Transforming Growth Factor-Beta Signaling in Cancer: Therapeutic Implications, Challenges, and Pathways to Progress
Faizah A. Alabi and Izuchukwu F. Okpalanwaka have equal contribution.
ABSTRACT
The transforming growth factor-β (TGF-β) family consists of evolutionarily conserved cytokines that regulate various physiological processes across nearly all tissue and cell types. While TGF-β signaling plays a critical role in immune homeostasis and tissue repair, its dysregulation is implicated in multiple diseases, particularly cancer. Given its dual role in tumor suppression and promotion, TGF-β has emerged as a promising yet challenging therapeutic target. Preclinical studies have demonstrated significant tumor inhibition through TGF-β signaling blockade using diverse classes of inhibitors. However, despite extensive research and clinical trials spanning over two decades, no TGF-β inhibitors have been approved for cancer therapy, underscoring a significant disconnect between preclinical promise and clinical efficacy. This review systematically examines the tumorigenic mechanisms driven by TGF-β and evaluates the therapeutic landscape of anti-TGF-β inhibitors, including receptor kinase inhibitors, neutralizing antibodies, bifunctional ligand traps, integrin-mediated TGF-β therapy, antisense oligonucleotides, TGF-β-targeted vaccines, and various combination strategies. By comparing preclinical and clinical findings, we highlight key challenges and propose novel approaches to improve the translational success of TGF-β-targeted therapies. These insights provide a foundation for optimizing future research and advancing the clinical application of TGF-β inhibitors in oncology.
Graphical Abstract
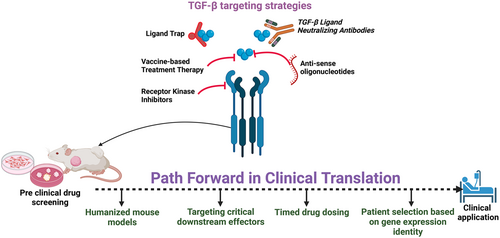
TGF-β inhibition is emerging as a promising cancer therapy, yet translating laboratory success to bedside implementation has suffered significant setbacks. The associated challenges include adverse drug reactions, inadequate predictive models, and activation of alternative signaling pathways. To bolster the therapeutic efficacy of TGF-β blockade, strategies such as optimized dosing regimens, adoption of humanized mouse models that better replicate human disease, and refined patient selection based on genetic profiles are crucial. These approaches aim to address current limitations and improve the translation of TGF-β inhibition from preclinical promise to clinical reality.
1 Introduction
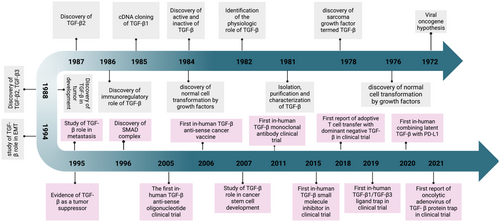
Nearly 40 years ago, researchers made significant strides in understanding how normal cells and fibroblasts transition to a malignant phenotype through overactive signaling pathways. One noteworthy discovery was the heightened secretion of growth factors observed in cancer cells displaying increased anchorage-independent growth In Vitro, mirroring that of cancer cells In Vivo [1]. The growth factor known as the sarcoma growth factor (SGF) led to the identification of the transforming growth factor (TGF) family, which has since been categorized into two types: alpha and beta (TGF-α and TGF-β). Beyond their role in malignant transformation, TGF-α exhibits colony-forming activity when they bind to epidermal growth factor (EGF) in non-neoplastic tissues. TGF-α was specifically differentiated from TGF-β due to its ability to bind EGF. At the same time, TGF-β does not interact with EGF receptors but is essential for inducing malignant transformation in conjunction with EGF receptors [1-3].
By 1981, researchers from two independent groups had successfully isolated, purified, and characterized TGF-β from normal fibroblast tissues (NRK-2B clone 49F) using various techniques, including soft agar colony-forming assays and chromatographic methods [1]. Following this achievement, the TGF-β molecule was identified in humans as comprising two linked 12,500-dalton subunits connected by a disulfide bond. The cloning of the first isoform, TGF-β1 complementary deoxyribonucleic acid (cDNA), revealed a monomer of 112 amino acids synthesized from a 390-residue C-terminal domain of a polypeptide precursor. It soon became evident that TGF-β generally exists in a biologically latent form due to non-covalent associations with the N-terminal domain, which masks the receptor binding domain (RBD) [1]. Activation of the complex requires specific treatments, such as acidification, to unmask the RBD. By the late 1980s, the other two isoforms, TGF-β2 and TGF-β3, were identified, and subsequently, the three receptors for TGF-β were discovered through competitive binding assays [1].
The following decade saw significant advancements in understanding the role of TGF-β in both physiological and pathological contexts such as cancer, summarized in Figure 1. In normal cells, TGF-β is crucial for tightly regulated signaling that governs embryonic development, cell differentiation, and proliferation [3]. However, when this regulation becomes aberrant, it promotes various pathologies, including cancer [4]. TGF-β exhibits multifunctional activities that can suppress or promote tumors, depending on the cellular context. Its tumor-suppressing effects primarily occur in the early phases of tumorigenesis, where it activates tumor suppressor genes and inhibits pro-tumor gene expression, leading to enhanced apoptosis, cell cycle arrest, and autophagy in normal and premalignant cells [4]. Conversely, in the later stages of tumor development, dysfunction in the TGF-β pathway can promote tumorigenesis by enhancing oncogenic characteristics such as stemness, motility, angiogenesis, EMT, metastasis, and immune evasion [1, 5-8]. Consequently, targeting TGF-β has become a critical focus in developing anti-cancer therapies. Yet, despite formulating numerous preclinical strategies to inhibit its activity, pursuing effective treatments for patients has proven challenging, revealing a significant gap in anti-TGF-β therapeutic options.
Over the past two decades, extensive research has focused on developing effective anti-TGF-β therapies for cancer treatment, including small-molecule kinase inhibitors, neutralizing antibodies, ligand traps, vaccines, antisense oligonucleotides, and combinatorial strategies. Despite promising preclinical outcomes, none of these therapies have gained clinical approval, highlighting a crucial gap between experimental success and clinical translation. This review first provides an in-depth overview of the TGF-β signaling pathways, emphasizing their dual role in tumor suppression and promotion and their involvement in epithelial-mesenchymal transition, metastasis, and immunosuppression. We then examine the current landscape of clinical trial strategies, critically analyzing the successes and limitations of existing therapies. Finally, we identify key challenges impeding clinical progress, discuss overlooked factors in therapeutic development, and propose innovative strategies to enhance the effectiveness of anti-TGF-β treatments.
2 Canonical and Non-Canonical Pathways of TGF-β: The SMAD-Dependent and SMAD-Independent
TGF-β signaling operates through multiple pathways, broadly categorized into canonical (suppressor of mothers against decapentaplegic [SMAD]-dependent) and non-canonical (SMAD-independent) mechanisms. This section explores these pathways’ distinct features and functional implications, highlighting their roles in mediating the diverse cellular responses to TGF-β stimulation.
2.1 Canonical Pathway: SMAD-Dependent Signaling
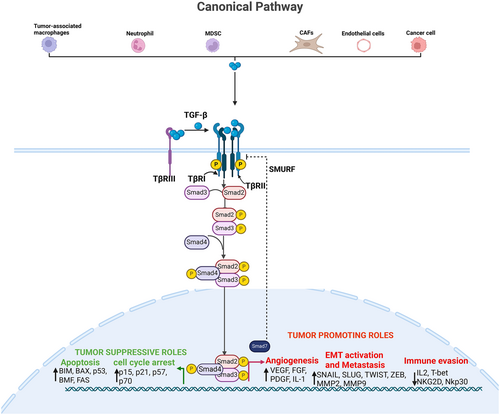
The TGF-β signaling is triggered by phosphorylation and activation of its transmembrane receptors, which then deploy SMAD transcription factors to regulate critical cellular processes. These mechanisms have been comprehensively reviewed elsewhere [3, 9]. Briefly, the TGF-β ligands initially bind to the TGF-β Type III receptors (TGF-βRIII), which then present TGF-β to the TGF-βRI/TGF-βRII complex, which has a high affinity for TGF-β. Activated TGF-β receptors phosphorylate SMAD2/3 on two C-terminal serine residues (SSXS motif). Phosphorylated SMAD2/3 bind SMAD4 to form a heterotrimeric complex, which translocates to the nucleus to activate or restrain target gene expression [10-13]. This is called canonical TGF-β signaling. The biological effects of this signaling are context and tissue-dependent. It could induce growth [14], cell death [15], metastasis [16, 17], differentiation [18, 19], remodeling [20-22], and stress response [23-25] (Figure 2). The concept of context-dependent biological activities of TGF-β is further discussed in Section 3 of this article.
2.2 Non-Canonical Pathway: SMAD-Independent Signaling
Apart from this SMAD-mediated effect, TGF-β signaling can also mediate its biological effect in a SMAD-independent mechanism. This is called non-canonical TGF-β signaling, which occurs via pleiotropic pathways (Figure 3). Multiple SMAD-independent (non-canonical) signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, which comprises extracellular signal-regulated kinase (ERK), jun N-terminal kinase (JNK), and p38 MAPK, phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) are activated in response to TGF-β [11, 12, 23-31]. The TGF-β-activated transforming-beta receptor-I (TβRI) can directly phosphorylate an adapter protein called Src homology domain 2-containing protein, forming a complex with growth factor receptor-bound protein 2 (GRB2) and son of sevenless (SOS) [30]. This cascade activates rat sarcoma (Ras), which sequentially phosphorylates rapidly accelerated fibrosarcoma, mitogen-activated protein kinase (MEK), and ERK, ultimately regulating cell survival, proliferation, adhesion, migration, and metabolism. ERK activation is implicated in multiple diseases, including cancer, making it a crucial pathway in TGF-β signaling.
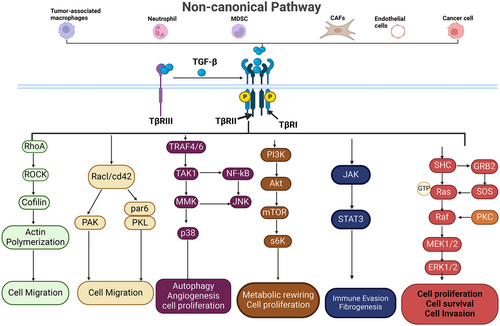
TGF-β also triggers Rho guanosine triphosphate hydrolases (Rho GTPase) signaling to influence cytoskeletal dynamics and cell migration, usually through epithelial-to-mesenchymal transition. For instance, TGF-β activates Ras homolog gene family member A (RHOA), which activates its key effector Rho-associated protein kinase 1 (ROCK1), leading to the activation of Lin-11, Isi-1, and Mec-3 (LIM kinases) to phosphorylate cofilin, a constitutive actin-depolymerizing factor. This phosphorylation cascade ultimately results in the stabilization of the actin cytoskeleton [32, 33]. TGF-β activates other Rho GTPases, such as cell division control protein 42 (CDC42), to engage cytoskeletal rearrangement, EMT, and invasion [34].
Collectively, activation of Rho GTPases by TGF-β reprograms cell dynamics in a way that facilitates cell migration, invasion, and metastatic spread. The TβRI kinase activity can also activate the PI3K/AKT signaling pathway through the classical mechanism involving activation of the PI3K- AKT-MTOR-S6k axis. This signaling is prominent for promoting cancer metabolic reprogramming, cell survival, and proliferation [35].
Another critical signaling cascade activated by TGF-β is the Janus kinase-signal transducer and activator of transcription (JAK/STAT) signaling, particularly involving JAK1 and JAK2 in certain contexts. This includes hepatic stellate cells, where activated JAK phosphorylates STAT3 to mediate the fibrogenic effects of TGF-β signaling [36]. Beyond fibrosis, JAK/STAT signaling is also implicated in immune regulation, wound healing, and tumor progression [37], making it a key pathway in TGF-β-mediated physiological and pathological processes.
The TGF-β/SMAD signal transduction pathway is tightly regulated through positive and negative mechanisms at multiple stages. A key negative feedback mechanism involves SMAD7, an adaptor protein induced by TGF-β transcriptional activity, which recruits SMAD ubiquitination regulatory factor (SMURF) ubiquitin ligases to the receptor complex, leading to its degradation via proteasomal and lysosomal pathways (Figure 2). In addition, autophosphorylation of serine residues on Type II receptors results in positive and negative regulation [38]. For instance, Luo and Lodish, using mouse and human cell lines, showed that phosphorylation of Ser213 activates transforming-beta receptor II (TβRII) while phosphorylation of Ser416 inhibits its kinase activity [39]. The appropriate regulation of the TGF-β signaling pathway prevents congenital and acquired musculoskeletal disease [3, 40, 41], like the accumulation of extracellular matrix (ECM)—loss of its regulation results in impaired regeneration of both inherited and acquired neuromuscular disorders. Overexpression of TGF-β in mice has been reported to induce bronchopulmonary dysplasia [42, 43], hydrocephalus [44-46], and impaired skin [47]. In humans, overactivation of TGF-β results in heritable Camurati-Engelmann disease (CED), seen as hyperostosis, sclerosis of the bones [48, 49] and cancer. When TGF-β function is lost in humans due to mutations in TGF-β or its receptor, aortic aneurysms and skeletal fragility are seen [50-52]. These findings underscore the importance of tightly regulated TGF-β signaling in maintaining tissue homeostasis, which is essential for preventing various pathological conditions.
3 TGF-β in Tumorigenesis: A Double-Edged Sword
TGF-β plays a paradoxical role in cancer progression, acting as a tumor suppressor in early-stage cancers and a promoter of metastasis in advanced malignancies—a phenomenon known as the “TGF-β paradox”. This section explores the diverse functions of TGF-β in tumorigenesis, emphasizing its context-dependent signaling and dual impact on cancer development and progression.
3.1 The Anti-Tumor Roles of TGF-β
The anti-tumorigenic role of TGF-β is predominantly highlighted in the early phases of cancer development, wherein it acts to suppress cancer cell proliferation, induce programmed cell death (apoptosis), ensure genomic stability, and augment immune surveillance. These functions are critical for maintaining cellular homeostasis and preventing the development of malignant cells. The subsequent section delineates this activity in greater detail.
3.1.1 TGF-β Promotes Apoptosis of Tumor Cells
Apoptosis is a cellular self-destruction process that eliminates unwanted or damaged cells, vital for developing and maintaining tissue health. Additionally, it serves as a crucial mechanism for suppressing tumor growth by eliminating severely damaged and dysfunctional cells. Apoptosis can be triggered through the intrinsic or extrinsic pathways, activating caspases, which destroy cells [53]. The process is regulated by B cell lymphoma (Bcl-2) family proteins, which consist of anti-apoptotic (Bcl-2 and Bcl-xl) and pro-apoptotic members; Bcl-2-associated protein x (Bax), Bcl-2-associated agonist of cell death (Bad), BH3 interacting-domain death (Bid) [54]. These proteins control programmed cell death by regulating the permeabilization of the outer mitochondrial membrane [55]. TGF-β induces apoptosis in numerous cell types via SMAD-dependent and -independent mechanisms. SMAD-dependent pathway proapoptotic effects are modulated by specific transcription factors, such as p53, which is coregulated by p38 and SMAD proteins [13, 56]. Pro-apoptotic proteins induced by the SMAD-dependent pathway, including transforming growth factor-beta inducible early response gene (TIEG1), cause oxidative stress via the death-associated protein kinase (DAPK) pathway, which triggers apoptosis through the release of mitochondrial cytochrome c and phosphatidylinositol-3,4,5-triphosphate 5-phosphatase (SHIP), a member of inositol-5-phosphatase that suppresses survival-promoting cell death through the PI3K/AKT pathway [57]. Other SMAD-dependent pro-apoptotic genes include Bcl-2-interacting mediator of cell death (BIM), Bcl-2-modifying factor (bBMF), BAX, and caspase 9, expressed via the stress-activated protein kinase/c-Jun NH(2)-terminal kinase (SAPK/JNK) pathway when TGF-β activates SMAD4 [57]. The adapter death-associated protein 6 (DAXX) modulates the Fas-mediated apoptotic pathway by stabilizing TβRII, leading to JNK activation. Apoptosis-related protein (ARTS) in the TGF-β signaling pathway is a septin-like protein that is necessary for the TGF-β apoptotic response in carcinoma cells that are resistant to TGF-β-induced growth inhibition by translocation of this mitochondrial protein to the nucleus [3, 58]. TGF-β has been reported to induce apoptosis in Burkitt's lymphoma (BL), prostate cancer, pancreatic ductal adenocarcinoma (PDAC), colon cancer, B cell lymphoma, lung cancer, hepatoma, and melanoma [59-63].
3.1.2 TGF-β Promotes Cell Growth Arrest
Cell cycle regulation, frequently described as the “accelerating and braking” process, plays a central role in cell growth. From a mechanistic perspective, a typical cell cycle consists of four distinct phases: Gap (G1), synthesis (S), Gap (G2), and mitosis (M). These phases are tightly controlled by two groups of regulatory proteins—cyclins and cyclin-dependent kinases (CDKs), which orchestrate the progression through each cell cycle stage [64, 65]. While CDKs 2, 4, and 6, along with cyclins A2, B1, B2, D1, D2, D3, E1, E2, and G1, drive the cell through the cell cycle to promote cell growth, CDK inhibitors (CDK-Is) such as p16, p21, p27, and p57 antagonize these activities to induce growth arrest [66]. TGF-β is a tumor-suppressor that prevents cell proliferation and promotes differentiation in normal or premalignant cells [67]. For instance, TGF-β causes growth arrest of most cell types, such as in normal epithelial and hematopoietic cells [57]. Targeting CDKs and their inhibitors (CDK-Is), which regulate cell cycle progression after the G1 phase and suppress c-Myc—a critical transcriptional inducer of cell growth and protein expression that helps mediate cell differentiation—are mechanisms through which transforming TGF-β suppresses cell growth [68, 69]. The CDK inhibitors involved are cell-type and context-dependent. CDK-Is inhibit CDK complexes in hematopoietic stem cells, leading to G1 phase cell cycle arrest [70]. TGF-β induced SMAD3-SMAD4 protein complex mediates the downregulation of Myc in keratinocytes and mammary epithelial cells [71]. Other SMAD-independent pathways downstream of TGF-β have also been implicated in the antiproliferative response. For example, dephosphorylation of p70S6K by PP2A, thus leading to cell cycle arrest [67, 72]. In UV-induced skin cancers, TGF-β exerts a growth-inhibitory effect on the keratinocytes via SMAD3 signaling [73].
3.2 The Pro-Tumor Roles of TGF-β
In malignant and advanced tumors, the overexpression of TGF-β cells is common in most tumor microenvironments (TME), leading to various tumor-promoting processes [22]. Increased oncogene expression, the release of immunosuppressive cytokines, and epithelial plasticity are all linked to TGF-β overexpression during tumorigenesis [74]. In the TME, stromal fibroblasts and other cancer cells release TGF-β, which shapes the tumor's architecture while suppressing the immune cells’ anti-tumor function, ultimately leading to tumor proliferation and progression [74]. TGF-β's pro-tumor activities result from its involvement in various tumor developmental processes, which we will discuss below. Here, we will explore the effects of TGF-β on different aspects of tumor growth.
3.2.1 TGF-β Supports Tumor Angiogenesis
To proliferate and metastasize, tumor cells often acquire and retain the ability to form blood vessels due to the increased demand for oxygen and nutrients in the TME [75, 76]. This phenomenon, known as angiogenesis, is the formation of new blood vessels from pre-existing vessels and the primary mechanism tumors use to increase their oxygen and nutrient supply. TGF-β plays a vital role in tumor angiogenesis in several ways. TGF-β influences the growth of endothelial cells, which form blood vessels, resulting in an angiogenic effect [77]. It is important to note that the impact of TGF-β on endothelial cells depends on the context. In a normal physiological state, TGF-β inhibits endothelial cell growth [78]. However, in an inflammatory or diseased state, such as in many cancer environments, TGF-β promotes endothelial cell growth, movement, and survival by increasing the expression of angiogenic factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), interleukin-1alpha (IL-1α), basic fibroblast growth factor (bFGF), and tumor necrosis factor-alpha (TNF-α) [79]. The ability of TGF-β to promote tumor proliferation in the TME is attributed mainly to its proangiogenic effects. TGF-β indirectly modulates the expression of VEGFs by stimulating pro-angiogenic proteolytic enzymes such as matrix-metalloproteinases (MMPs), particularly MMP2 and MMP9 [80]. These MMPs are essential for releasing VEGF from the ECM by degrading the ECM and basement membrane, thus facilitating endothelial cell migration and vessel sprouting [80]. The released VEGF, through VEGFR1 and VEGFR2, drives tumor angiogenesis. MMPs play a crucial role in regulating the availability of VEGF, a well-known angiogenic factor. VEGF, in turn, promotes the migration of endothelial cells into hypoxic tissues, thereby necessitating the formation of new blood vessels to supply oxygen and nutrients [81]. In Vitro assays with retinoblastoma cells have demonstrated reduced angiogenesis and VEGF expression following inhibition of MMP-2 and MMP-9 [82].
3.2.2 Metastasis Through Epithelial-Mesenchymal Transition
Metastasis, the dissemination of cancer from its original location to a distant organ, is the foremost cause of fatality among cancer patients. A pivotal process in a metastatic spread is the EMT [83]. EMT plays a critical role in cancer progression and metastasis as the TME is known to harbor cancer-associated fibroblasts (CAFs), inflammatory cells, and other tumor growth components [84, 85], potent producers of TGF-β. During the mid to late stages of tumor growth, an increase in TGF-β expression leads to the formation of CAFs and the activation of MMPs [86, 87]. MMPs are zinc-dependent endopeptidases known to disrupt the basement membrane and facilitate cell migration, which is crucial for tumor metastasis [81]. Due to their ability to break down collagen and laminin-5, MMPs play a role in remodeling the basement membrane, ultimately enhancing cell migration and invasion [88]. Among the MMP family, MMP2 is particularly significant in promoting cell migration [82]. Overexpression of MMP2 has been linked to lymph node metastasis and poor prognosis in NSCLC patients [89]. TGF-β-induced, MMP2-mediated cell migration and metastasis occur through SMAD2/3 signaling [90]. Recent findings indicate that the inflammatory cytokine TNF-α plays a crucial role in CAF-mediated metastasis. TNF-α increases the expression of the Type 1 TGF-β receptor, which induces EMT through SMAD2/3 Signaling involving activin A and integrin alpha-V (ITGAV). As indicated earlier, SMAD2/3-signaling pathways are the central pathways in which TGF-β exerts biological influences [91]. Evidence shows that TGF-β promotes tumor metastasis through SMAD2/3 pathways [92]. In the presence of TNF-α and TGF-β, cultured oral squamous cell carcinoma (SCC)-derived epithelial cells undergo an epithelial-to-mesenchymal transition in response to signaling factors produced by TGF-β [74, 93]. The importance of SMAD2/3 signaling in the TGF-β-induced EMT was further highlighted when the inhibition of the TGF-β1/SMAD2/3 pathway by Glaucocalyxin reverses EMT in osteosarcoma [94]. TGF-β induced EMT through SMAD-mediated activation of EMT transcription factors such as SNAIL, SLUG, TWIST, zinc finger E-box binding homeobox (ZEB1), ZEB2, forkhead box C2 (FOXC2), forkhead box transcription factor (FOXA1), FOXA2, peroxiredoxin 1 (PRX1), and high mobility group AT-hook 2 (HMGA2), which repress the expression of epithelial markers while promoting mesenchymal phenotypes [95]. Gong et al. noted a decrease in brain metastasis of MDA-MB-231 breast cancer following the knockdown of angiopoietin-like 4 (ANGPTL4) that led to an increase in TGF-β2 via SMAD3/4 signaling [96]. SMAD3/4 is crucial in promoting the TGF-β-induced EMT [97, 98]. In a similar observation, the expression of dominant negative versions of SMAD2/3 has been shown to inhibit EMT [99, 100]. This role of SMAD2 is supported by the understanding that the SNAIL gene is upregulated in the absence of SMAD2, leading to increased fusion of the promoter of the SNAIL gene with SMAD3/4 complex, which enhances EMT [98]. A complex of SNAIL1, SMAD3, and SMAD4 represses the activities of genes promoting the expression of CAR, a tight-junction protein, and E-cadherin, hence supporting the TGF-β-induced EMT in breast epithelial cell metastasis [101]. Patients with metastatic skin cancer often show SMAD2 deficiency, suggesting an association between SMAD2 deficiency and skin tumor formation and metastasis [102].
Although TGF-β plays a vital role in inducing EMT in a SMAD-dependent manner, recent data suggest that TGF-β can also promote tumor metastasis through non-SMAD-independent mechanisms [31]. For instance, the activation of the MAPK pathway enhances TGF-β-dependent EMT. In addition, TGF-β promotes detachment of adherent junctions and cell migration by the ERK signaling in various tissues such as epithelial and endothelial cells, fibroblasts, colon cancer, and breast cancer cells [103-105]. Increased gene expression of Ras, MEK1/2, and ERK1/2 was observed when a murine mammary epithelial cell was treated with TGF-β1 and increased levels of phosphorylated ERK and ERK kinase activity [106, 107]. Reduced phosphorylated ERK and ERK kinase activity levels were evident in the presence of MEK inhibitors associated with TGF-β1-induced EMT inhibition [29]. This supports the notion that the activation of the ERK signaling pathway by TGF-β is required for TGF-β1-induced EMT [108]. In the In Vivo model of cervical cancer, TGF-β signaling promoted metastasis via circular RNA (CDR1as) [16]. Also, TGF-β has been shown to enhance cluster of differentiation 36 (CD36)-mediated EMT in different cervical In Vitro and In Vivo models [109]. With an increasing understanding of the role of TGF-β in tumor metastasis, this presents an important area in targeted therapies hoping to limit tumor metastasis.
3.2.3 Immune Evasion
Since sustained immune system activation can induce inflammation and tissue damage, the immune system is often rewired to prevent excessive activation. Tumor cells and other cells in the TME take advantage of these immunological safeguards by overproducing immunosuppressive cytokines, particularly TGF-β. By inhibiting various components of both innate and adaptive immunity, TGF-β creates a favorable environment for tumor growth (Figure 4). Hence, TGF-β signaling is an essential part of tumor immunosuppression.
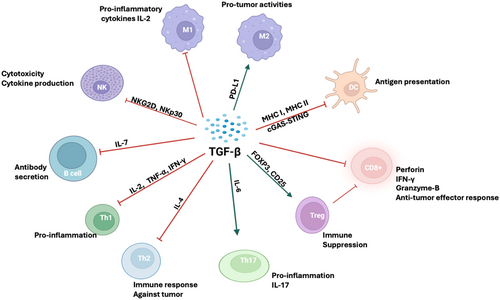
3.3 TGF-β-Driven Immune Tolerance in T Cells
In the adaptive arm of the immune response, TGF-β activation in the TME is associated with significant suppression of T-cell differentiation, activation, proliferation, and cytokine production, leading to loss of T-cell effector function (Figure 4). This T cell-dependent anti-tumor inhibition occurs via various mechanisms. In an In Vitro study evaluating the co-culture of naïve T cells with TGF-β, it was found that naïve T cells could not differentiate into a Th1 response. In contrast, mice lacking TGF-βR2 exhibited a significant Th1 response and increased sensitivity to TCR stimulation [22, 110]. Mechanistically, the absence of TGF-βR2 in CD4+ CD8+ T cells results in enhanced TCR-dependent activation in response to weak antigenic stimulation, leading to improved functionality characterized by increased expression of IFN-γ and granzyme B [22, 110] that infiltrates into the tumor beds and tumor-draining lymph nodes [tDLN], indicating a role of TGF-β in preventing tumor-specific T cell priming. Transgenic mice with dominant-negative TGF-βR2 in CD4+ and CD8+ T cells rejected cancer cells upon inoculation, with expansion of tumor-specific CD8+ T cells [22].
Additionally, TGF-β silences critical cytokines such as IL-2, which induces T cell proliferation, interferon-γ (IFNγ), and cytolytic molecules like granzymes [22, 111]. This also inhibits the expression of T-Box expressed in T cells (T-BET) and eosodermin (EOMES), which support effector and memory T cell function [79, 112]. Activating the nuclear factor of activated T cells (NFAT) is vital for differentiating helper T cells, particularly Th1 and Th2 cells, via the interleukin-2 inducible T cell kinase (ITK). TGF-β can inhibit ITK phosphorylation, reducing calcium influx and impairing NFAT activation, contributing to reduced T cell differentiation and proliferation [113]. Furthermore, TGF-β can also downregulate the expression of key transcription factors critical for the development of Th1 and Th2 cells, such as GATA-binding protein-3 (GATA-3) and T-Box expressed in T cells (TBET), in CD4+ T cells [22].
Moreover, it is well established that prolonged antigen stimulation of cytotoxic T lymphocytes (CTLs) results in exhaustion of T cell function, marked by high expression of inhibitory receptors, including programmed cell death protein 1 (PD-1). TGF-β transcriptionally enhances PD-1 expression by recruiting SMAD3 to the programmed cell death protein 1 (PDCD1) promoter region [114]. In another instance, TGF-β-mediated T memory cell exhaustion is due to the upregulation of CD70 on effector T cells [115].
The induction of forkhead box P3 (FOXP3) by TGF-β can promote the development of CD4+CD25+ regulatory T cells (Tregs), a known mechanism of T cell immunosuppression [22]. In a seminal study by Yang and colleagues [116], TGF-β secreted by lymphoma cells promoted regulatory T (Treg) cells’ function, mediated by the enhanced FOXP3.
3.4 TGF-β as a Modulator of B Cell Immunosuppression
The humoral response of B cells is driven by the secretion of immunoglobulins (Igs). For this process to take place, a transition from membrane-bound Igs to soluble Igs is essential. This switching mechanism can be inhibited by TGF-β, which selectively suppresses immunoglobulin lambda (Ig λ) light chains and the isotypes immunoglobulin M (IgM) and immunoglobulin G (IgG) while preventing class switching to immunoglobulin A (IgA). However, the IgA induced by TGF-β is insufficient to support robust immune responses, such as antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis (ADCP) [3]. Mechanistically, TGF-β1 and TGF-β3 hinder key molecules involved in cell cycle division and transcription factors critical for the differentiation into antibody-secreting cells, such as interferon regulatory factor 4 (IRF4), B-lymphocyte induced maturation protein-1 (Blimp-1), and x-box binding protein-1 (XBP1) [117]. Additionally, TGF-β downregulates the secretion of the stromal factor IL-7, which is vital for the proliferation and survival of early lymphoid progenitors of B cell precursors. Furthermore, TGF-β reprograms the balance of pro-apoptotic and anti-apoptotic genes by upregulating BIK and downregulating BCL-XL, ultimately leading to B cell apoptosis [111].
3.5 TGF-β as a Suppressor of Innate Myeloid Function: Antigen-Presenting Cells (APCs), Macrophages, Neutrophils
In addition, TGF-β signaling suppresses the innate immune response against tumors, facilitating their progression. Studies have shown that it inhibits cell death mediated by macrophages and monocytes by promoting a shift in macrophage polarization from pro- to anti-inflammatory (M1 to M2), which reduces their effectiveness and increases the release of anti-inflammatory cytokines, thus worsening the immunosuppressive environment of the tumor [118, 119]. TGF-β also diminishes the anti-tumor activity of neutrophils as blockage of TGF-β leads to recruitment of neutrophil-attracting chemokines C-X-C motif chemokines (CXCL12 and CXCL5) and cytokines (TNF-α and IFNγ) that expresses a high level of lymphocyte antigen 6 complex locus G (CD11b+/Ly6G+) neutrophils [120]. In colorectal cancer (CRC), TGF-β mediates the polarization of tumor-associated neutrophil (TAN) from pro-tumor (N2) to anti-tumor (N1) via PI3K/AKT signaling on TANs and TGF-β/SMAD signaling in the tumor cells [121]. Additionally, on dendritic cells (DCs), TGF-β suppresses the expression of major histocompatibility complex II (MHCII), hindering antigen recognition [122].
In the TME, TGF-β-mediated immunosuppression can also be facilitated through paracrine or autocrine induction of CAF [123] and activation of MMPs [22]. The primary mechanism through which TGF-β facilitates immune exclusion is via CAF signaling, which leads to increased ECM deposition within the TME, subsequently impeding the infiltration of CD8+ T cells. In a sophisticated study, a class of ECM genes called cancer-associated ECM (C-ECM) has been identified [124]. These genes, present in hot and cold tumors, were significantly more abundant in ICB non-responders than in responders [124]. In addition, TGF-β-induced CAFs can exert inhibitory effects on anti-tumor immune cells through the polarization of anti-tumor myeloid and NK cells into a pro-tumor phenotype. This process may also occur via the secretion of physical barrier proteins, such as fibrils in the ECM, which have the potential to impede the infiltration of cytotoxic T cells [125, 126]. Taken together, these observations indicate that TGF-β signaling undermines the immune function of various cell types crucial to the body's anti-tumor defense system, including natural killer (NK) cells, CD4+ and CD8+ T cells, macrophages, neutrophils, and B cells [59, 127].
Additionally, the increased secretion of factors such as indoleamine 2,3-dioxygenase (IDO), interleukin-10 (IL-10), and arginase can redirect DCs toward an immature phenotype characterized by immunosuppressive features [128]. In melanoma, breast cancers, and other solid tumors, TGF-β signaling upregulates IDO in plasmacytoid DCs and CCL22 in myeloid DCs. This DC phenotype enhances the migration of regulatory T cells (Tregs) and contributes to overall immunosuppression [129].
Another crucial aspect of the innate immune system is the cyclic GMP-AMP synthase (cGAS-STING) pathway. Activating this pathway produces Type I IFNs and various inflammatory cytokines, significantly enhancing anti-tumor immunity [130, 131]. The cGAS-STING pathway is acknowledged as a tumor-suppressive mechanism that can facilitate the maturation and mobilization of APCs [131, 132]. When its activation is altered, it results in increased tumor survival and metastasis. For example, in multiple myeloma (MM), Tregs-induced TGF-β1 secretion was shown to inhibit the cGAS-STING signaling pathway, suppressed major histocompatibility complex (MHC-I) and upregulate programmed death ligand 1 (PD-L1), thereby contributing to a tumor-progressive microenvironment [133]. Interestingly, either neutralizing TGF-β1 or activating cGAS-STING pathway rescued the effect of Tregs on the tumor antigen presentation and immunosuppressive signal in MM cells, an effect that was faithfully recapitulated in the mouse model. This model highlights the critical role of the Treg-TGF-β1-cGAS-STING axis in mediating TGF-β1-driven immunosuppression, particularly in MM. Furthermore, accumulation of TGF-β in the TME of advanced tumors has been found to shift the balance of cGAS-STING from tumor surveillance to tumor progression by downregulating cGAS-STING levels in gamma-delta (γδ) T cells, aiding immune evasion [134].
3.6 TGF-β as a Negative Regulator of NK Cells
NK cells possess a remarkable ability to kill, making them a powerhouse of the innate immune system. However, TGF-β signaling diminishes the cytotoxic capacity of NK cells by downregulating NK group-2 member D (NKG2D) and NK cell protein 30 (NKp30), two critical activating surface receptors involved in eliminating cancer cells [135]. Furthermore, the inhibition of E4-promoter binding protein-4 (E4PBP4) by SMAD3 can further impede the development of NK cells [136]. Additionally, the negative regulation of cytolytic proteins such as granzymes and perforin via SMAD signaling can also compromise the cytotoxic potential of NK cells [137]. Another notable observation is how tumor cells exploit the plasticity of NK cells. Analysis of tumor samples revealed that the presence of TGF-β in the TME encourages the transdifferentiation of NK cells into innate lymphoid cells Type-1 (ILC1), which lack cytotoxic function, in a SMAD4-independent TGF-β signaling pathway [138, 139].
4 Targeting TGF-β for Cancer Therapy
The inhibition of TGF-β in cancer therapy is of great interest due to its multifaceted role in cancer development and progression. Various preclinical and clinical trials have explored approaches such as blocking TGF-β binding to its receptor, inhibiting TGF-β receptor kinase, and preventing its activation. Figure 5 shows various classes of anti-TGF-β that have been explored for cancer therapy. TGFβ-1 is the most dominant isoform and has demonstrated superior effectiveness in targeting TGF-β in cancer. However, blocking all three isoforms has been linked to adverse effects such as heart complications, skin issues, and increased susceptibility to new malignant tumors, highlighting the importance of understanding the diverse roles of these isoforms in different cancer scenarios [71, 92].
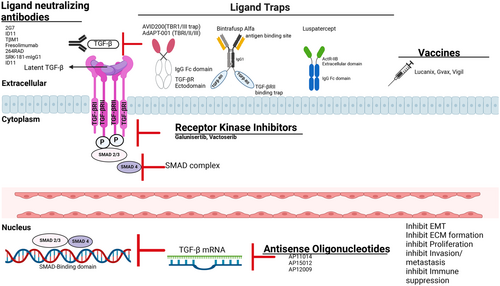
4.1 Small Molecule Kinase Inhibitors
The binding of the TGF-β receptor to its receptors (TGF-β1, 2, 3) initiates a cascade of phosphorylating events leading to the activation of SMAD2/3 and SMAD4 [140]. The phosphorylated SMADs then form a “SMAD complex” and translocate into the nucleus to increase the expression of genes that regulate tumor growth and division. Galunisertib (LY2157299) is a small molecule experimental inhibitor of TGF-β receptor kinase Type 1 that prevents the binding of adenosine triphosphate (ATP) to TGF-β thus, blocking the downstream activation of SMAD2/3 complexes and activation of TGF-β [141]. As demonstrated in the Phase 1 trial, the inhibition of these complexes prevents the development of lung and breast cancer with a safe therapeutic profile in various solid tumors [142]. It has a selective capacity to inhibit the phosphorylation of serine/threonine residue, thereby preventing the downstream “SMAD complex” formation. In addition to its primary function, these inhibitors also act on other TGF-β-related protein targets such as p-P38 MAPK, PI3K/AKT, nodal, activin, and myostatin [142, 143], endowing them with pleiotropic activity against both canonical and non-canonical TGF-β signaling. However, the safety and pharmacokinetics were evaluated in a Phase 1 clinical trial of Galunisertib with Durvalumab, an anti-PD-L1. The combined treatment failed to elicit a superior efficacy over individual drugs. The median overall survival (OS) and progression-free survival (PFS) were 5.72 months and 1.87 months, respectively [144]. A non-randomized Phase II/III study of LY2157299, in patients with very low, low, medium, and intermediate risk-myelodysplastic syndromes (MDS) showed an acceptable pharmacological and safety profile with a linked hematological recovery (24.4%) in patients with lower and intermediate-risk MDS, as well as responses in patients that depend hugely on transfusion and those with evidence of early stem cell differentiation block [145]. In addition, 43.9% attained erythroid response, and 44% had a consequential debility reduction.
The therapeutic activity of Galunisertib (NCT01246986) was examined in second-line hepatocellular carcinoma (HCC) patients in baseline serum alpha-fetoprotein (AFP). Median time-to-progression (TTP) and OS were 2.7 months, 7.3 months (n = 102), and 4.2 months, 16.8 months (n = 40), respectively, majorly observed in patients with a normal fetoprotein and reduced TGF-β1. The commonly seen treatment-related adverse effects were increased bilirubin, hypoalbuminemia, embolism, anemia, fatigue, and neutropenia. Overall, treatment with Galunisertib displayed a decent safety profile and more prolonged survival in AFP and TGF-β1 responders [146]. A Phase II research combination of Galunisertib and Lomustine (NCT01582269) in patients with recurrent glioma failed to elicit a better OS than placebo plus Lomustine [147]. In a Phase II trial of resected pancreatic cancer patients, a combination of Galunisertib and gemcitabine resulted in a boost in OS in patients treated with the combination (10.9) as against those treated with Gemcitabine and placebo (7.2 months) [144]. Another Phase 1b dose-finding/escalating study evaluated the safety, tolerability, and efficacy of Galunisertib (LY2157299) in combination with Durvalumab, an anti-PD-L1 in recurrent/refractory metastatic pancreatic cancer, displayed a limited therapeutic efficacy, with OS of and PFS of 5.72 and 1.87 months, respectively. The combination pharmacokinetic profile commensurate to the individual drugs and lack of association between probable biomarkers and treatment outcomes suggests treatment regimen as an earlier treatment line and in patients with predictive TGF-β1 inhibition [144].
Another possible anti-cancer agent, Vascosertib, is an orally available inhibitor of activin-receptor-like kinase 5 (ALK5) that has been implicated in aiding many tumor growths [148]. By inhibiting TGF-βR1 overexpression, tumor development slows down, and the host's immune response is restored. With IC50 of 17.3 nM, Vactosertib inhibits ALK-4 in different mouse models, including osteosarcoma [149-153]. Through tumor intrinsic and extrinsic mechanisms, Vactosertib exhibited potent anti-tumor effects in various solid tumors, including MM [154]. Numerous completed studies have reported on the safety and effectiveness of Vactosertib against different cancer types delineated in the table (Table 1). In a Phase I first-in-human dose escalation of Vactosertib in subjects with advanced malignant tumors, the safety, tolerability, pharmacokinetics, and an expansion phase to determine the recommended dosing for Phase II was carried out. Due to its short half-life, multiple once-per-day dosing was postulated [155]. Several ongoing and completed trials are now in the pipeline to evaluate the efficacy of Vactosertib as a combination therapy. Vactosertib, in combination with pembrolizumab, underwent an open-label multi-center trial to evaluate its safety, tolerability, and anti-cancer effect in patients with microsatellite-stable metastatic CRC [156].
| Target | Treatment type/phase | Cancer type | Major adverse events | Key outcome measures | Trial ID |
|---|---|---|---|---|---|
| SMALL MOLECULES | |||||
| LY32000882: TGF-βR1 inhibitor | LY32000882 + Pembrolizumab | Advanced cancer | / | / | NCT04158700 (W) |
| LY32000882 + Capecitabine monotherapy | CRC | / | / | NCT04031872 (US) | |
| LY32000882 + Pembrolizumab/Gemcitabine + Paclitaxel + Cisplatin + radiation | Advanced cancer | Grade 3-related toxicities observed in combination arm; cardiovascular toxicity | Safe and well tolerable with anti-tumor activity | NCT02937272 (C) | |
| Galunisertib + Capecitabine (I/II) | CRC | / | Dose, response rate, OS, PFS | NCT03470350 (W) | |
| Galunisertib (I) | Cancer | / | Immunotherapeutic intervention, pharmacokinetics | NCT02304419 (R) | |
| Galunisertib + Durvalumab (I/Ib) | Metastatic pancreatic cancer | No reported serious AEs | Disease control rate (25%0, PFS 1.87 months), OS (5.72 months) | NCT02734160 (CWR) | |
| Galunisertib + Nivolumab | Solid tumor, NSCLC | Fatigue, decreased appetite, pruritus, Grades 1–3 treatment-related AEs | Duration of response (7.43 months), PFS (5.26 months), OS (11.99 months) | NCT02423343 (CWR) | |
| Galunisertib (II) | Myelodysplastic syndrome | Grades 1–2 AEs, fatigue, diarrhea, pyrexia, vomiting | Erythroid response (43.9%), improved hematologic response (32.1%), reduced fatigue (44%) | NCT02008318 (CWR) | |
| Galunisertib + chemoradiation (II) | Advanced rectal adenocarcinoma | Grades 1–2 treatment-related AEs | 2 years PFS (81.5%0, 2 years OS (97%), 1 year CR (5 patients), improved immunologic response | NCT02688712 (CWR) | |
| Galunisertib + stereotactic body radiotherapy (SBRT) (I) | Advanced hepatocellular cancer | Fatigue, nausea, abdominal pain, vomiting, decrease white blood cell count, Grades 1–2 AEs | PFS (3.68 months), PR (13.3%), DCR (53%) | NCT02906397 (CWR) | |
| Galunisertib + Sorafenib (Ib) | Hepatocellular carcinoma | / | Dose-Limiting toxicities and pharmacokinetics to be measured | NCT02240433 (US) | |
| Galunisertib + Sorafenib (II) | Hepatocellular carcinoma | / | OS and pharmacokinetics to be measured | NCT02178358 (US) | |
| Galunisertib + Radiotherapy (I) | Breast cancer | / | AEs, treatment-related response, immunologic response | NCT02538471 (T) | |
| Galunisertib + Paclitaxel/Carboplatin (IB) | Ovarian, uterine, fallopian tube or peritoneal carcinosarcoma | / | Proportion of patients that completed the treatment, PFS, OS, OR | NCT03206177 (US) | |
| Galunisertib + Paclitaxel | Triple negative breast cancer | / | AEs, MTD, ORR, PFS | NCT02672475 (US) | |
| Galunisertib + Gemcitabine (II) | Pancreatic cancer | Grades 3–4 AEs, neutropenia, thrombocytopenia | OS (10.9 months), PFS (3.65 months), ORR (8.7 months) | NCT01373164 (CWR) | |
| Galunisertib (I) | Advanced solid tumor | AEs including natriuretic peptide increased, leukopenia, rash | Acceptable safety profile | NCT01722825 (CWR) | |
| Galunisertib + Enzalutamide (II) | Prostate cancer | / | PFS, OS, treatment-related AEs | NCT02452008 (R) | |
| Galunisertib + Lomustine (II) | Glioblastoma | / | OS, Pharmacokinetics, anti-tumor activity | NCT01582269 (ANR) | |
| Galunisertib + Temozolomide-based radio chemotherapy (Ib/IIa) | Malignant glioma Brain glioma |
Safety, tolerability, pharmacodynamic profile, PK, efficacy, changes in T cells subset | OS (18.2 months), PFS (6 months), DCR (80%) | NCT01220271 (CWR) | |
| Galunisertib (II) | Advanced hepatocarcinoma | Grades 3–4 AEs, hypoalbuminemia, neutropenia, thrombocytopenia, abdominal pain, constipation, drug eruption, pruritus, gall bladder perforation | OS (16.8 months), OS (21.8 months) | NCT01246986 (CWR) | |
| Galunisertib + Lomustine (I) | Glioma | / | Dose, PK, tumor response | NCT01682187 (ANR) | |
| Galunisertib + Capecitabine (I/II) | CRC | / | Recommended dose, response rate, AEs, OS | NCT04031872 (ANR) | |
| Galunisertib + Palbociclib (II) | Nasopharyngeal carcinoma | / | PFS, OS, AEs | NCT04605562 (NYR) | |
| Vactosertib TβR1 | Vactosertib + 5-fluorouracil (Ib) | Pancreatic ductal adenocarcinoma | / | Dose, PK, PFS, ORS, DCR | NCT04258072 (R) |
| Vactosertib (II) | Myeloproliferative neoplasm | / | Dose, safety, and efficacy | NCT04103645 (T) | |
Vactosertib + Paclitaxel + Ramucirumab (IIa) |
Gastric adenocarcinoma | / | DCR, ORR | NCT04656002 (US) | |
| Vactosertib + Pomalidomide (Ib) | Multiple myeloma | Grades 2–3 AEs, fatigue, sinus bradycardia | Safe and tolerable, PFS (80%) | NCT03143985 (CWR) | |
| Vactosertib + Pembrolizumab (II) | Acral and mucosa melanoma | / | ORR | NCT05436990 (NYR) | |
| Vactosertib + Imatinib (II) | Desmoid tumor | / | PFS, safety | NCT06219733 (W) | |
| Vactosertib + Durvalumab (II) | Urothelial cancer | / | ORR, PFS, OS | NCT04064190 (W) | |
| Vactosertib + Durvalumab (II) | Gastric cancer | / | ORR | NCT04893252 (US) | |
| Vactosertib + Pembrolizumab (Ib/IIa) | CRC, gastric cancer, gastroesophageal junction adenocarcinoma | / | MTD, safety and tolerability, ORR, PFS, OS | NCT03724851 (NYR) | |
| Vactosertib + Pembrolizumab + hepatectomy (II) | CRC, hepatic cancer, | / | Increase in TIL, AEs, irAEs | NCT03844750 (R) | |
| Vactosertib + Paclitaxel (Ib) | Gastric adenocarcinoma | Anemia, anorexia, fatigue, and urticaria, Grades 1–2 AEs | ORR (16.7%), DCR (83.3%) | NCT03698825 (CWR) | |
| Vactosertib (IIb) | Esophageal adenocarcinoma | / | Metabolic response, pathological response, | NCT06044311 (R) | |
| Vactosertib + Pembrolizumab | NSCLC | / | ORR | NCT04515979 (T) | |
| Vactosertib + Fludarabine phosphate + Cyclophosphamide + donor NK cells + Aldesleukin (IL-12) (I) | CRC, hematologic cancer, rectum cancer | / | Safety and tolerability, persistence of donor NK cells, clinical response | NCT05400122 (R) | |
| Vactosertib (I) | Advanced solid tumor | / | MTD, dose-dependent toxicity | NCT02160106 (C) | |
| Vactosertib (I/II) | MDS | / | MTD, hematologic improvement, | NCT03074006 (C) | |
| Vactosertib + Durvalumab (I/II) | NSCLC | / | MTD, ORR, PK, treatment-related AEs | NCT03732274 (ANR) | |
| NEUTRALIZING ANTIBODIES | |||||
Fresolimumab TGF-β1, 2, and 3 |
Fresolimumab (II) | Glioma | No major toxicity | PFS (61 days), OS (106 days) | NCT01472731 (CWR) |
| Fresolimumab + radiotherapy (II) | Breast cancer | / | Abscopal response rate | NCT01401062 (C) | |
| Fresolimumab + (II) | Pleural mesothelioma | Grades 2–4 AEs, | PFS (23.1%), OS (7.17–14.3 months), systemic humoral anti-tumor response (46.2%) | NCT01112293 (CWR) | |
| Fresolimumab + Stereotactic ablative radiotherapy (I/II) | NSCLC | DLTs, radiation-induced fibrosis | / | NCT02581787 (C) | |
| Fresolimumab (I) | Melanoma, Renal cell carcinoma | Reversible cutaneous keratoacanthomas, hyperkeratosis | PFS (24 weeks, partial response (one patient), stable disease (six patients) | NCT00356460 (CWR) | |
| Fresolimumab | Advanced melanoma | MTD, PK, DLTs | / | NCT00923169 (C) | |
NIS793 TGF-β1 and 2 |
NIS793 + Nab-Paclitaxel + Gemcitabine (III) | Metastatic pancreatic ductal adenocarcinoma | / | Safety, OS, PFS, ORR, DCR | NCT04935359 (ANR) |
| NIS793 + Bevacizumab + FOLFOX6 (II) | CRC | / | Dose, PFS, safety, OS, anti-tumor activity | NCT04952753 (ANR) | |
| NIS793 + Spatalizumab + Gemcitabine + nab-Paclitaxel (II) | PDAC | / | PFS, safety and tolerability, anti-tumor activity, OS, changes to CD8 and PD-L1 | NCT04390763 (ANR) | |
| NIS793 + MBG453 + Canakinumab (I) | MDS | / | DLTs, AEs, reduction in red blood cells, best overall response | NCT04810611 (T) | |
| NIS793 + FOLFIRINOX (Ib) | PDAC | / | Disease-free survival, PFS, OS, CR | NCT05417386 (R) | |
| NIS793 + Ruxolitinib + Siremadlin + Crizanlizumab + Sabatolimab + Rineterkib (I, II) | Myelofibrosis | / | DLTs, improved hemoglobin level, PFS, PK | NCT04097821 (C) | |
| NIS793 + Spartalizumab (I/Ib) | Advanced tumors | No DLTs, AEs; rash, pruritus, fatigue | PR (three patients), mechanism of action proved with engagement with TGF-β initiation pathways. | NCT02947165 (CWR) | |
| LIGAND TRAP | |||||
| TGF-β Ligand trap | AVID200 (TGF-β1) (I) | Malignant solid tumors | MTD not reached, treatment-related AEs: diarrhea, lipase elevation | MTDs not reached | NCT03834662 (CWR) |
AVID200 Bintrafusp Alfa (Ib) |
Myelofibrosis | No DLTs, Grades 3–4 AEs: anemia and thrombocytopenia | Well tolerated, clinical benefit (2 patients), | NCT03895112 (C) | |
| Bintrafusp Alfa (IIb) | Cervical cancer | Treatment-related AEs: anemia, rash, hypothyroidism, pruritus | ORR (21.9%) | NCT04246489 (CWR) | |
| Bintrafusp Alfa + Doxorubicin (II) | Urothelial carcinoma | / | Pathologic complete response rate, changes to TGF-β and effector T cells, OS | NCT04878250 (US) | |
| Bintrafusp Alfa (II) | Advanced sarcoma | / | Anti-tumor activity, ORR, safety | NCT04874311 (R) | |
| Bintrfusp Alfa + Carboplatin + Paclitaxel (II) | Olfactory neuroblastoma | / | ORR, safety and tolerability, OS, PFS. | NCT05012098 (ANR) | |
| Bintrafusp Alfa + Bevacizumab (I) | HER2 positive breast cancer | / | Changes to TIL, eye symptoms, vital signs | NCT03620201 (ANR) | |
| Bintrafusp Alfa + Radiation (Ib/Ib/II) | Cervical cancer | DLTs; Grade 4 amylase, Grade 3 menorrhagia, Grades 1–2 AEs; anemia, bleeding | ORR (75%), 44.4% and 62.5% | NCT04551950 (CWR) | |
| Bintrafusp Alfa (II) | Biliary tract cancer | Grade ≥ 3 AEs, hepatic failure, anemia, pruritus, increased alanine aminotransferase | OR (6.4%–16.6%), PFS (1.8 months), OS (7.6 months, 57.9%) | NCT03833661 (CWR) | |
Bintrafusp Alfa + PDSO1ADC + Entinostat (I/II) |
HPV+ cancers, small bowel, and colon cancers | / | ORR, PFS, DoR. | NCT04708470 (R) | |
| Bintrafusp Alfa + radiation (I) | HER2 negative breast cancer | / | Recommended dose, safety, and tolerability, PFS, OS, immunologic response. | NCT03524170 (C) | |
| Bintrafusp Alfa + hypofractionated radiation (I) | Cholangiocarcinoma | / | AEs, ORR, PFS, OS | NCT04708067 (ANR) | |
| Bintrafusp Alfa + Pimasertib (I/II) | Brain metastases | / | Dose, DLTs, OS, intracranial progression | NCT04789668 (C) | |
| Bintrafusp Alfa + chemoradiation | Esophageal cancer, squamous cell carcinoma | / | Feasibility, DLTs, percentage completion and withdrawal, PFS, OS, AEs | NCT04481256 (R) | |
| Bintrafusp Alfa (II) | Thymoma, Thymic carcinoma | / | ORR, DoR, PFS, OS safety and tolerability | NCT04417660 (R) | |
| Bintrafusp Alfa + TriAd vaccine + N-803 (I/II) | HPV-negative Head and neck squamous cell carcinoma | / | Dose, DLTs, safety and tolerability, ORR, OS, PFS, DoR. | NCT04247282 (CWR) | |
| Bintrafusp Alfa + SX-682 + MVA-BN-CV301 + (FPV)-CV301 (I/II) | Advanced solid tumors | / | / | NCT04574583 (ANR) | |
| Bintrafusp Alfa + stereotactic radiation (I/II) | Head and Neck carcinoma | / | DLTs, PFS, OS, AEs | NCT04220775 (T) | |
| Bintrafusp Alfa + Cisplatin + Carboplatin + Pemetrexed (I/II) | NSCLC | / | DLTs, treatment-related AEs, PFS, OS, DoR, PK | NCT03840915 (C) | |
| Bintrafusp Alfa (I/II) | CRC, MSI-H cancer | Grades 1–5 AEs; adrenal insufficiency, hepatic failure. | DCR (21%), PFS (1.8 months, 9.1 months), | NCT03436563 (CWR) | |
| Bintrafusp Alfa + IL-12 + SBRT (I) | Genitourinary cancer | No DLTs, treatment-related AEs; fatigue, lipase increase, anemia, hematuria, increased creatine | ORR 936.4%) | NCT04235777 (CWR) | |
| Bintrafusp Alfa + Paclitaxel (Ib/II) | Gastric cancer | No DLTs, Grade > 3 treatment-related AEs; anemia, urticaria, neutropenia, rash, pneumonitis | PFS (3.8 months), OS (9.6 months) | NCT04835896 (CWR) | |
| Pembrolizumab vs. Bintrafusp Alfa (III) | NSCLC | Treatment-related AEs; Grades 3–4 | PFS (7 months vs. 4.2 months), OS (21.1 vs. 22.1 months) | NCT03631706 (CWR) | |
| Bintrafusp Alfa (I) | Advanced solid tumors | / | Dose, AEs, PK, | NCT02517398 (C) | |
| Bintrafusp Alfa (I) | Locally advanced urothelial cancer | / | Manageable tolerated profile, limited anti-tumor effects | NCT04349280 (T) | |
| AdAPT-001 + ICI (I) | Sarcoma, Solid tumor | / | DLT, MTD, safety and tolerability, ORR, anti-tumor activity. | NCT04673942 (R) | |
| INTEGRINS | |||||
| α5β8 | PF-06940434 (I) | Solid tumor | / | DLTs, AEs, ORR, DoR, PK | NCT04152018 (T) |
| VACCINES | |||||
| Vaccines | Belagenpumatucel-L (II) | NSCLC | Anemia, fatigue | 15% partial response rate | NCT01058785 (CWR) |
| Belagenpumatucel-L vs. placebo (III) | NSCLC | No difference in OS and PFS vs placebo | NCT00676507 (CWR) | ||
| GVAX (I) | Melanoma | Treatment-related toxicities | Safe and well tolerable | NCT01435499 (CWR) | |
| GVAX + Pembrolizumab (II) | CRC | Grade ≥ 3 related toxicities | Failed to meet ORR, PFS, OS | NCT02981524 (CWR) | |
| GVAX + Ipilimumab vs. chemotherapy (II) | Pancreatic cancer | Treatment-related adverse event | Failed to meet OS, but showed high immune infiltration | NIL | |
| Gemogenovatucel (Vigil) (III/IV) | Ovarian cancer | Grade ≤ 3 related toxicities | Failed to meet OS | NCT02346747 (CWR) | |
| ANTI-SENSE OLIGONUCLEOTIDES(ASOs) | |||||
| TGF-β2 anti-sense | Trabedersen (AP12009) (I/II) | Pancreatic cancer, melanoma, CRC | Excellent safety except for transient thrombocytopenia | Encouraging survival results with complete response in one patient. | NIL |
- Abbreviations: AE, adverse events; ANR, active not recruiting; C, completed; CR, complete response; CRC, colorectal cancer; CWR, completed with result; DCR, disease control rate; DoR, duration of response; HER2, human epidermal growth factor; HPV, human papilloma virus; IRAE, immune-related adverse events; MDS, myelodysplastic cyndrome; MSI-H, microsatellite instability-high; MTD, maximum tolerable dose; NSCLC, non-small cell lung cancer; NYR, not yet recruiting; ORR, objective rate response; OS, overall survival; PDAC, pancreatic ductal adenocarcinoma; PFS, progression-free survival; PK pharmacokinetic 9; PR, partial response; R, recruiting; TGF-β, transforming growth factor beta-2; TIL, tumor infiltrating lymphocytes; TβR, transforming beta receptor; TWR, terminated with result; US, unknown status; W, withdrawn.
- Source: United States National Library of Medicine (ClinicalTrials.gov).
4.2 Targeting TGF-β With Neutralizing Antibodies
The multifunctional role of TGF-β in both tumorigenesis and non-tumorigenesis status makes it an attractive target in cancer. However, it could also be a source of systemic toxicity, as confirmed by several studies [157]. On this note, it is paramount to understand and evaluate the role of the three isoforms in tumor progression, serving as a basis for selective toxicity. Although the dominant form in tumors is TGF-β1, the role of other isoforms is yet to be fully elucidated. In two pre-clinical models evaluating the blockade and efficacy of TGF-β1 or TGF-β3 isoforms in colon and melanoma cancer, neutralization of TGF-β1, but not TGF-β3, increased the prophylactic efficacy against a colon cancer vaccine, promoting tumor-infiltrating CD8+ T cell, and reducing regulatory T-cells and myeloid-derived suppressor cells (MDSCs) [158]. In this same study, TGF-β1 isoform blockade, as a single agent, improved mouse survival by delaying tumor intrinsic phenotypic EMT [158]. This evaluated the critical role of TGF-β1 against other isoforms and its therapeutic potential either as a monotherapy or combined with other immunotherapeutic or targeted therapies. In another randomized tamoxifen trial study of 564 invasive pre-menopausal breast cancer carcinoma, TGF-β2 expression in CAF was associated with improved recurrence-free survival (RFS), suggesting TGF-β2 could have an anti-tumorigenic effect in CAF+ breast cancer patient, thereby serving as a novel prognostic marker, especially in transformed-CAF+ cancer [159]. It is, however, not surprising that the blockade of TGF-β1,2,3 to TGF-βR2 attenuated the growth and metastasis of cancer stroma, suggesting a conflicting dual role of these isoforms in different cancer models. This further supports the need for extensive studies of the role of the three isoforms in cancer and characterizing the TME as part of diagnostic tools in cancer patients. This could inform if a patient will benefit from TGF-β inhibitors.
In the Phase 1 clinical trial (NCT00356460) of Fresolimumab in advanced and malignant melanoma or renal carcinoma, Fresolimumab, a human IgG4 neutralizing monoclonal antibody against the three TGF-β isoforms, demonstrated a tolerable safety profile [160]. However, this trial was conducted in a small cohort of 29 patients and may not accurately represent this therapy's safety profile. In a discontinued Phase 2 trial of the immunological effect of Fresolimumab in malignant pleural mesothelioma, 3 out of 13 patients recruited showed stable disease with improved OS but no impact in effector adaptive immune cells [122].
During a Phase I clinical trial of anti-TGF-βII IgG1 monoclonal antibody in patients with advanced tumors (NCT01646203), a treatment aimed at inhibiting receptor-mediated signaling activation of TGF-β was evaluated in 14 patients. The study revealed various treatment-related adverse events, ranging from uncontrolled cytokine release syndrome to infusion-related reactions. Although three dose ranges were tested, they failed to determine the maximum tolerable dose (MTD). Furthermore, escalating the dose beyond 25 mg/dose was deemed unsafe due to the observed adverse events [161]. Other neutralizing antibodies were tested in preclinical. These include 2G7, an antibody that inhibits the three TGF-β isoforms in nude mice of human breast cancer and metastatic lung cancer [162]. In addition, 2G7 showed increased NK cell activity implicated in the anti-tumor effects. 1D11 is another anti-TGF-β monoclonal antibody that inhibits the three isoforms and was investigated in preclinical models of lung and breast cancers. However, at higher doses, 1D11 induced epithelial hyperplasia of the tongue in mice, observed with extreme weight loss and dysphagia in a mouse model of familial adenomatous polyposis, 1D11 increased cancer progression. Hence, it is necessary to determine if blockade of TGF-β could induce tumor progression, especially in genetically related cancers [163].
4.3 Ligand Trap
The constitutive ligand-receptor interaction of TGF-β that follows when the cytokine binds to its surface receptor, causing dimerization and further downstream signal transduction, can be further inhibited with either anti-ligand or anti-receptor antibodies or soluble TGF-β receptor inhibitors known as ligand traps or sequesters [164]. Most of these inhibitors are designed to isolate TGF-β1 and TGF-β3 receptors by preventing their binding and activation to TGF-β2 [164]. Although these antibodies displayed remarkable pharmacokinetic parameters via high binding capacity and superior specificity to the receptors, they have less capacity to efficiently infiltrate and distribute evenly in tumor mass [3, 4]. So far, the primary strategies employed in developing ligand trap sequesters include anti-ligand/receptor. The first soluble TGF-βII trap was developed in a transgenic metastatic breast cancer mice model [165]. The antagonist was able to confer lifetime protection by preventing metastases in mice when rechallenged with orthotopic tumor implantation [165]. A novel tetravalent TGF-βII trap showed comparable inhibition to a pan TGF-β kinase inhibitor or neutralizing antibody in different prostate cancer cells [166]. Since then, several other antagonist and/or ligand traps have been developed, although with poor activity, and some are already in clinical trials (Table 1).
4.3.1 AVID200
AVID200 (NCT03834662), a soluble TGF-βI and III dimerized protein trap, which comprises an IgG-ectodomain fused to a human Fc fusion domain, can reverse the immunosuppressive ability of TGF-β by preventing the exclusion of immune cells from the TME [167]. It is currently being studied in clinical trials for malignant solid tumors. Because it does not bind to TGF-β2, which is involved in the normal regulation of hematogenesis and cardiovascular function, and due to its nature of binding to just the undesirable isoform of TGF-β, it has higher ligand-binding selectivity than previously developed agents and is also employed in the In Vitro and In Vivo treatment of myelodysplastic-related anemia, due to its ability to reduce excessive TGF-β1 signaling, attenuation of SMAD2 phosphorylation, and reduction in collagen expression, which are all involved in the pathobiology of myelofibrosis (4) [168].
Although AVID200 showed optimal activity in preclinical studies, much could not be said for its non-randomized interventional Phase I trial in patients with malignant solid tumors. This study measured and evaluated the safety, tolerability, and dose-limiting toxicity profile, with a single dose of 180 mg administered every 3 weeks. As of when this paper is written, there is no data on the study's outcome.
4.3.2 Bintrafusp Alfa
Since its discovery, checkpoint inhibitor-based immunotherapies have successfully treated several cancers. However, only a small percentage of patients attained measurable clinical benefits due to several setbacks, such as primary and acquired resistance to treatment [169]. Recently, the dysregulated TGF-β signaling landscape has been indicated to contribute to Immune checkpoint inhibitor (ICI) resistance and immune escape [124, 170-172]. By changing the tumor immunological landscape, TGF-β reduced T-lymphocyte infiltration, tumor killing effectiveness, enhanced the formation of T cell exclusion phenotypes [171, 173], and antigen presentation. Concerning this, several researchers have proven that the selective inhibition of TGF-β1 activity overcomes the initial resistance to checkpoint blockade activity [174-177]. Therefore, concurrent TGF-β-blocking with ICI may be a workable method to improve effectiveness and reduce resistance to ICBs.
To further simplify the treatment workflow, as well as dosage load, Lan et al. [178], designed a bifunctional fusion protein, M7824, capable of selectively blocking PD-L1 with a human TBRβII extracellular domain. Initial In Vitro and In Vivo mice studies showed satisfactory anti-tumor control and prolonged survival in mice while improving and increasing the tumor-infiltrating lymphocytes (TIL) [178]. This innovative bifunctional human fusion protein monoclonal antibody targets programmed death ligand 1 (PD-L1) and is linked to the extracellular domain of TGF-βR2. It is designed to serve as a checkpoint inhibitor and TGF-β trap within the. However, the success seen in preclinical studies of this agent has not yet been fully replicated in clinical trial studies. Based on ongoing and completed clinical trials of this therapy, the main inclusion criteria focused on patients on previous treatment regimens, PD-L1-high tumors, immune-excluded tumors, and patients with no prior immunotherapy treatment.
In a Phase 1 dose-escalation clinical trial, 80 patients with advanced NSCLC who had received prior platinum-based treatment or had not previously undergone immunotherapy were enrolled. Using a 1:1 randomization treatment design of either 1200 mg Phase II dose or 500 mg every 2 weeks, Bintrafusp alfa displayed good efficacy and tolerable toxicity. The primary endpoint measured by objective response rate (ORR) was 21.3% for both doses. It is worth noting that the treatment success rate was higher in patients with PD-L1 high and PD-L1 positive tumors.
In another Phase 1 clinical trial in previously/heavily treated advanced esophageal adenocarcinoma patients, Bintrafusp Alfa displayed clinically significant effectiveness and a controllable safety profile with an ORR of 20% lasting 1.3–8.3 months [179]. The highest response was recorded in patients with the immune-excluded phenotype [179]. Several clinical trials have begun or completed that focused on the unique combination that Bintrafusp Alfa offers in different cancer types such as esophageal SCC [180], advanced, human papillomavirus-related malignancies [181], Glioblastoma [182], NSCLC resistant or refractory to ICIs [183], ctDNA-positive metastatic CRC [184], pre-treated CRC [185], pre-treated recurrent or refractory gastric cancer [186] pre-treated biliary tract cancer (BTC) [187], advanced solid tumors [180], NCT04246489.
The international single-arm Phase II trial will evaluate Bintrafusp alfa's treatment efficacy and safety. In a Phase II trial in patients with locally advanced-metastatic BTCs, Bintrafusp Alfa failed to elicit the required anti-tumor efficacy nor meet the primary endpoint [188]. While several other Phase I trials are still ongoing, the result so far has been quite confusing and mixed, coupled with the Phase II result in which a meager 10.1% ORR was recorded in BTC. These results indicate the need for more in-depth research to justify inclusion criteria in subsequent trials. Alexander and Xiao highlighted critical suggestive criteria that may be employed to refine subsequent clinical trials of Bintrafusp Alfa [189].
4.3.3 Luspatercept
To treat severe types of anemia and MDS, a recombinant fusion protein called Luspatercept was created by fusing the human activin receptor's extracellular domain with the immunoglobulin's Fc component. This activin receptor ligand trap improves late-stage erythropoiesis by inhibiting SMAD2 and SMAD signaling, leading to erythroid maturation [190]. Luspatercept has now been approved in treating anemia-associated MDS after a Phase III randomized, double-blinded trial, where a significant reduction in transfusion burden was recorded in transfusion-dependent β-thalassemia patients. Patients with lower-risk MDS placed on frequent red-blood-cell transfusion or those resistant to prior erythropoiesis-stimulating medications were shown to benefit the most from Luspatercept by having less severe anemia [191-193]. Another notable ligand trap construct is the immunoglobulin fragment crystallizable (fc) fused to the extracellular domain of both TGF-βRII and beta glycan. Such a construct elicited a potent anti-tumor effect metastasis in preclinical models of transplanted 4T1 and EMT-6 mammary tumors [194].
4.3.4 AdAPT-001
This is a first Phase I in-man oncolytic adenovirus TGF-β receptor ectodomain-IgG Fc fusion protein trap designed to counteract the immunosuppressive and profibrotic effects of TGF-β in solid tumors [195]. AdAPT-001 targets non-tumor cells by deleting a small 50 base-pair segment upstream of the E1A initiation site, which results in non-productive infection and minimal to no cytolytic effect on normal cells while exhibiting strong cytolytic and replicative activity in tumor cells. Additionally, the construct includes an attenuated Bcl-2 adenovirus homolog, a gene known to inhibit apoptosis. In this study, AdAPT-001 was administered intratumorally using a 3 + 3 dose escalation and expansion of different doses [195]. The primary endpoint was the evaluation of safety and tolerability, with efficacy as the secondary endpoint. None of the nine patients enrolled experienced dose-limiting toxicities or treatment-related adverse events. For the second part of the trial, which focused on measuring efficacy through dose expansion, 19 patients were enrolled, with three showing partial response, five experiencing prolonged disease, and 13 progressing diseases. One limitation of the trial was the inability to distinguish between pseudo progression and true progression observed in some patients before a favorable response was observed. This was due to the lack of pathological results from biopsies, which could have accurately measured clinical endpoints such as the kinetics of replication of the oncolytic virus, extent of immune infiltration, degree of tumor apoptosis, and shrinkage. This underscores the importance of on-treatment screening of tumor biopsies to correlate with treatment endpoints. Nevertheless, AdAPT-001 is currently being evaluated in several Phase II trials, either alone or combined with checkpoint inhibitors [195].
4.4 Antibodies Against Integrin-Mediated TGF-β Signaling
Recruitment of cell adhesion molecules to ECMs is vital to regulating homeostasis. Integrins are the central cell adhesion receptors and migratory molecules crucial in cell movement [196, 197]. Altered integrins have been implicated in almost all cancers, and they not only contribute to cancer progression but play a significant role in tumor metastasis [196, 198-200]. They have also been linked to aberrant oncogenic signaling, growth factor signaling (GFR), T cell resistance, colocalization, and anchorage-independent survival of tumors in metastatic sites [201, 202]. Additionally, integrins could remodel the tumor stroma, which supports the invasion, acquisition, and maintenance of cancer stem cells (CSCs), ultimately leading to resistance to therapy [203]. There is increasing evidence that integrins play a role in activating TGF-β1 ligand [204]. When integrins bind to TGF-β1, they can negatively impact the tumor stroma, which can cause the tumor to progress [205]. Therefore, targeting integrins could be a viable strategy for blocking TGF-β signaling. When TGF-β1 interacts with β, av-integrin complexes, it activates latent TGF-β signaling. Notable integrins that activate TGF-β include αvβ1 (40), αvβ3 [206], αvβ5 [206], αvβ6 [207], and αvβ8 [208]. Each of these integrins has specific expressions in different tissues. By disrupting this interaction, it may be possible to provide targeted, tissue-specific therapy, preventing systemic inhibition, which could be harmful, as TGF-β has pleiotropic effects, thus, monoclonal antibodies against αvβ1 (44,45), αvβ6 [209], αvβ8 [210, 211] have shown optimal inhibition of TGF-β1. Few preclinical studies evaluating the blocking of these interactions have been reported with αvβ8 in colon carcinoma and breast cancers [212], where the blockade of αvβ8 using engineered antibodies blocks the release of TGF-β1, mediating an anti-tumor immune effect. A neutralizing antibody, 264RAD, against αvβ6 also inhibits TGF-β1 signaling and suppresses the tumor growth in αvβ6+ human PDAC xenografts [213].
Another antibody, SRK-181-mIgG1, has been developed to target the TGF-β large N-terminal of the precursor polypeptide, also known as latency-associated peptide (LAP). This prevents the detachment of mature TGF-β1 from latency-associated proteins, keeping TGF-β1 latent and averting the activation of TGF-β2 and TGF-β3. This, in turn, prevents systemic activation of TGF-β. The antibody was tested for its ability to prevent checkpoint blockade resistance [214], thus preventing systemic activation of TGF-β. This agent, tested as a monotherapy or combined with anti-PD1 blockade, produced a robust anti-tumor effect via CD8+ T cells and decreased immunosuppressive myeloid population. Providing a rationale for selective blockade of TGF-β [215]. In addition, a clinical trial evaluation (NCT04152018) of PF-06940434 that targets αvβ8 is currently ongoing.
4.5 Vaccine-Based Treatment Therapy
Gene-based vaccine therapy is a notable strategy against TGF-β. Belagenpumatucel-L (Lucanix) is a non-viral allogeneic tumor-cell vaccine containing four lung cancer cell lines transfected with a TGF-β2 antisense transgene that downregulates TGF-β2 signaling [216]. In Phase II studies in 83 and 75 NSCLC patients of various stages, Belagenpumatucel-L showed improved survival, especially in patients with advanced-stage NSCLC [217]. This prompted a Phase III randomized trial, where Belagenpumatucel-L was given intradermally to 270 patients. However, the study failed to reach a significant difference in OS and PFS (median OS: 20.3 months) to placebo (17.8 months). Although, the study showed that early treatment in patients who received chemotherapy and radiation may be associated with better benefits, suggesting that the vaccine may be beneficial in this category of patients [218].
Using a principle similar to Belagenpumatucel-L, GVAX was developed to deliver the whole tumor cell vaccine and granulocyte-monocyte colony-stimulating factor (GM-CSF). Combined with cyclophosphamide (GVAX-Cy), this vaccine was tested in 20 patients with stage IIB-IV melanoma, with patients receiving four doses over 28 days. Elevated levels of activated monocytes, PD-1+ lymphocytes, and eosinophils were observed. However, there was no decrease in Tregs level [219]. Several other Phase I/II studies in NSCLC patients displayed promising benefits with increased granulocytes, DCs, lymphocytes, and macrophages [218, 220]. However, the treatment failed to elicit a similar effect in two Phase III trials with prostate cancer patients [221, 222]. In another Phase II study, with pembrolizumab in patients with mismatch repair-proficient (MMR-p) advanced CRC, ORR was measured. The study failed to meet its ORR, with PFS and OS of 82 days and 179–441 days, respectively. However, some patients exhibited an increased immune response [223]. Another Phase II study evaluated GVAX with ipilimumab as a maintenance therapy for metastatic pancreatic cancer. The study failed to improve the overall response or survival of metastatic pancreatic cancer as maintenance therapy [224].
Another notable vaccine is Gemogenovatucel (Vigil), which is derived from the patient's tumor cells during the initial de-bulking. Vigil is engineered with a plasmid that encodes GM-CSF and a furin short-hairpin (shRNA) that knocks down furin gene expression. GM-CSF acts to promote antigen immunogenicity and presentation by DCs, and furin is a crucial convertase needed for the secretion of TGF-β1 and TGF-β2. Vigil has been tested in several trials such as a Phase II/III multicenter randomized double-blinded trial of 80 patients with different high-risk stages (3b-4) of ovarian cancer [225], a Phase II cross-over trial of 42 cancer patients that tested for OS and RFS [226]. In a separate Phase II study, the safety and efficacy of Vigil in women with advanced gynecological cancers were examined, both as a standalone treatment and in conjunction with checkpoint inhibitors like Atezolimumab [226]. This ongoing study aims to gauge biomarkers of response, including anti-tumor immune response. Another preliminary study assessed the safety, tolerance, and efficacy of Vigil, either alone or combined with Durvalumab, and measured PFS in the combination arm for patients with advanced gynecological cancers [226]. Overall, Vigil was well tolerated and met some of the endpoints such as OS and RFS.
4.6 Anti-Sense Oligonucleotides (ASOs) Therapy
Oligonucleotides are a novel class of agents used to treat various diseases, including cancer. However, they are highly unstable, and due to this, the modified version of ASOs using nanoparticles or chemical modification has been developed to aid delivery and stability [227]. In a few preclinical studies using AP11014 and AP15012 oligonucleotides in mouse models of prostate cancer, CRC, non-small cell lung cancer (NSCLC), and MM, suppression of TGF-β was adequately achieved [228, 229]. Additionally, AP12009 (Trabedersen), which inhibits TGF-β2, demonstrated the ability to impede cell proliferation and migration in a preclinical study involving human pancreatic cells and an orthotopic xenograft model. Consequently, a Phase I/II trial was conducted to assess the safety and efficacy of Trabedersen in various cancers, including pancreatic cancer, malignant melanoma, and CRC [230]. The prosecution revealed favorable safety and survival outcomes, with the primary adverse reaction being non-serious, short-term thrombocytopenia [231]. Furthermore, AP12009 was studied in an open-label Phase I/II dose-escalation trial in recurrent high-grade glioma, with a satisfactory median survival profile [230].
4.7 Combination-Based Therapy
Advances in cancer immunotherapy, specifically ICIs and chimeric antigen receptor T cell therapies (CAR-T) have transformed cancer treatment since discovering PD-1. These agents are classified into two categories: co-stimulatory agonists such as CD26, CD28, CD40, OX40, inducible costimulatory (ICOS) CD134, and CD137, and co-inhibitory antagonists like PD-1, cytotoxic T-lymphocyte associated antigen-4 (CTLA4), lymphocyte activation gene 3 (LAG3), T cell immunoglobulin and mucin domain 3 (TIM3), and T cell immunoreceptor with Ig and ITIM domain (TIGIT). However, these therapies have recorded a sub-optimal response rate of only 15% in patients, partly due to TGF-β-immunosuppression [232]. TGF-β has been found to contribute to anti-PD-1/PD-L1 treatment resistance by promoting tumor evasion and downregulating key inflammatory cytokines and cytolytic proteins such as granzyme A, B, perforin, IFN-γ, and Fas ligand via SMAD-mediated mechanisms that attenuate CD4+ and CD8+ T cell responses [170, 233, 234]. Furthermore, immune-excluded tumors exhibit significant elevation in TGF-β signaling in the stromal compartment, which might play a role in preventing immune infiltration. Therefore, inhibiting TGF-β could suppress immunosuppressive factors that promote angiogenesis and EMT [233-235]. Given the similar roles of PD1/L1, CTLA4, and TGF-β in the immunosuppression of T cells, macrophages, and NK-cells, treatment strategies that combine ICIs with TGF-β inhibition are more likely to improve therapeutic outcomes.
Additionally, there is growing evidence that both TGF-β and PD-L1 are parallel immunosuppressive pathways that can activate each other, as observed in some tumor cells where TGFβ-induced EMT increases the expression of PD-L1 and TGF-β-SMAD3 signaling enhances PD-1 levels on TILs [114, 236, 237]. Therefore, there is growing interest in combining anti-TGF-β and ICIs. Moreover, targeting TGF-β in the TME before or after adoptive cell therapy leukapheresis might significantly reduce T cell immunosuppression [238]. Based on this, several preclinical combination studies are being carried out, with targets demonstrating more significant superiority when combined with anti-TGF-β. In a mouse xenograft model of triple-negative breast cancer (TNBC) and melanoma, a combined effect of TβRII chimera with anti-CTLA4 demonstrated profound anti-tumor responses compared to anti-CTLA4 alone [177]. The same is true of a bispecific decoy of CD4+ TGF-β trap, which blocks TGF-β signaling on CD4+ T cells, leading to tumor vasculature normalization in a transgenic model of breast cancer [239]. In a mouse breast cancer model of Galunisertib (LY2157299) with anti-PD-1/L1, the combined treatment displayed superior anti-tumor efficacy than the single arm [143]. Other similar combinations of preclinical models have been tested with most currently in clinical trials, such as AVID200 in homografts tumor models [240], SRK-181-mIgG1 with anti-PD1 in ICI-resistant syngeneic mouse models showed a superior infiltration of CD8+ T cells [215], a decoy of CD4+ TGF-β trap with a selective blockade of TGF-β signaling in CD4+ T cells and reduced immunosuppressive myeloid cells [241], and pH-sensitive nanoparticles for Galunisertib (LY2157299), with small interfering PD-L1 RNA have been used to suppress tumor growth in PDAC mouse models [242], and finally is TβR1 inhibitor SM16A, which enhances adoptive T cells infiltration and anti-tumor effect in mesothelioma mouse models [243]. Shou et al. have recently developed a hemagglutinin-tagged decoy comprising the TGF-βRII ectodomain and a glycophosphatidylinositol (GPI) anchor. This construct demonstrated elevated expression of IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) alongside other cytokines. It exhibited proficient capability in trapping and deactivating TGF-β [244]. Numerous studies have explored the combination of TGF-β inhibitors with other cancer therapies. Some of these investigations have progressed to clinical trials, as delineated in Table 1, and have exhibited manageable safety, tolerability, and anti-tumor efficacy. However, none of these interventions have received approval for clinical use. A promising strategy for solid tumors is CAR-T cell therapy, which entails the modification of T cells to target tumor antigens. While CAR-T cell therapy has exhibited considerable success in hematologic malignancies, its efficacy against solid tumors has been limited, partly attributed to TGF-β-mediated immunosuppression [245-247]. Researchers have developed strategies to overcome this hurdle, including co-expressing the negative dominant form of TGF-β (TβII) [248] and the constitutively active AKT (caAKT) [249], which provides resistance against tumor-associated inhibitory signals. Additionally, co-expressing the TβIII-41BB chain has prevented immunosuppression by converting the TGF-β immunosuppressive signal to a 41BB stimulatory signal [250].
Our analysis of nearly two decades of anti-TGF-β treatment development highlights the need to review unsuccessful clinical trials for insights. This evaluation is crucial for future therapeutic advancements. Nisevokitug (NIS793), a human IgG2 monoclonal antibody aimed at pancreatic adenocarcinoma (PDAC), showed inadequate tumor shrinkage, leading to its discontinuation (NCT04935359). Additionally, a Phase II trial of Bintrafusp Alfa, intended for metastatic biliary tract and lung cancers, did not meet its primary endpoint compared to Nivolumab, showing a lower ORR and more severe adverse events (NCT03838661) [1]. Integrin inhibitors also showed limited efficacy: an antibody targeting integrins αvβ3 and αvβ5 in glioblastoma (NCT00689221) and head and neck SCC (NCT00705016) did not improve survival or anti-tumor efficacy compared to chemotherapy. Similarly, a trial for patients with advanced solid tumors failed due to severe cytokine release syndrome and challenges in determining the MTD (LY3022859) (NCT01646203). As previously articulated, the favorable outcomes from numerous clinical trials involving anti-TGF-β therapies have yet to be realized. Even in instances where noteworthy results have been documented, they remain neither universal nor sufficiently satisfactory when juxtaposed with data derived from preclinical investigations. Drawing upon recent discoveries and an exhaustive review of the literature, we posit several significant considerations regarding TGF-β signaling that are frequently overlooked in the majority of clinical assessments. In particular, we underscore: (i) the off-target effects, (ii) the limitations of preclinical models, (iii) the activation of redundant and compensatory mechanisms, (iv) the absence of patient selection criteria, and (v) the dual opposing roles of TGF-β at both contextual and cellular levels.
5 Addressing Challenges and Advancing Anti-TGF-β Therapy for Effective Cancer Treatment
Despite the enthusiasm following the discovery of potent TGF-β inhibitors in preclinical studies, the expectation of effective cancer therapy by blocking TGF-β signaling has been marked with considerable limitations in clinical trials for about two decades(Table 2). This disappointing performance can be attributed to many factors. In this section, we will explore these challenges and discuss potential strategies to overcome them, paving the way forward for anti-TGF-β cancer therapies, aiming to unlock the full potential of anti-TGF-β therapies in cancer treatment.
| Target | Cancer type | Outcome measures | Trial outcome | Trial ID |
|---|---|---|---|---|
TβRII |
Advanced solid tumors | Dose-Limiting Toxicities (DLTs), Immunogenicity, Complete Outcome, Pharmacokinetics | Failed to establish a safe dose due to uncontrolled cytokine release syndrome (CRS) | NCT01646203 |
| NIS793 (Nisevokitug) | First-line metastatic pancreatic adenocarcinoma (mPDAC) | Dose-Limiting Toxicities (DLTs), Progression-Free Survival (PFS), Overall Survival (OS) | Failed to meet the primary endpoint | NCTO4935359 |
| M7824 (Bintrafusp) | Biliary tract cancer | Objective Response Rate, Progression-Free Survival, Safety OS | ORR, increased deaths | NCT03833661 |
| EMD121974 | GliobGlioblastoma Head and neck squamous cancer |
Poor OS and anti-tumor activity | NCT NCT00689221 NCT00705016 |
- Source: National Center for Biotechnology Information (https://clinicaltrials.gov/).
5.1 Off-Target Effects
Compelling evidence suggests that cancer patients treated with TGF-β blockers may experience significant organ toxicity. This is because TGF-β is ubiquitously expressed in nearly all healthy cell types where it regulates critical homeostatic functions [251, 252]. For instance, while TβRI kinase inhibitors showed therapeutic effects in cancer patients, there was significant cardiac toxicity at high doses. These include hemorrhagic, degenerative, and inflammatory lesions in heart valves. Additionally, skin toxicity manifested as eruptive keratoacanthomas, hyperkeratosis, cutaneous squamous-cell carcinomas, and basal cell carcinoma, limiting the safe therapeutic window of these inhibitors [253]. Addressing these challenges may require a focused strategy to identify and target definite genes modulating the tumorigenic activity of TGF-β, such as targeting specific epitopes of TGF-β on cancer cells and other relevant cell types to minimize off-target toxicity while preserving normal homeostatic function. In addition, implementing an intermittent dosing schedule, where patients experience planned breaks from the medication (called drug holidays), may be crucial for Patients' safety. For instance, the treatment protocol for alisertib in patients with glioma in 2 weeks on and 2 weeks off schedules significantly reduced cardiac toxicity [254]. In addition, the timing of anti-TGF-β therapy is crucial for optimal outcomes. Patients with premalignant or early-stage malignant tumors may derive more significant benefits from this therapy, as the tumor-suppressive capacity of TGF-β remains active during these stages [71]. In contrast, advanced tumors may have developed uncontrollable signaling networks, rendering them less responsive to anti-TGF-β treatment. This rationale is evidenced in mouse and animal studies where the TGF-β was inhibited before exposing the mouse to tumors. The study observed a significant decrease and, in some cases, complete eradication of tumor growth with high infiltration of CD8+ T cells [255]. Given the safety of plant-derived compounds and their potent anticancer activities [256, 257], exploring naturally derived compounds as inhibitors of TGF-β in cancer therapies may be rewarding. Interestingly, a plant-derived agent such as 8-Oxo-epi berberine (OPB) was shown to reverse TGF-β1-triggered morphological changes, EMT biomarkers expression, migration, adhesion, and invasion in cell culture assays [258]. In animal models, OPB also dramatically decreased the metastatic nodules in the lung without affecting the growth of primary tumors. In conclusion, OPB inhibited TGF-β1-induced EMT, possibly by interfering with SMAD3. This study further revealed that OPB suppressed TGF-β1-induced SMAD2/3 activation, SMAD3 phosphorylation and nuclear translocation, and interaction of SMAD3 with SMAD4.
5.2 Limitations of Preclinical Testing Models
Another potential factor responsible for the poor clinical performance of TGF-β inhibitors could be the misrepresentation of preclinical data that do not faithfully recapitulate the complexity of the disease in humans. For instance, Maier et al investigated the effectiveness of Galunisertib in patient-derived tumor xenografts (PDXs) and found impressive tumor inhibition in lung and prostate cancer PDX models [259]. However, this agent failed to demonstrate improved OS compared to placebo in clinical studies (104). Despite providing a crucial platform for preclinical research and personalized medicine, PDXs have significant limitations. First, the fidelity of cancer cells in the PDX models is compromised due to loss of heterogeneity, clonal evolution within the tumors, and selection bias for engraftment. A study analyzed 1110 PDX samples across 24 cancer types to understand the dynamics of copy number alterations (CNAs) dynamics during PDX passaging [260]. The findings revealed that CNAs rapidly accumulate during the passaging of PDXs, likely due to the selection of preexisting minor clones within the tumors. Notably, the accumulation of CNAs in PDXs differed from that acquired during tumor evolution in patients. Notably, several CNAs recurrently observed in primary tumors tended to disappear over time in PDXs. These findings suggest that the genomic aberrations in PDXs are dynamic and do not necessarily capture the genomic landscape of primary tumors. This raises a big concern about the preclinical data generated from PDX models that predict the clinical efficacy of Galunisertib. Another critical limitation of PDX models that may explain why Galunisertib was ineffective in humans despite encouraging results in PDX models is the lack of immune cells in the TME compared to human tumors. This means that the efficacy of Galunisertib as reported in PDX models disregards the contribution of the immune components in tumor progression. Stressing the critical role of the TME in TGF-β signaling, it was found that the cytotoxic activities of Galunisertib are dependent on factors emanating from the TME [259]. Humanized mouse models offer a more sophisticated platform for anticancer drug assessment, closely simulating human disease conditions. These advanced models, which incorporate human immune system components into mice, offer improved predictive capabilities for drug screening within a human-like immune environment. This innovative method signifies a significant leap forward in preclinical research, generating findings that more accurately forecast potential outcomes in human patients.
5.3 Activation of Redundant and Compensatory Mechanisms
Clinical insights suggest that one of the key resistance mechanisms to anticancer therapy is utilizing functionally redundant pathways or activating compensatory mechanisms, leading to adaptive and intrinsic drug resistance. The complexity of the TGF-β signaling pathway may promote redundancy [261]; for instance, five TGF-β receptors, two TβR1 (ACVRI, BMPIa), and three BMP-SMAD receptors (SMAD1, SMAD5, SMAD8) can redundantly transduce anti-mullerian hormone signal needed for male infertility [262]. Also, reports have shown that chemoresistance is a major clog in the wheel of TGF-β inhibitors for cancer therapy [263]. While the molecular mechanisms underlying this resistance are not fully clear, it is tempting to speculate that activation of compensatory mechanisms would be a major culprit. This can occur at receptor or pathway levels. At the receptor level, TGF-β has 14 signaling receptors, 42 ligands, and several proteases (serine protease, plasmin MMPs, plasma kallikrein, etc) that can activate it [264, 265]. Presently, clinical trials primarily target a select few components of this pathway, potentially leading to variations in patient responses. Excluding other signaling mechanisms might result in variation in anti-TGF-β responses in patients depending on ligand/receptor availability and accessibility. Therefore, comprehensive patient stratification, informed by advanced bioinformatic and biotechnological tools to profile the expression and activation of these components, is essential to discern which patients are likely to benefit from TGF-β therapy. Additionally, efforts should be geared toward identifying TGF-β redundant pathways in cancer progression that can lead to treatment failure and resistance. It might also be noteworthy to determine which type of TGF-β signaling receptors/ligands/proteases are expressed by patients and define their role in cell and tissue-dependent manner. Multiple signaling pathways can compensate for the loss of TGF-β activities at the pathway level. A prime example is observed in angiogenesis, a crucial oncogenic process that ensures continuous nutrient supply to tumor cells. While inhibiting TGF-β-mediated angiogenesis is a key objective of anti-TGF-β therapies, numerous alternative factors can effectively induce angiogenesis in its absence. These include FGFs, angiopoietins, EGFs, fibroblast growth factors, hepatocyte growth factors, placental growth factors, and stromal cell-derived factor 1 [266]. This redundancy underscores the limitations of using anti-TGF-β agents as monotherapy. Corroborating this concept, both preclinical and clinical studies have demonstrated that therapeutic strategy targeting other angiogenic factors, such as using anti-VEGFA antibodies or tyrosine-kinase inhibitors against VEGF receptors, paradoxically stimulates the production of alternative growth factors [267]. This compensatory mechanism further complicates the efficacy of single-pathway targeted treatments and highlights the need for more comprehensive therapeutic strategies. These observations justify that anti-TGF-β therapy may yield more significant benefits when combined with other treatments instead of being used as a standalone therapy. Hence, its efficacy may be enhanced when administered concomitantly with chemotherapeutic or immunotherapeutic agents. Notably, combination with immunotherapies may mitigate immunosuppression and prevent cancer invasion and metastasis.
5.4 Lack of Patients' Selection Criteria
Choosing the right treatments for an individual patient is complex but is central to achieving remission and increasing survival. Most clinical studies that investigate the effectiveness of TGF-β blockers did not have specific selection criteria, which may partly explain modest clinical benefits. As we gain a deeper understanding of the effects of TGF-β on the TME components, it becomes evident that the cellular and molecular characteristics influencing responsiveness to TGF-β blockade can vary between different tumor types and across individual patients. For instance, the response to TGF-β blockade in PDAC, which is characterized by extensive stromal development, frequent inactivation of SMAD4, and a low tumor mutation burden [268] differs from that in head and neck SCCs, which have high tumor mutation burdens and significant CD4+ T cell presence In parallel studies [269], transcriptional profiling of patient samples from many different cancer types, including glioblastoma, pancreatic cancer, breast cancer, ovarian cancer, (CRC), and NSCLC, revealed that cancer patients with mesenchymal subtypes have high expression of TGF-β target genes and that this correlates with poor prognosis. Therefore, subgroups of patients with cancers that carry a mesenchymal phenotype may particularly benefit from anti-TGF-β therapies [270-272] According to these results, the effectiveness of TGF-β blockers appears to be influenced by both the type of cancer and the stage of progression, with late-stage patients possibly seeing more significant improvements. In sum, using predictive biomarkers to determine patients who are most likely able to benefit most from treatment with anti-TGF-β agents may increase the clinical benefit of TGF-β targeted therapy. Factors such as mesenchymal gene expression signature, SMAD4 status, and tumors with extensive stromal development, such as pancreatic cancer, may be good candidates for TGF-β blockade. It should be noted that serial biopsies may be required to fine-tune treatment strategies as tumors may rewire their gene expression profile in response to treatment or with advanced tumor stage.
5.5 Dual Role of TGF-β
The dual nature of TGF-β poses significant challenges in developing therapeutic interventions, as inhibiting its tumor-promoting signaling may compromise its tumor-suppressive functions. Consequently, responses to TGF-β therapy across nearly all types of cancer are markedly context-dependent. The tumor-promoting characteristics of TGF-β have been extensively documented [16, 17, 59, 101, 149, 273-275]. Nevertheless, TGF-β exhibits tumor-suppressive activities during the early stages of tumor development; however, the extent to which this property is preserved in advanced tumors remains undetermined. Therefore, disrupting TGF-β signaling necessitates a more nuanced approach. In a controlled In Vitro study assessing the effects of TGF-β neutralizing antibodies on 12 metastatic breast cancer models, only 5 (42%) of the models exhibited a suppression of metastasis, whereas 4 (33%) displayed no response, and 3 (25%) experienced progressive stimulation of metastasis [276]. The inhibition of metastasis was found to be immune-dependent, while the stimulation of metastasis appeared to be primarily associated with tumor cells, particularly CSCs. Further transcriptomic analysis of the pre-treated tumors demonstrated differential gene enrichment; the responder group was enriched with genes related to inflammation and anti-tumor activity, characteristics of immunologically active tumors. In contrast, non-responders presented an immunologically inactive state, exhibiting heightened levels of rapamycin complex 1 (mTORC1) mammalian targets and tumor-associated macrophages (TAMs), recognized for their role in promoting immunosuppression [276].
Furthermore, the metastatic-stimulating properties of anti-TGF-β are attributed to the interference with TGF-β's tumor-suppressive effects, subsequently promoting the activities of CSCs. Notably, TGF-β inhibits CSC activity in certain melanoma and breast cancer models [277]. An additional study investigating the TGF-β tumor-suppressive gene signature indicated that the persistent expression of these genes is associated with a reduction in metastasis among breast cancer patients in this category. Collectively, this information suggests that the prevailing notion that the tumor-suppressive effects of TGF-β are diminished in advanced metastatic cancers may not be universally applicable across all patient categories within the context of breast cancer. This also implies that a subgroup of breast cancer patients with cold tumors, particularly those that are estrogen receptor-positive, may not derive benefits from anti-TGF-β therapies. The study underscores the significance of examining multiple targets rather than a singular target and the necessity of analyzing the transcriptomic profiles of cancer patients to identify candidates more likely to respond favorably to anti-TGF-β therapies. It remains to determine if a similar scenario exists in other cancer types.
Furthermore, TGF-β is recognized for its role in promoting metastasis by enhancing the activity of EMT-promoting genes. However, emerging studies from breast cancer [278], HCC [279], lung cancer [280], and CRC [281] indicate that anti-TGF-β treatment can also augment mesenchymal-epithelial transition (MET) activity, thereby enhancing colonization and metastasis, further complicating anti-TGF-β therapeutic application. Given that the majority of cancer diagnoses occur at an advanced stage when the disease has already disseminated into lymphatics and undergone EMT, the administration of anti-TGF-β therapies may hinder the maintenance of the mesenchymal phenotype while facilitating the seeding and spread of secondary tumors through MET. This observation is particularly evident in a breast cancer model exhibiting distinct phenotypes; administration of the TβR1 kinase inhibitor led to two disparate outcomes: treated and untreated epithelial-like breast cancer cells metastasized at comparable rates, whereas the mesenchymal-like breast cancer treated with anti-TGF-β exhibited an increased incidence of lung metastases, suggestive of enhanced MET activity in this context [278]. Thus, it can be inferred that TGF-β may potentially obstruct MET activity by impeding colonization in secondary organs. This further underscores the necessity of elucidating the precise role of TGF-β at the contextual level and at various cellular stages of cancer metastasis.
By and large, dual and context-dependent roles of TGF-β in cancer provides a strong rationale to consider localized blockade strategy may offer a more effective and safer therapeutic approach compared to systemic inhibition. This approach would balance efficacy with safety, ensuring that therapeutic benefits are maximized while systemic side effects are minimized. localized delivery methods, such as nanoparticle-based drug carriers, intratumoral injections, or tumor-targeted antibodies, could selectively inhibit TGF-β activity within the TME without affecting systemic signaling.
6 Perspectives and Future Direction
Following the mixed outcome with anti-TGF-β therapies in the clinic, recent efforts have been geared toward strategies that improve the anti-tumor efficacy of TGF-based therapies. Current studies are progressing in unraveling the different mechanisms of resistance involved in the TGF-β pathway in cancer [4, 282, 283]. The success of these studies will help usher in new therapeutic options in cancer TGF-β targeting. Combining anti-TGF-β therapies with immunotherapies is the most attractive strategy among oncology researchers [284, 285]. Early results from the combined blockade of TGF-β and immune checkpoint have shown promise [286, 287]. More research is expected as scientists rally to evaluate different ICIs for their combinatorial effects with anti-TGF-β therapies.
TGF-β and PD-L1 bispecific antibodies have demonstrated preclinical anti-tumor efficacy in murine colon cancer, lung cancer, and TNBC models [288, 289]. Also, a better understanding of TGF-β role in the progression of PDAC has led to a successful combination of anti-TGF-β therapies with chemotherapies in the preclinical models of PDAC. Pre-clinical and clinical studies (NCT04935359) of anti-TGF-β antibodies and chemotherapy are currently being explored [290, 291]. As we await results from these studies, many more clinical evaluations are expected for the anti-TGF-β plus ICI combinations in many cancer types, focusing on the safety and the anti-tumor efficacy in human subjects.
Future studies should consider several factors highlighted above (Section 5) to improve the efficacy of anti-TGF-β therapy. In addition, the dynamic nature of TGF-β signaling should be a key consideration for future research. Contrary to static activation patterns, TGF-β signaling fluctuates temporally due to complex interactions within the tumor TME [2], as illustrated in Figure 6. These fluctuations are believed to arise from a combination of factors originating in the TME and intrinsic cellular regulatory mechanisms. Specifically, inflammation, hypoxia, and mechanical cues in the TME can induce transient surges in TGF-β activity [292, 293]. Additionally, internal feedback loops, such as those involving SMAD7 and receptor dynamics, may modulate the intensity of signaling [294]. This dynamic signaling enables TGF-β activation levels to alternate between high (green box) and low (red box) activity states. Such fluctuations may significantly impact tumor behavior and therapeutic responses.
While cellular heterogeneity may result in varied responses to TGF-β signaling across different cells—such as Cell #1 versus Cell #2 in Figure 6—we speculate that carefully timed drug dosing to coincide with high-activity windows, as illustrated by Treatment A, is a promising strategy for enhancing anti-TGF-β therapy outcomes in cancer treatment. However, we acknowledge that this model may be oversimplified; cells with low TGF-β activity could escape inhibition and “rebound,” to create resistant clones. Unfortunately, traditional preclinical drug screening methods—where anti-TGF-β therapies are administered after tumors are established in mouse models—fail to account for the dynamic and heterogeneous responses to TGF-β signaling. Therefore, more advanced preclinical models are needed to experimentally validate the dynamic signaling patterns and provide deeper insights into TGF-β activation status across heterogeneous tumor states. To address this, we envision leveraging spontaneous tumor models with TGF-β gain-of-function alterations. From these models, researchers can employ serial tumor sampling to improve our understanding of how TGF-β activity evolves over time and how this evolution influences treatment response. Of note, tumor cells exhibiting high-, low-, and mixed-TGF-β signaling activity can be isolated and evaluated for their differential responses to anti-TGF-β therapies In Vitro. This approach would allow researchers to assess whether the activation level of TGF-β signaling determines its response to pathway inhibition, whether resistant cell populations emerge due to low initial TGF-β activity, and how these cells adapt post-treatment.
Furthermore, TGF-β activation levels likely vary depending on the stage of tumor progression. This underscores the need for non-invasive biomarkers capable of predicting TGF-β activity In Situ. These biomarkers would facilitate real-time monitoring of TGF-β signaling dynamics, which could be used to predict tumor stage and guide treatment planning. Emerging technologies—such as single-cell RNA sequencing (scRNA-seq) and FRET-based biosensors—offer powerful means to track how TGF-β signaling varies across individual cells within a tumor over time, how these patterns evolve throughout tumor progression. Insights gained from these approaches will not only deepen our understanding of TGF-β signaling but also guide the development of more effective therapies tailored to the unique temporal dynamics of the TME.
7 Concluding Remark
The biology of TGF-β signaling pathway and its biological implications have been extensively studied over the past four decades, emphasizing its role in physiological and pathological conditions, including cancer. Consequently, numerous anti-TGF-β therapies have been clinically tested for cancer therapies. For instance, neutralizing antibodies like Vactosertib and Galunisertib are being explored in combination with chemotherapy or immunotherapy. Ligand traps such as AVID200 are being studied alongside ICIs. Bifunctional antibodies like Bintrafusp Alfa, which selectively block TGF-β receptors and checkpoint inhibitors, are also under investigation. Additionally, the incorporation of the dormant negative form of TGF-β into CAR-T constructs is being developed to prevent immunosuppression. Vaccines and antisense oligonucleotides are also being explored. Despite these, none of these approaches have been able to appeal regulatory approval. In fact, only a few have progressed with acceptable safety and therapeutic profiles. To replicate the success seen in preclinical studies in clinical trials, more efforts are required to understand the complexity of TGF-β signaling such as its dynamic activity, paradoxical roles, non-invasive predictive biomarkers which will facilitate patient selection for optimal therapeutic benefits.
Author Contributions
Faizah Alabi: conceptualization (equal), data curation (equal), investigation (equal), project administration (equal), resources (equal), supervision (equal), validation (equal), visualization (equal), writing – original draft (lead), writing-review and editing (lead). Francis F. Okpalanwaka: conceptualization (equal), data curation (equal), investigation (equal), project administration (equal), resources (equal), supervision (equal), validation (equal), visualization (equal), writing – original draft (equal), writing-review and editing (equal). Sikiru O. Imodoye: conceptualization (equal), data curation (equal), investigation (equal), project administration (equal), resources (equal), supervision (equal), validation (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Adenike Oyegbesan: data curation (equal), investigation (equal), writing – original draft (equal), writing – review and editing (equal). Ebenezar Okoyeocha: data curation (equal), investigation (equal), writing – original draft (equal), writing – review and editing (equal). Mariam Oladejo: data curation (equal), writing- original draft (equal), writing – review and editing (equal). All authors have read and approved the final manuscript.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.