252]. CTLA-4 is broadly expressed, primarily on activated Tregs [253]. The CTLA-4 molecule contains a lead peptide and three structural domains that are associated with exons 2, 3, and 4, respectively [254]. And the first structural domain functions to bind ligands, while the second and third structural domains are transmembrane and cytoplasmic structural domains [255]. The first identification of CTLA-4 was obtained by screening a cDNA library derived from mouse CTL, which revealed a high degree of homology between the mouse and human CTLA-4 genes [256, 257]. In vivo, CTLA-4 serves as an important immune checkpoint receptor for the co-stimulatory ligands B7-1 (CD80) and B7-2 (CD86) on T-cells, exerting a negative regulatory effect on T-cell activation in immunotherapy [258].
Pioneering murine genetic ablation models in the mid-1990s first established CTLA-4's critical inhibitory role in modulating T lymphocyte activation cascades [259]. Research established CTLA-4 as a potent CD80/CD86-binding receptor, functioning as a critical inhibitory checkpoint that suppresses adaptive immunity through competitive ligand interactions [260]. This molecule has been extensively studied in immune response therapy and has been successfully utilized in clinical therapy, playing a pivotal role in impeding anticancer immunity [261].
CTLA-4 exhibits significant structural homology with the ectodomain of CD28, which is another co-stimulatory receptor for CD80/CD86 [260]. CTLA-4 demonstrates a higher binding affinity for the ligand compared to CD28. In fact, its affinity for the CD80/CD86 ligand is 10 to 100 times higher than that of CD28 [256, 262]. CTLA-4 is initially present in intracellular vesicles of inactivated T cells and can only be detected 24 h after activation [263]. Its expression reaches a maximum at 36 to 48 h but can also be constitutively expressed on Tregs (Figure 5) [262, 264]. Furthermore, it was shown that CTLA-4 is closely associated with the differential expression of T cell subsets and that a portion of its biological function is also reliant on the stage of T cell differentiation [256, 265]. In addition, the expression of CTLA-4 serves as a mechanism to maintain the balance of T-cell activation intensity [266]. This not only ensures the normal functioning of the body's immune response but also facilitates the survival of CTLA-4 expressing cells within the body [267, 268]. The interaction between CD28 on T-cells and CD80/CD86 on APCs initiates a co-stimulatory signal essential for T-cell activation. This, together with the first signal generated by the recognition of MHC by the antigen-specific T-cell receptor, constitutes a dual signal for T-cell activation [269]. Due to the high degree of homology between the extracellular domains of CTLA-4 and CD28, CTLA-4 can antagonize the interaction of CD28 with its ligands. This action reduces the production of co-stimulatory signals and further hinders the promotional function of CD28 molecules on T-cell activation [270]. Ultimately, this leads to T-cell suppression, reduced cytokine production, and proliferation restriction [264, 271]. This type of inhibition of T cell activation is a cell-intrinsic mechanism.
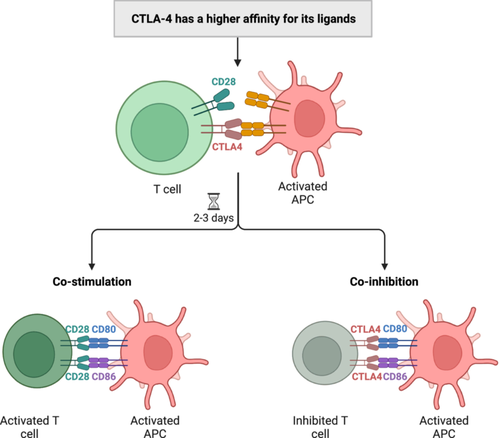
CTLA-4 can transmit inhibitory signals into APCs via CD80/CD86 ligands, leading to the inhibition of the production of the tryptophan-depleting enzyme indoleamine-2, 3-dioxygenase and T-cell effector responses [251, 272]. In addition, binding of CTLA-4 to CD80/CD86 ligands can also result in transcytosis of APC ligands [273]. Furthermore, CTLA-4 expressing T cells have the ability to deplete ligands from APCs, thereby decreasing the level of ligands on the surface of APCs and subsequently inhibiting co-stimulation of opposing T cells [274]. This type of inhibition represents an extracellular mechanism for regulating T cell activation. In contrast, CTLA-4 also enhances T cell/APC adhesion through an LFA1-mediated pathway and leads to attenuated T cell activation by reducing the duration of APC/T cell interactions [275].
In addition, CTLA-4 can also disrupt TCR signaling by interacting with PP2A (protein phosphatase 2) and SHP2 [276]. This interaction is believed to downregulate TCR signaling on T cells upon ligand binding between the TCR and the MHC and antigenic peptide on DCs [277, 278]. The inhibitory effect of CTLA-4 on T cells is further demonstrated by the physiological function of CTLA-4-deficient mice [279]. These mice develop normally before birth but are born with various lymphoproliferative disorders of the thymus, spleen, and lymph nodes [280]. These lymphoid organs enlarge 5 to 10 times larger than those of normal mice and usually die by the third or fourth week [281]. Studies showed that T cells in these mice were in a highly activated state and would proliferate more compared to normal mice when triggered by TCR/CD3, leading to a significant increase in CD4 and CD8 numbers [282]. The aforementioned examples further illustrate that the deficiency of CTLA-4 leads to uncontrolled activation of T cells, highlighting the pivotal role of CTLA-4 in inhibiting T cell activation. Additionally, the disease observed in CTLA-4-deficient mice is not attributed to active T-cell defects [283]; rather, normal T cells triggered by CTLA-4 produce factors that inhibit disease induced by CTLA-4 deficiency [284]. CTLA-4 serves as the target gene for Forkhead box protein P3 (Foxp3), a master regulator of Tregs. Consequently, inactivation of the gene encoding Foxp3 in CTLA-4-deficient mice results in fatal autoimmune disease [251], underscoring the essential role of CTLA-4 in enabling Treg cells to suppress immune responses and maintain immune self-tolerance [284].
2.3.2 Diversity, Structural Characteristics of Killer Immunoglobulin-Like Receptor (KIR) and Its Function in Immune Regulation
KIR is an important class of receptors expressed on the surface of NK cells and T cells, which play a key role in regulating the activity of NK cells [285]. The KIR gene family is highly polymorphic, showing random, diverse, single allele expression patterns, and this diversity may lead to different KIR expression patterns and functions [286, 287]. KIRs are categorized as activating KIRs (aKIRs) and inhibitory KIRs (iKIRs), and their family members are classified into two types: the KIR2D and KIR3D [288-290]. The naming of KIRs is based on the structural characteristics of their extracellular regions (“2D” and “3D” represent the number of extracellular Ig-like domains) and the length of their cytoplasmic tails (“L” for long and “S” for short). These structural domains endow them with specificity for particular HLA molecules (Figure 6) [291]. The function of KIR can be deduced based on the extent of the cytoplasmic region; L receptors are usually inhibitory, and S receptors are usually activating [292, 293]. Currently, iKIRs play a dominant role in controlling NK cell function by interacting with specific MHC-I molecules (human HLA-I molecules) and modulating immune activity by regulating either killing or inhibitory signals in the context of recognizing different HLA-like molecules and other ligands [288].
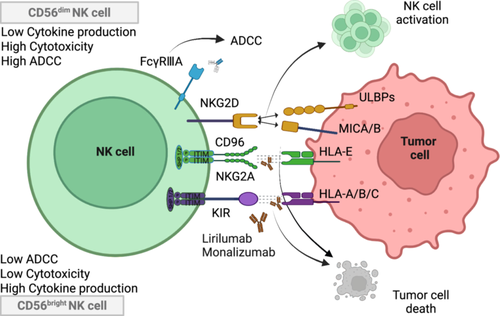
iKIRs are type I transmembrane receptors targeting polymorphic HLA-A, B, and C molecules [294]. For example, KIR2DL1 primarily recognizes the HLA-C C2 population with high affinity, whereas KIR2DL2 and KIR2DL3 recognize the HLA-C C1 allotype [295-297]. KIR3DL1 recognizes HLA-B and some HLA-A carrying Bw4 [298, 299]. When iKIRs bind to their ligands, they transmit inhibitory signals through ITIMs in cytoplasmic tails [300, 301]. These ITIMs are phosphorylated by specific tyrosine kinases (e.g., Src family kinases) upon binding [162, 302, 303]. Phosphorylated ITIMs recruit and activate phosphatases containing the SH2 structural domain, such as SHP-1 and SHP-2 [304, 305]. These phosphatases dephosphorylate and inhibit signaling molecules associated with NK cell activation, such as Lck, Fyn, and Syk, which downregulate PI3K, MAPK, and NF-κB signaling [303, 306, 307]. The above mechanisms lead to downregulation of NK cell-mediated cytotoxicity, attenuation of cell proliferation, and significant reduction in cytokine production [302, 304]. In addition, NK cells do not mount an attack on cells expressing normal HLA-class I molecules. Under normal conditions, iKIRs help NK cells distinguish between self and non-self-cells, thus playing an important role in immune surveillance [303]. If cells (e.g., tumor cells or virus-infected cells) reduce the expression of HLA-class I molecules, the inhibitory signals of iKIRs are weakened, and NK cells may then be activated and kill these abnormal cells.
2.3.3 NKG2A/CD94: A Key Inhibitory Receptor Complex in NK Cell and T Cell Regulation
NKG2A (CD159) is a heterodimeric inhibitory receptor of the C-type lectin family expressed predominantly on the surface of NK cells and also present in certain T cell subsets [308]. NKG2A is expressed in several organ tissues of the human body, and especially the highest expression is found in peripheral blood cells. CD94 is a 70 kDa type II transmembrane glycoprotein belonging to the C-type lectin superfamily, which, like NKG2A, is mainly expressed on the surface of NK cells and some T cells [309, 310]. The NKG2A/CD94 molecule, after being linked by disulfide bonds to form a heterodimeric complex, is recognized by HLA-E, a nonclassical MHC-I molecule on the target cell, which induces cascading inhibitory signals and suppresses cytotoxic activity and cytokine secretion from NK cells(Figure 6) [37, 311]. The N-terminus of NKG2A is located in the cytoplasm, and like KIR, it contains ITIMs [312]. After NKG2A binds to CD94 to form a heterodimer, it can recognize the target cell-specific ligand HLA-E and transmit inhibitory signals to NK cells by recruiting SHP-1 and SHP-2 [312-314]. In addition, during the activation of CD8+ T cells, the NKG2A on the surface of CD8+ T cells can bind to the HLA-E molecule, thereby inhibiting the activation process of CD8+ T cells [315, 316].
In the tumor-infiltrated areas, the expression of HLA-E and NKG2A on the surface of NK and CD8+ T cells increases, and the ability of local NKG2A to bind to HLA-E in the tumor increases, thereby inhibiting the function of NK and CD8+ T cells, which helps tumor cells evade immune surveillance [317, 318]. Therefore, NKG2A targeting agents are being studied for the intervention of various cancer types, including, but not limited to, colorectal, head and neck, ovarian, Hodgkin's lymphoma, and lung cancers [319-321]. Investigating the inhibitors of NKG2A holds significant clinical potential.
2.3.4 Lymphocyte Activation Gene-3 (LAG-3): Structure, Function, and Its Critical Role in the Immune System
LAG-3, also referred to as CD223, was first identified in 1990 from a screen of CD3-negative IL-2-dependent NK cell line F5 cells [322]. LAG-3, a protein classified within the IgSF, is specifically identified as a mature type I transmembrane glycoprotein. It is located on human chromosome 12 and serves as a critical regulator of the immune system [91, 323]. This biomolecule features three distinct topological segments: a membrane-spanning segment, an ectodomain, and an intracellular module containing functional motifs [324]. The extracellular structure is composed of four IgSF structural domains (D1–D4). The significance of the extracellular domain in binding ligands to LAG-3 is primarily attributed to the presence of a proline-rich ring structure and an intrachain disulfide bond within the D1 domain [325]. This domain belongs to the IgSF and exhibits species-specific characteristics [91]. In the transmembrane-cytoplasmic fraction, LAG-3 undergoes proteolytic shedding from the plasma membrane, mediated predominantly by family metalloproteases (ADAM10 and ADAM17). This, in turn, regulates the function of LAG-3 [325]. The intracellular domain contains three structural elements: (1) a phosphorylatable serine residue at position S454 (substrate of protein kinase C), (2) a conserved KIEELE amino acid sequence, and repeating glutamic acid-proline dipeptide units [326, 327]. The highly conserved “KIEELE” motif is crucial for the function of LAG-3, and mutation of KIEELE can result in complete loss of LAG-3 function [328]. LAG-3 can co-localize with CD4, CD8, and CD3 molecules. However, structurally, LAG-3 is not identical to CD3 and CD8 but shares approximately 20% amino acid sequence homology with CD4 in the extracellular region [329]. LAG-3 is predominantly expressed on the surface of effector T cells and regulatory Tregs, NK cells, B cells, and DCs. Similar to CTLA-4, LAG-3 is not expressed on naive T cells but is induced by CD8+ and CD4+ T cells in response to sustained antigenic stimulation [330].
Studies have indicated that the LAG-3 ligands identified in the TME consist of MHC-II molecules, galactose lectin-3 (Gal-3) [331], fibrinogen-like protein 1 (FGL1), and liver sinusoidal endothelial cell lectin (LSECtin) [323]. While LAG-3 and CD4 both engage MHC-II molecules,346 LAG-3 demonstrates superior binding affinity over CD4, competitively impairing CD4-MHC-II complex formation [332]. Moreover, LAG-3 selectively binds to the MHC-II/peptide (pMHC-II) complex on the surface of APCs [333]. leading to signaling of MHC-II molecules in DCs [334]. Upon binding of LAG-3 to MHC-II, inhibitory signals can be transmitted through the cytoplasmic structural domains, leading to the inhibition of CD4 T cell activation and cytokine production [335, 336]. This ultimately impacts the immune response. Furthermore, the interaction between LAG-3 and MHC-II promotes the recruitment of tumor-specific CD4T cells and facilitates tumor escape [337]. Gal-3 is a soluble molecule secreted by tumor-associated stromal cells. It is predominantly expressed on the surface of tumor cells and activated T cells [338]. It is binding to LAG-3, which results in the inhibition of IFN-γ production by CD8+ T cells and plays a critical role in suppressing the expansion of plasmacytoid DCs [339]. FGL1 is produced and released by both tumor cells and hepatocytes, functioning to suppress IL-2 LSECtin production. This cytokine, primarily located in the liver, has also been detected on tumor cell surfaces [333]. Upon binding to LAG-3, both FGL1 and LSECtin have demonstrated significant inhibition of antitumor responses [340, 341]. To date, LAG-3 has been identified as a key factor in various pathological conditions and is pivotal in the progression of malignancies. The relationship between LAG-3 expression and clinical outcomes differs significantly among cancer types.
2.3.5 T Cell Immunoreceptor With Immunoglobulin and ITIM Domains (TIGIT): Emerging Immunotherapy Targets in the IgSF
TIGIT is an IgSF receptor that has become a target for cancer immunotherapy [342, 343]. It was discovered in 2009 through genome-wide analysis of genes expressed on immune cells [344], revealing that these genes contain structural domains commonly found in inhibitory receptors, thus identifying TIGIT as an inhibitory receptor [345, 346]. The human TIGIT gene is located on chromosome 3 at q13. 31 and has a cDNA length of 2, 926 bp, which can encode 244 amino acids with 6 TIGIT isoforms [345]. TIGIT is structurally organized into three distinct regions: an extracellular IgV domain, a type I transmembrane topology, and a cytoplasmic segment housing both an ITIM and an immunoglobulin tyrosine tail [347]. TIGIT functions as an inhibitory checkpoint modulator for cytotoxic immune responses, with preferential expression observed across innate lymphoid populations (NK cells/γδ T cells) and adaptive subsets including CD4+/CD8+ T lymphocytes, regulatory T cells, and Th cell populations [348]. However, its expression is weak when CD4+ and CD8+ T cells are in the naive state but can be highly expressed when they are activated [349].
TIGIT has three primary ligands: CD155 (also known as poliovirus receptor, PVR), CD112, and CD113, all of which are part of the connexin and NECL families [342]. CD155 contains Ig domains, C1-like and C2-like domains in its extracellular regions, a long C-terminal domain, and ITIM in its transmembrane region [350]. Demonstrating potent TIGIT-binding specificity, this molecule modulates multifaceted immunoregulatory pathways within TME [351]. TIGIT binds to CD155 on DCs and induces phosphorylation of CD155, initiating a biochemical transduction chain that reduces IL-12 production and suppresses T cell function [352]. The binding of TIGIT to its ligands inhibits the activity of T cells and NK cells, as well as reduces antigen presentation and anti-inflammatory cytokine secretion [353]. In addition to binding to these ligands, CD226 also plays a crucial role in the mechanism of action of TIGIT [354]. CD226 is a co-stimulatory receptor expressed on T cells and NK cells, which binds to LFA-1 and mediates the activation of immune cell function [355]. The binding of TIGIT to CD155 interferes with CD226 activation signaling and suppresses tumor immune response [356, 357]. Notably, highly TIGIT-expressing Tregs have a potent inhibitory effect on the immune response. They can upregulate various Tregs gene markers such as CTLA-4, LAG-3, PD-1, Foxp3, and so forth, and attenuate Th1 and Th17 functions [358]. Furthermore, these highly TIGIT-expressing Tregs have also been found to attenuate Th1 and Th17 functions [359]. TIGIT inhibits NK cell degranulation and cytokine production. Additionally, the binding of TIGIT+NK cells to CD155+ MDSCs inhibits the phosphorylation of ZAP70/SYK and ERK1/2, ultimately attenuating NK cell cytolysis [306, 360]. TIGIT is widely expressed on lymphocytes and is currently a popular immune checkpoint, particularly when combined with PD-1/PD-L1 [361, 362]. This combination has demonstrated improved therapeutic effects in clinical settings.
2.4 Other Novel Immune Checkpoints
In recent years, there has been a rapid rise in research on novel immune checkpoint molecules. In addition to the more widely studied immune checkpoints mentioned above, there are a number of other novel immune checkpoints. CD161 directly inhibits CD8+ T-cell function by binding to the ligand CLEC2D (alternatively named lectin-like transcript 1; LLT1), which signals independently of the PD-1 pathway and is significantly associated with T-cell depletion in hematologic malignancies and melanoma. Its signaling is independent of the PD-1 pathway and is significantly associated with T-cell depletion in hematological malignancies and melanoma [363]. Currently, a fully human monoclonal antibody against CD161 effectively blocks the binding of CLEC2D to CD161, thereby releasing the antitumor activity of T cells and reversing PD-1 resistance [363]. Herpes virus entry mediator B- and T-lymphocyte attenuator acts as a T and B-cell co-suppressor receptor to inhibit the NF-κB pathway through activation of SHP-1/2 phosphatase, which is negatively correlated with PD-L1 expression in lymphomas, and the combination of its monoclonal antibody tifcemalimab/icatolimab with PD-1 inhibitors reduces immune-related adverse effects [364]. In addition, ectonucleoside triphosphate diphosphohydrolase-1, also known as CD39, can inhibit T and NK cells by hydrolyzing ATP to generate adenosine to activate the A2aR pathway, and in combination with anti-PD-L1, synergistically blocks the adenosine-PD-1 dual inhibitory signaling [365]. The study of emerging immune checkpoint molecules has significant theoretical and clinical value in the field of tumor immunotherapy. Based on the discovery of emerging targets, future research needs to focus on multi-target synergistic blockade strategies, tissue-selective drug delivery, and the construction of dynamic biomarker systems to break through the limitations of existing immunotherapy and achieve safer and longer-lasting tumor control.
3 Roles of Immune Checkpoints in Cancer Development
Immune checkpoint is a key regulatory molecule in the immune system. By binding with the corresponding ligand, it conveys suppressive cues to prevent the function of immune cells from being strongly stimulated, thus maintaining immune balance, avoiding the occurrence of autoimmune diseases, and controlling the intensity and duration of immune response [8, 9]. However, in TME, tumor cells exert their immune checkpoint function through multiple mechanisms: on the one hand, their continuous upregulated reservoirs of inhibitory molecules (such as PD-L1) leads to T cell depletion; on the other hand, by reshaping the cellular composition, metabolic characteristics and physical barrier of TME, the immunosuppressive ecology is constructed and the immune escape is synergically promoted. For example, the PD-1/PD-L1 pathway not only inhibits the cytotoxic activity of T cells but also blocks the migration and infiltration of T cells to the tumor site, reducing the number of effective T cells [41]. At the same time, malignant cells evade immune detection by downregulating antigen expression, inducing antigen mutations, or epigenetic modifications such as deletion of MHC-I class molecules [75, 366]. Under immune stress, tumor cells can alter the antigen epitope structure (similar to the strategy of SARS-CoV-2 puncture protein mutation escape T cell recognition), reduce TCR affinity, or inhibit the expression of MHC molecules through gene deletion and DNA methylation, preventing CTL recognition [367]. These mechanisms promote tumor progression by impairing the immune system's ability to recognize it [368].
The heterogeneity of TME further exacerbates immune escape. Overexpression of PD-L1 is not only a direct immunosuppressive strategy for tumor cells, but is also positively regulated by inflammatory factors (such as IFN-γ) in TME through the JAK-STAT pathway, forming a self-reinforcing inhibitory cycle [369]. In addition, tumor cells secrete inhibitory cytokines such as TGF-β and IL-10 by recruiting Treg and M2 macrophages, and induce tumor-associated fibroblasts (CAFs) to secrete collagen to form a physical barrier to prevent immune cell infiltration [75, 369]. Finally, by reducing antigen presentation, activating immune checkpoints, and reshaping the physical and metabolic barriers of TME, tumor cells construct a multi-level escape network that not only evades immune killing but also drives metastasis and drug resistance, forming a vicious cycle.
Therefore, in view of tumor heterogeneity and the complexity of immune escape mechanisms, targeting immune checkpoints provides a key intervention target for overcoming tumor drug resistance and immunosuppression. By blocking inhibitory signals (such as PD-1/PD-L1) or activating co-stimulatory pathways (such as CD28), such strategies can reverse T cell depletion, reshape the immunosuppressive microenvironment, and even restart antitumor immune responses. At present, the medical adoption of checkpoint blockade has expanded from single drug therapy to combination regimens with chemotherapy, radiotherapy, and targeted drugs, focusing on their clinical progress, drug resistance mechanism, and combination therapy optimization strategy, providing a new direction for breaking through the existing treatment bottleneck.
4 Clinical Translation of Immune Checkpoint Inhibitors (ICIs) in Cancer Therapeutics
Targeting the prominent role of immune checkpoints in mediating tumor immune evasion, the development of drugs that inhibit immune checkpoint activity has emerged as an effective antitumor strategy [370, 371]. These drugs are collectively known as ICIs [372]. Several ICIs have already demonstrated promising outcomes, underscoring their potential impact on tumor treatment [373].
4.1 Clinical Transitions of ICIs
The CTLA-4-blocking monoclonal antibody ipilimumab, functioning as a pioneering immune checkpoint blockade agent, achieved regulatory clearance from the Food and Drug Administration (FDA) in 2011 for managing progressive melanocytic neoplasms under accelerated approval protocols [374]. Emerging clinical evidence reveals ipilimumab's therapeutic efficacy extends beyond melanoma to encompass renal carcinoma, NSCLC, and colorectal malignancies [375]. AstraZeneca's tremelimumab is another extensively studied CTLA-4 antibody that is currently available in the United States, following approval by the FDA in 2022. In addition, drugs that have been approved for marketing and target CTLA-4 include cadonilimab, belatacept, and abatacept (Table 1).
| Target | Drug | Other study ID numbers | First approved indication | Company name (sponsor) | Time of first approval for marketing | References |
|---|---|---|---|---|---|---|
| CD80/CD86 (APCs) | Abatacept | BMS-188667; BMS-188667IV; BMS-188667SC; BMS-1888667; ONO-4164; ONO-4164IV; ONO-4164SC | Rheumatoid arthritis | Bristol-Myers Squibb Company | 2005 | [376] |
| CTLA-4 (T cells) | Tremelimumab | CP-675206; MEDI-1123; PF-06753388 | Hepatocellular carcinoma | AstraZeneca under license from Pfizer | 2022 | [377] |
| Ipilimumab | BMS-734016; MDX-010; MDX-101; MDX-CTLA4; ONO-4480 | Malignant melanoma | Medarex | 2011 | [378] | |
| Belatacept | LEA-029; BMS-224818; LEA-29Y | Kidney transplant rejection | Bristol-Myers Squibb Company | 2011 | [379] | |
| CTLA-4/PD-1 | Cadonilimab | AK-104 | Relapsed or metastatic cervical cancer | Akeso Inc. | 2022 | [380] |
| PD-1 (T cells, B cells, DCs, macrophages) | Toripalimab | JS-001; TAB-001 | Metastatic melanoma | Shanghai Junshi Bioscience Co. Ltd. | 2018 | [381] |
| Tislelizumab | BGB-108; BGB-A317; VDT-482 | Hodgkin's lymphoma | BeiGene | 2019 | [382] | |
| Pembrolizumab | MK-3475; SCH-900475 | Malignant melanoma | Merck & Co. | 2014 | [383] | |
| Sintilimab | IBI-308 | Hodgkin's lymphoma | Innovent Biologics and Eli Lilly and Company | 2018 | [384] | |
| Cemiplimab | REGN-2810; SAR-439684 | Squamous cell carcinoma | Regeneron Pharmaceuticals and Sanofi Genzyme | 2018 | [385] | |
| Retifanlimab | INCMGA-00012; INCMGA-0012; MGA-012 | Merkel cell carcinoma | Incyte Corporation | 2023 | [386] | |
| Camrelizumab | INCSHR-1210; SHR-1210 | Hodgkin's lymphoma | Jiangsu Hengrui Medicine Co. Ltd. | 2019 | [387] | |
| Serplulimab | HLX-10 | Advanced unresectable or metastatic microsatellite instability-high solid tumors | Shanghai Henlius Biotech Inc. | 2022 | [388] | |
| Enlonstobart | SG-001 | Cervical cancer | CSPC Zhongqi Pharmaceutical Technology Co. Ltd. | 2024 | [389] | |
| Penpulimab | AK-105 | Hodgkin's lymphoma | Akeso Biopharma, in collaboration with Chia Tai Tianqing | 2021 | [390] | |
| Dostarlimab | ANA-011; ANA-11; ANB-011; GSK-4057190; GSK-4057190A; TSR-042 | Endometrial cancer | AnaptysBio Inc. | 2021 | [391] | |
| Zimberelimab | AB-122; GLS-010; GS-0122; WBP-3055 | Hodgkin's lymphoma | Gloria Biosciences, Arcus Biosciences, and Taiho Pharmaceutical Co. | 2021 | [392] | |
| Prolgolimab | BCD-100 | Melanoma | Biocad Ltd. | 2020 | [65, 393, 394] | |
| Pucotenlimab | AK-103; HX-008 | Solid tumor | Lepu Biopharma | 2022 | [395] | |
| PD-1/LAG-3 | Nivolumab/Relatlimab | BMS-986213; ONO-7121 | Unresectable or metastatic melanoma | Bristol Myers Squibb | 2022 | [396] |
| PD-1/VEGFR | Ivonescimab | AK-112; SMT-112 | Non-small cell lung cancer | Akeso Biopharma | 2024 | [397] |
| PD-L1 (tumor cells, DCs, macrophages) | Durvalumab | MEDI-4736 | Urothelial carcinoma | AstraZeneca | 2017 | [398] |
| Sugemalimab | CS-1001; EQ-165; WBP-3155 | Metastatic non-small cell lung cancer | CStone Pharmaceuticals | 2021 | [399] | |
| Atezolizumab | L-01XC32; MPDL-3280A; RG-7446; RG-7746; RO-5541267 | Metastatic bladder cancer; metastatic uroepithelial carcinoma | Genentech Inc. | 2016 | [400] | |
| Avelumab | MSB-0010718C; MSB0010682; PF-06834635 | Merkel cell carcinoma | EMD Serono Inc. | 2017 | [401] | |
| Envafolimab | 3D-2-02-0015; ASC-22; KN-035 | Solid tumor | Suzhou Alphamab | 2021 | [402] |
Furthermore, targeting PD-1/PD-L1 immune checkpoints has also shown significant antitumor effects in numerous clinical trials [403]. Numerous malignancies, including melanoma, NSCLC, renal cell carcinoma, and Hodgkin's lymphoma, can be treated with PD-1 antibodies [404, 405]. These antibodies enhance T-cell attack on tumors by inhibiting the linking of PD-1 to PD-L1, thereby alleviating the suppression of the immune system by tumor cells [406, 407]. The FDA has approved a number of PD-1 antibodies to date, including cemiplimab, tislelizumab, pembrolizumab, and nivolumab [65] (Table 1). There have also been clinical trials on several PD-1 antibodies. The PD-1/PD-L1 pathway is considered the most promising in immunotherapy [408], and therefore, PD-L1 antibody drugs entering clinical trials are an important aspect of current cancer immunotherapy research [409, 410]. These therapeutic medications are being tested in clinical trials both alone and in combination with other therapies such as chemotherapy, radiotherapy, and other immune checkpoint blockers [411, 412].
Another immune checkpoint that has received increased attention is LAG-3. A phase I clinical trial of HLX26 has demonstrated a favorable safety and tolerability profile in patients with metastatic solid tumors or lymphomas. Additionally, the FDA has authorized the use of relatlimab and nivolumab together to treat patients with metastatic or incurable melanoma (Table 1).
Based on the tremendous success of CTLA-4 and PD-1/PD-L1 antibody drugs, there is an increasing use of ICIs in clinical settings. The clinical trials investigating checkpoints on macrophages have increasingly emerged as a prominent area of research. One of the most notable phagocytic checkpoints is CD47. Antibody drug development against CD47 mainly focuses on three areas: targeting the CD47-SIRPα axis, targeting SIRPα-Fc fusion proteins, and combining with other ICIs for synergistic antitumor purposes [413]. Additionally, the fusion protein can both neutralize signals inhibiting CD47 phagocytosis through the SIRPα segment and bind to the FcR on macrophages through the Fc region [191], thereby promoting the activation of phagocytosis. The clinical study TTI-621 (NCT02663518) was well tolerated in diffuse large B-cell lymphoma and T-cell non-Hodgkin's lymphoma [414]. Furthermore, a phase I clinical trial of IMM2520 (NCT05780307), a PD-L1 and CD47-targeting bispecific antibody for patients with advanced solid malignancies, is also currently in progress [415]. To date, other clinical trials of CD47 antibodies are ongoing. Clinical trials involving acute myeloid leukemia, myelodysplastic syndrome, lymphoma, and numerous solid malignancies have demonstrated a satisfactory safety and tolerability profile for the humanized antibody Hu5F9-G4 (magrolimab, an anti-CD47 monoclonal antibody) [416, 417]. A large number of Hu5F9-G4-based clinical trials are currently underway (Table 2). In addition, CD24, another target of significant interest on macrophages, has also been associated with clinical trials. A Phase I clinical study was conducted to investigate the role of IMM47 in advanced solid tumors (NCT05985083). In addition, ONC-841, a humanized antibody targeting Siglec-10 and featuring the human Ig G4 Fc structural domain, is also currently in a Phase I clinical trial exploring efficacy in advanced solid tumors (NCT06352359). There are some clinical trials exploring the effect of CD24Fc on COVID-19, efficacy of human immunodeficiency virus (HIV), and graft-versus-host disease (Table 2).
| Target | Drugs | Combination drugs | Disease | Sponsors | Clinical phase | Study start (actual) | Status | National Clinical Trial number (NCT no.) |
|---|---|---|---|---|---|---|---|---|
| CD47 (DCs, macrophages) | Hu5F9-G4 (magrolimab) | Acalabrutinib, AZD9150, AZD6738, AZD5153, Rituximab | Non-Hodgkin's lymphoma | Acerta Pharma BV | Phase 1 | 2018-06-19 | Completed | NCT03527147 |
| Single agent | Solid tumor | Gilead Sciences | Phase 1 | 2014-08 | Completed | NCT02216409 | ||
| Single agent | Acute myeloid leukemia, myelodysplastic syndrome | Gilead Sciences | Phase 1 | 2015-11 | Completed | NCT02678338 | ||
| Atezolizumab | Acute myeloid leukemia | Hoffmann-La Roche | Phase 1 | 2019-10-08 | Terminated (sponsor decision) | NCT03922477 | ||
| Olaparib | BRCA-mutated metastatic or recurrent breast, castrate-resistant prostate cancer | National Cancer Institute | Phase 1 | 2024-03-05 | Withdrawn (No subjects enrolled in the study) | NCT05807126 | ||
| Pembrolizumab | Relapsed or refractory classic Hodgkin lymphoma | Stanford University | Phase 2 | 2022-06-21 | Active, not recruiting | NCT04788043 | ||
| Cetuximab | Solid tumor colorectal cancer | Gilead Sciences | Phase 1/2 | 2016-11-02 | Completed | NCT02953782 | ||
| Dinutuximab | Recurrent osteosarcoma, refractory neuroblastoma | National Cancer Institute | Phase 1 | 2021-08-31 | Active, not recruiting | NCT04751383 | ||
| Avelumab | Solid tumor | Gilead Sciences | Phase 1 | 2018-05-23 | Completed | NCT03558139 | ||
| Mogamulizumab | Relapsed/refractory treated T-cell lymphoma | National Cancer Institute | Phase 1/2 | 2021-04-06 | Active, not recruiting | NCT04541017 | ||
| Rituximab, Gemcitabine, Oxaliplatin | Non-Hodgkin's lymphoma | Gilead Sciences | Phase 1/2 | 2016-11-21 | Terminated | NCT02953509 | ||
| Atezolizumab, Enfortumab Vedotin, Niraparib, Tiragolumab, Sacituzumab Govitecan, Tocilizumab, Cisplatin, Gemcitabine | Urothelial carcinoma bladder cancer | Hoffmann-La Roche | Phase 1/2 | 2019-06-01 | Active, not recruiting | NCT03869190 | ||
| Cisplatin, Gemcitabine | Advanced urothelial carcinoma | Insel Gruppe AG, University Hospital Bern | Phase 1 | 2023-06-21 | Withdrawn (IMP security Issues reported by Sponsor) | NCT05738161 | ||
| Decitabine/Cedazuridine | Myelodysplastic syndromes | Astex Pharmaceutical Inc. | Phase 2 | 2023-06-27 | Terminated | NCT05835011 | ||
| 7+3 (cytarabine/daunorubicin chemotherapy), CPX-351 | Acute myeloid leukemia, myelodysplastic neoplasm | Uwe Platzbecker | Phase 2 | 2024-03-31 | Withdrawn | NCT05829434 | ||
| Azacitidine | Hematological malignancies | Gilead Sciences | Phase 1 | 2017-09-08 | Terminated | NCT03248479 | ||
| Azacitidine, Placebo | Myelodysplastic syndromes | Gilead Sciences | Phase 3 | 2020-09-09 | Terminated | NCT04313881 | ||
| Azacitidine, Venetoclax | Acute myeloid leukemia | M.D. Anderson Cancer Center | Phase 1/2 | 2020-07-28 | Active, not recruiting | NCT04435691 | ||
| Azacitidine | Acute myeloid leukemia, myelodysplastic syndrome | City of Hope Medical Center | Phase 1 | 2024-10-09 | Withdrawn | NCT05823480 | ||
| Siglec-10 (APCs) | ONC-841 | Single agent | Advanced solid tumor | OncoC4 Inc. | Phase 1 | 2024-06 | Withdrawn | NCT06219499 |
| Single agent | Advanced solid tumor | OncoC4 Inc. | Phase 1 | 2024-08-23 | Recruiting | NCT06352359 | ||
| CD24 (tumor cells, DCs) | IMM-47 | Single agent | Advanced solid tumor | ImmuneOnco Biopharmaceuticals (Shanghai) Inc. | Phase 1 | 2023-08-18 | Recruiting | NCT05985083 |
| CD24Fc (MK-7110, efprezimod alfa) | Single agent | Advanced solid tumor | Tianhong Li, University of California, Davis | Phase 1/2 | 2020-10-30 | Terminated | NCT04552704 | |
| Ipilimumab, Nivolumab | Metastatic melanoma | Oncoimmune Inc., a subsidiary of Merck & Co. Inc. | Phase 1/2 | 2021-06-15 | Withdrawn (business reasons) | NCT04060407 | ||
| Single agent | HIV infections, dyslipidemias | Oncoimmune Inc., a subsidiary of Merck & Co. Inc. | Phase 2 | 2020-08-31 | Terminated | NCT03960541 | ||
| Methotrexate, Tacrolimus | Graft versus host disease, hematopoietic stem cell transplantation, leukemia | Oncoimmune Inc., a subsidiary of Merck & Co. Inc. | Phase 2 | 2016-09-19 | Completed | NCT02663622 | ||
| Single agent | Coronavirus Disease 2019 (COVID-19) | Oncoimmune Inc., a subsidiary of Merck & Co. Inc. | Phase 3 | 2020-04-24 | Completed | NCT04317040 | ||
| KIR (T cells, NK cells) | Anti-KIR (1-7F9) | Single agent | Multiple myeloma | Innate Pharma | Phase 1 | 2007-05 | Completed | NCT00552396 |
| IPH2101 | Single agent | Multiple myeloma | Innate Pharma | Phase 2 | 2009-09 | Completed | NCT00999830 | |
| Single agent | Acute myeloid leukemia | Innate Pharma | Phase 1 | 2007-02 | Completed | NCT01256073 | ||
| Single agent | Smoldering multiple myeloma | Innate Pharma | Phase 2 | 2010-09 | Completed | NCT01222286 | ||
| Lenalidomide | Patients with multiple myeloma experiencing a first or second relapse | Innate Pharma | Phase 1 | 2010-09 | Completed | NCT01217203 | ||
| Lirilumab (IPH2102) | Single agent | Acute myeloid leukemia | Innate Pharma | Phase 2 | 2012-10 | Completed | NCT01687387 | |
| Ipilimumab | Selected advanced tumor | Bristol-Myers Squibb | Phase 1 | 2012-12 | Completed | NCT01750580 | ||
| Elotuzumab, Urelumab | Multiple Myeloma | Innate Pharma | Phase 1 | 2014-12-09 | Completed | NCT02252263 | ||
| Rituximab | Leukemia, chronic lymphocytic leukemia, lymphocytic leukemia | M.D. Anderson Cancer Center, Bristol-Myers Squibb | Phase 2 | 2015-06-23 | Completed | NCT02481297 | ||
| Nivolumab, Ipilimumab | Advanced solid tumors | Bristol-Myers Squibb | Phase 1 | 2017-07-14 | Completed | NCT03203876 | ||
| Nivolumab, Ipilimumab | Advanced solid tumors | Bristol-Myers Squibb | Phase 1/2 | 2012-10-07 | Completed | NCT01714739 | ||
| IPH4102 | Single agent | Relapsed/refractory cutaneous T-cell lymphomas | Innate Pharma | Phase 1 | 2015-10 | Completed | NCT02593045 | |
| Single agent | T-cell lymphoma, cutaneous lymphoma | Innate Pharma | Phase 2 | 2019-05-22 | Active, not recruiting | NCT03902184 | ||
| NKG2A (T cells, NK cells) | Monalizumab (IPH2201) | Single agent | Squamous cell carcinoma | Innate Pharma | Phase 1/2 | 2014-12 | Terminated (lack of recruitment) | NCT02331875 |
| Single agent | Gynecologic malignancies | Canadian Cancer Trials Group, Innate Pharma | Phase 1 | 2015-09-17 | Completed | NCT02459301 | ||
| Single agent | Hematologic malignancies | Institut Paoli-Calmettes, Innate Pharma | Phase 1 | 2016-11-28 | Unknown status | NCT02921685 | ||
| Ibrutinib | Chronic lymphocytic leukemia | Innate Pharma | Phase 1/2 | 2015-11-09 | Terminated (sponsor decision) | NCT02557516 | ||
| Cetuximab, Anti-PD(L)1 | Human papillomavirus (HPV) (+) and HPV (−) recurrent or metastatic SCCHN (squamous cell carcinoma of the head and neck) | Innate Pharma, AstraZeneca | Phase 1/2 | 2015-12 | Completed | NCT02643550 | ||
| Durvalumab (MEDI4736), Cetuximab, mFOLFOX6, Bevacizumab | Advanced solid tumors | MedImmune LLC | Phase 1/2 | 2016-02-22 | Active, not recruiting | NCT02671435 | ||
| Durvalumab, Afatinib, Palbociclib, standard of care, Niraparib, INCAGN01876 | Recurrent/metastatic SCCHN | European Organization for Research and Treatment of Cancer – EORTC | Phase 2 | 2017-11-16 | Active, not recruiting | NCT03088059 | ||
| Durvalumab, Oleclumab (MEDI-9447) | Non-small cell lung cancer | MedImmune LLC | Phase 2 | 2018-12-19 | Completed | NCT03822351 | ||
| Durvalumab, Oleclumab, Ceralasertib, DOCETAXEL, Savolitinib | Non-small cell lung cancer patients with PD-1 ICI resistance | Assistance Publique Hopitaux De Marseille | Phase 2 | 2019-10-08 | Active, not recruiting | NCT03833440 | ||
| TIM-3 (T cells, NK cells, APCs) | Sym023 (anti-TIM-3) | Single agent | Advanced solid tumors, lymphomas | Symphogen A/S | Phase 1 | 2018-05-24 | Completed | NCT03489343 |
| TSR-022 | Nivolumab, Docetaxel, Pemetrexed, Cisplatin, Carboplatin, TSR-042 (dostarlimab), TSR-033 | Advanced solid tumor | Tesaro Inc. | Phase 1 | 2016-07-08 | Recruiting | NCT02817633 | |
| TSR-022 | TSR-042 | Liver cancer | University of Hawaii | Phase 2 | 2019-12-19 | Recruiting | NCT03680508 | |
| TSR-022 | TSR-042 | Resectable stage III or oligometastatic stage IV melanoma | Diwakar Davar, Tesaro Inc. | Phase 2 | 2020-06-12 | Recruiting | NCT04139902 | |
| LY3321367 | LY3300054 | Advanced relapsed/refractory solid tumors | Eli Lilly and Company | Phase 1 | 2017-04-12 | Completed | NCT03099109 | |
| BGB-A425 | BGB-A425, LBL-007, Tislelizumab | Advanced solid tumors | BeiGene | Phase 1/2 | 2018-11-13 | Recruiting | NCT03744468 | |
| MBG453 | Spartalizumab | Advanced malignancies | Novartis Pharmaceuticals | Phase 1/2 | 2015-11-23 | Completed | NCT02608268 | |
| MBG453 (sabatolimab) | Decitabine, PDR001(spartalizumab), Azacitidine | Acute myeloid leukemia or high-risk myelodysplastic syndrome | Novartis Pharmaceuticals | Phase 1 | 2017-07-06 | Completed | NCT03066648 | |
| MBG453 | Spartalizumab | Glioblastoma multiforme | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Phase 1 | 2020-02-18 | Active, not recruiting | NCT03961971 | |
| TIGIT (T cells, NK cells) | Tiragolumab (MTIG7192A) | Atezolizumab, Carboplatin, Cisplatin, Pemetrexed, Paclitaxel, Etoposide, Capecitabine, Bevacizumab, Pembrolizumab | Advanced or metastatic tumors | Genentech Inc. | Phase 1 | 2016-05-23 | Active, not recruiting | NCT02794571 |
| Atezolizumab | Non-small cell lung cancer | Genentech Inc. | Phase 1 | 2018-08-10 | Active, not recruiting | NCT03563716 | ||
| Etigilimab (OMP-313M32) | Nivolumab | Solid tumors | OncoMed Pharmaceuticals Inc. | Phase 1 | 2017-05-02 | Terminated (sponsor decision) | NCT03119428 | |
| Domvanalimab (AB154) | Zimberelimab (AB122) | Advanced solid malignancies | Arcus Biosciences Inc. | Phase 1 | 2018-09-12 | Active, not recruiting | NCT03628677 | |
| LILRB1/2 | NGM707 | Pembrolizumab | Mesothelioma, renal cell carcinoma, non-small cell lung cancer, melanoma, breast cancer | NGM Biopharmaceuticals Inc. | Phase 1/2 | 2021-06-09 | Active, not recruiting | NCT04913337 |
| PF-07826390 | Sasanlimab | Non-small cell lung cancer, squamous cell carcinoma of the head and neck, renal cell carcinoma | Pfizer | Phase 1 | 2024-09-06 | Recruiting | NCT06546553 | |
| LILRB1 (T cells, NK cells, APCs) | BND-22 | Pembrolizumab, Cetuximab, Carboplatin, Pemetrexed | Advanced solid tumors | Sanofi | Phase 1/2 | 2021-04-11 | Recruiting | NCT04717375 |
| AGEN1571 | Balstilimab, Botensilimab | Advanced solid tumors | Agenus Inc. | Phase 1 | 2022-07-19 | Active, not recruiting | NCT05377528 | |
| HLA-G (tumor cells, DCs) | TTX-080 | Pembrolizumab, Cetuximab | Advanced solid refractory/resistant malignancies | Tizona Therapeutics Inc. | Phase 1 | 2020-07-14 | Active, not recruiting | NCT04485013 |
| JNJ-78306358 | Single agent | Advanced solid tumors | Janssen Research & Development, LLC | Phase 1 | 2021-10-24 | Completed | NCT04991740 | |
| VISTA (tumor cells, T cells, DCs, macrophages) | PMC-309 | Pembrolizumab | Advanced or metastatic solid tumors | PharmAbcine | Phase 1 | 2023-11-28 | Not yet recruiting | NCT05957081 |
| CA-170 | Single agent | Advanced solid tumors or lymphomas | Curis Inc. | Phase 1 | 2016-05 | Completed | NCT02812875 | |
| SNS-101 | Cemiplimab | Solid tumor, adult, advanced solid tumor, head and neck cancer, breast cancer | Sensei Biotherapeutics Inc. | Phase 1/2 | 2023-05-31 | Recruiting | NCT05864144 | |
| KVA-12123 | Pembrolizumab | Solid tumor, melanoma, carcinoma | Kineta Inc. | Phase 1/2 | 2023-03-03 | Recruiting | NCT05708950 | |
| HMBD-002 | Pembrolizumab | Solid tumor, non-small cell lung cancer, triple negative breast cancer | Hummingbird Bioscience | Phase 1 | 2022-01-01 | Recruiting | NCT05082610 | |
| CI-8993 | Single agent | Solid tumor | Curis Inc. | Phase 1 | 2020-09-22 | Completed | NCT04475523 |
- Note: Data resources come from https://clinicaltrials.gov.
Related inhibitors of the immune checkpoints KIR and NKG2A, which act primarily on NK cells, have also been extensively studied. There is growing evidence that KIR/HLA class-I and NKG2A are associated with the development of malignancies such as lymphoma, NSCLC, colorectal cancer, and leukemia [418-421]. Therefore, current research on monoclonal antibodies to KIR and NKG2A is also a hot topic in antitumor immunity research, often used as monotherapy or in combination with other drugs [422, 423]. For example, clinical evaluation of lirilumab (anti-KIR) in combination with nivolumab (anti-PD-1) or lirilumab in combination with nivolumab and Ipilimumab for advanced refractory solid tumors (NCT01714739). In addition, NKG2A has potential clinical value in viral infections, autoimmune diseases [316, 424]. For example, NKG2A expression is elevated in COVID-19 patients, suggesting that NKG2A inhibitors may be used in combination with potent drugs for the treatment of COVID-19 [425, 426]. NKG2A expression is commonly downregulated in autoimmune diseases. The development of NKG2A antibodies has shown some safety and potential efficacy in clinical trials. For example, the NKG2A antibody monalizumab blocks the interaction of NKG2A with HLA-E, thereby restoring the antitumor activity of NK cells. Clinical trials are currently being conducted to investigate its potential in cancer treatment (NCT06152523). Additionally, some clinical trials are now in progress (Table 2).
In the realm of immunotherapy, ICIs continue to be a focus of research. In addition to the extensively studied CTLA-4, PD-1/PD-L1, LAG-3 and CD47, other ICIs such as CD24, TIM-3, TIGIT, MHC-I and VISTA are also undergoing extensive research and development as well as clinical trials (Table 2). According to https://clinicaltrials.gov, various types of ICIs are being investigated in numerous clinical trials. These studies provide strong evidence that ICIs have the potential for broader applications.
4.2 Combination Therapy of ICIs
The combination of ICIs with conventional therapies (chemotherapy, targeted therapy, or radiotherapy) represents a transformative strategy in oncology, leveraging synergistic mechanisms to enhance antitumor immunity and overcome resistance [427]. Chemotherapy induces immunogenic cell death (ICD), releasing tumor-associated antigens and priming DCs to amplify T-cell activation, while radiotherapy modulates the TME through localized immune activation and systemic abscopal effects [428]. Targeted therapies disrupt immunosuppressive pathways (e.g., VEGF inhibitors normalize vasculature to improve T-cell infiltration) or directly inhibit oncogenic drivers that impair immune recognition [429]. These approaches collectively convert immunologically “cold” tumors into “hot” phenotypes by enhancing immune cell recruitment, functionality, and persistence. Clinically, such combinations address limitations of monotherapy but introduce challenges due to overlapping or amplified toxicities. For instance, in the KEYNOTE-189 trial (NSCLC), pembrolizumab combined with platinum-based chemotherapy significantly improved median overall survival and objective response rate, albeit with increased grade 3–4 treatment-related adverse events, including anemia and neutropenia [430, 431]. In melanoma (CheckMate 067 trial), dual ICI therapy (nivolumab + ipilimumab) achieved a 5-year survival rate of 52%, but severe irAEs such as colitis and hepatitis necessitated glucocorticoid intervention [432]. For triple-negative breast cancer (IMpassion130 trial), atezolizumab-nab-paclitaxel combination therapy demonstrated improved progression-free survival outcomes among PD-L1+ patients, though risks of peripheral neuropathy and neutropenia were elevated [433]. Targeted therapy combinations (e.g., BRAF/MEK inhibitors with ICIs) may exacerbate toxicities like pyrexia and hepatotoxicity [434]. Despite these risks, proactive toxicity management (e.g., early irAE recognition, corticosteroid protocols, and multidisciplinary monitoring) facilitates safer implementation [435]. The combination therapy provides sustained efficacy in a variety of malignancies, significantly enhancing T cell activity and reversing the immunosuppressive state of TME, thereby breaking the response rate limits and resistance of monotherapy, expanding the beneficiary patient population, and extending survival benefits.
The clinical transformation of immune checkpoints has rapidly expanded from monotherapy of classical targets (such as PD-1/PD-L1, CTLA-4) to multi-dimensional intervention of emerging molecules (such as LAG-3, TIGIT, CD47, LILRB family, and Siglec family). At present, PD-1/CTLA-4 dual antibody and LAG-3 inhibitor (Relatlimab) have been approved for melanoma. CD47/SIRRP α and LILRB1 inhibitors targeting myeloid immunosuppression (e.g., Magrolimab, IO-202) have shown advantages in overcoming microenvironmental resistance in hematoma and solid tumors. Emerging strategies such as dual/multispecific antibodies (PD-1 + TIGIT) and metabolically targeted combination therapies (CD73 inhibitors + PD-1) that synergistically block immune escape signals or remodel the metabolic barrier are being validated in clinical trials. With the in-depth analysis of immune checkpoint interaction and drug resistance mechanism, immune checkpoint clinical treatment is opening up a new way to improve the treatment effect of patients and break through the bottleneck of drug resistance.
5 Conclusions and Future Perspectives
Immune checkpoint research has achieved a milestone breakthrough in tumor immunotherapy, but its complexity still poses multiple challenges for clinical translation. For example, irAE associated with ICI treatment is noteworthy. irAE is an autoimmune response induced by ICI treatment, which can affect multiple organ systems, including the skin, intestine, endocrine glands, lung, and heart [436]. The incidence and severity of these adverse events vary according to the type of ICI, the treatment regimen, and individual patient differences [436]. Preventive measures include a comprehensive baseline assessment to identify risk factors before treatment. Explain in detail the symptoms and potential risks of irAE and emphasize the importance of seeking medical attention in a timely manner. Regular monitoring of patients' symptoms and signs during and after treatment for the timely detection and management of irAE. Treatment interventions are graded according to the severity of irAE. Mild irAE (grades 1–2) are usually treated with observation and supportive care, such as topical corticosteroids [436]. Moderate to severe irAE (grades 3–4) require ICIs to be suspended and systemic corticosteroids or other immunosuppressive agents should be used, and symptomatic treatment should be taken according to the specific type and severity [437]. In conclusion, patients' reactions and changes in adverse events should be closely monitored during treatment, and the treatment plan should be adjusted in a timely manner.
In addition to ICIs, ICDs are also worthy of attention. ICD has emerged as a crucial therapeutic strategy in oncology by mitigating immunosuppressive conditions within TME and enhancing tumor responsiveness to immunotherapeutic approaches [438]. ICD is a special mode of cell death, which can stimulate adaptive immune response in the host with perfect immune function [438]. The core mechanism of ICD is through the release of DAMPs, such as cell-surface exposed calreticulin, extracellular released adenosine triphosphate, and high mobility group box 1 protein [438, 439]. These DAMPs act as danger signals and are recognized by PRRs of the innate immune system to activate antitumor immune responses [440]. ICD critically enhances the therapeutic outcomes of ICIs. At the same time, ICI can enhance or complement ICD-mediated immune responses [441]. For example, ICI can relieve immunosuppression in TME and increase the infiltration of immune cells in tumor tissues, thereby enhancing ICD-induced immune responses [441]. The combination of ICD inducers and ICIs can relieve immunosuppression by ICIs, thus achieving better antitumor effects.
With the role of immune checkpoints in tumor therapy becoming more and more widely used, the discovery and research of new immune checkpoint targets is the core direction to break through the bottleneck of current immunotherapy, and its progress depends on the deep integration of multidisciplinary technological innovation and translational medicine. Using single-cell sequencing, spatial transcriptomes, and CRISPR screening techniques, researchers have revealed the unique mechanisms of LAG-3, TIGIT, and SIRPα-CD47 targets in T/NK cell depletion, metabolic regulation, and myeloid immunosuppression, and used artificial intelligence to predict protein interactions to accelerate drug design. However, the complexity of the target, preclinical model limitations (such as mouse immune system differences), and the risk of toxicity due to widespread expression (such as CD39) remain translational challenges. In the future, it is still necessary to integrate multiple disciplines to build a predictable and editable immune regulatory network to achieve precision immunotherapy.
In this review, we have discussed the immune checkpoints that are primarily found on macrophages and T cells, as well as a wide range of other immune cells. The main components, distribution, and mechanism of action of each immune checkpoint have been thoroughly examined. The article concludes with an in-depth discussion of the current major clinical applications of ICIs.
A significant number of ICIs are currently undergoing preclinical and clinical studies. Inhibitors targeting these checkpoints, such as anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies, have shown successful outcomes in the treatment of melanoma and NSCLC. In recent years, additional immune checkpoints have been identified, leading to a surge in research on inhibitors targeting these proteins. The development of these innovative ICIs has introduced new strategies and possibilities for tumor therapy. Despite the significant success of ICIs in clinical applications, several challenges and issues persist. For instance, a large proportion of patients may not derive benefit from ICIs therapy, highlighting the need for reliable biomarkers to identify individuals who are likely to respond to this treatment.
In conclusion, ICIs demonstrate significant potential and promise in tumor therapy. However, further in-depth research and clinical trials are necessary to address existing challenges and enhance therapeutic efficacy. In addition, the combination of multiple ICIs and the use of ICIs in conjunction with other immunotherapies is a major research hotspot in tumor immunotherapy, and future studies need to find more effective means of combining ICIs. With the implementation of more extensive clinical studies and trials, further investigation into tumor-mediated immune evasion mechanisms and targeting of immune checkpoints is poised to become a pivotal weapon in future cancer treatments.
Author Contributions
Han Sun: writing – original draft, methodology, data curation. Dan Huang: formal analysis. Huiling Zhang and Mingyuan Dong: validation. Simiao Wang and Man Sun: investigation. Jiayi Liu: software. Yiqi Wang and Xiaojie Qu: visualization. Xuefeng Li and Zhaogang Yang: writing – review and editing, project administration, resources, funding acquisition, supervision. All authors have read and approved the final manuscript.
Acknowledgments
Data for this study were obtained from https://clinicaltrials.gov. During the preparation of this study, we used ChatGPT-3.5 to improve language. We would like to acknowledge the support of BioRender in creating the figures. This manuscript is supported by the National Natural Science Foundation of China (82422041, X.L.; 21HAA01203, Z.Y.) and Jilin Science-Technology Committee (20230402042GH, Z.Y.).
Ethics Statement
This study did not involve human participants and/or animals or informed consent. Thus, ethical clearance is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.