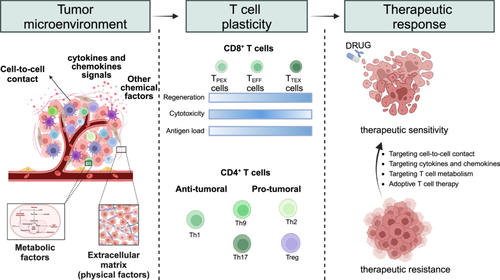
Unlocking T-Cell Plasticity in the Tumor Microenvironment: Implications for Cancer Progression and Therapeutic Strategies
Xiao-Hong Ding, Xue-Pei Li, and Fenfang Chen contributed equally to this study.
ABSTRACT
The tumor microenvironment (TME) is a complex and dynamic ecosystem crucial for cancer development and progression. Within this intricate milieu, T-cells constitute a heterogeneous population and serve as a cornerstone of antitumor immunity. Notably, T-cells can rapidly transition across a wide spectrum of phenotypic and functional states within the disrupted TME. Despite the crucial role of T-cells in cancer immunity, a comprehensive understanding of their plasticity within the TME remains limited. In this review, we delve into the functional plasticity and spatial distribution of T-cells in response to diverse microenvironmental conditions. Additionally, we review the plasticity of T-cell functional states during conventional therapies, highlighting their potential to enhance or limit therapeutic outcomes. Finally, we propose innovative therapeutic approaches that leverage T-cell plasticity to enhance clinical efficacy by regulating the immune response within the TME. By providing insights into the dynamics of T-cell behavior, this review highlights the promising potential of targeting T-cell plasticity as an immuno-sensitizer to refine therapeutic strategies and overcome current challenges in cancer treatment.
Graphical Abstract
The tumor microenvironment (TME) shapes T-cell plasticity, influencing cancer progression and therapeutic outcomes. Here, we summarize the phenotypic transitions and spatial distribution of T cells within the TME, highlighting their dual roles in therapeutic efficacy and resistance. Novel strategies leveraging T-cell plasticity offer the potential for optimizing cancer treatment.
1 Introduction
The tumor microenvironment (TME) is a structured ecosystem comprising tumor cells and various nonmalignant-cells, including immune cells, cancer-associated fibroblasts (CAFs), and endothelial cells [1]. Within this intricate TME, interactions between tumor cells and these various compartments form a sophisticated network that contributes to carcinogenesis, tumor progression, and metastasis [2, 3]. Furthermore, tumor cells can shift between cellular states, allowing them to adapt and survive under stress [4]. Other cells within the TME can either suppress or promote tumor development, depending on the context. For instance, neutrophils demonstrate this plasticity by acting as tumor fighters through direct cytotoxic activity or by activating T-cell-mediated immunity [5, 6]. However, they can also produce reactive oxygen species (ROS) and tumor necrosis factor (TNF), which suppress T-cell activity and result in chemotherapy resistance [7]. Alongside, while M1-like macrophages mediate tumoricidal effects and increase the efficacy of several pharmacological agents [8], M2-like macrophage populations impair T-cell function and thereby support tumor development [9, 10]. Overall, all components within the TME exhibit significant dynamism across the various stages of tumorigenesis, closely correlating with cancer progression and therapeutic response.
T-cells account for the largest portion of immune cells and are crucial for antitumor immunity in most solid tumors. CD8+ T-cells are the main responders to tumor cells, whereas CD4+ T-cells can exert both protumor and antitumor effects. The high heterogeneity of T-cells within the TME reflects their plasticity for reprogramming, allowing them to transition between functional states ranging from activation to exhaustion (Table 1). Consequently, the effectiveness of T-cell subsets in tumor eradication varies, which in turn leads to diverse prognostic outcomes for cancer patients [11]. Additionally, the localization of T-cells within the TME significantly influences their functional states. For example, T-cell populations that are spatially enriched at the tumor periphery usually display a diminished capacity to combat cancer cells compared with those within the tumor core, largely due to the presence of stromal barriers [12]. Interestingly, anticancer treatments can provoke a switch in the proportion or phenotype of CD8+ TEFF cells and TTEX cells in a response-associated manner [13, 14]. Specifically, discrepant dynamics of T-cell subsets have been observed among different groups of responsive patients. Therefore, an advanced understanding of T-cell plasticity could facilitate the exploration of its applications to improve clinical outcomes and therapeutic efficacy.
| CD8+ T-cells | Features | ||
|---|---|---|---|
| Regeneration | Cytotoxicity | Antigen load | |
| TPEX cells | + | — | + |
| TEFF cells | — | ++ | ++ |
| TTEX cells | — | +/− | + |
| CD4+ T-cells | Features | ||
|---|---|---|---|
| Lineage-special transcription factor | Effector cytokines | Major role | |
| Th1 | T-bet | IL-2, IFN-γ, TNF | Antitumoral |
| Th2 | Gata-3 | IF-4, IL-5, IL-13 | Pro-tumoral |
| Th9 | BATF | IL-9, IL-10 | Bidirectional |
| Th17 | ROR-γt | IL-17, IL-22 | Bidirectional |
| Tregs | Foxp3 | IL-10, TGF-β | Pro-tumoral |
- Abbreviations: IFN, interferon; IL, interleukin; TEFF cells, effector-like T-cells; TPEX cells, progenitor exhausted CD8+ T-cells; TTEX cells, terminally exhausted T-cells; TGF, transforming growth factor; Th cells, helper T-cells; TNF, tumor necrosis factor; Tregs, regulatory T-cells.
Here, we comprehensively summarized the intricate clues within the TME that trigger the functional alterations and spatial distribution of T-cells. We also discussed the T-cell plasticity in the context of conventional therapies, with an emphasis on its role in acquired drug resistance. Finally, we proposed potential opportunities and therapeutic strategies to mobilize T-cells for more effective tumor treatment.
2 Tumor Microenvironment (TME)-Mediated Regulation of T-Cell Functional Plasticity
T-cells exhibit significant functional plasticity in response to complex perturbations within the TME. Through interactions with various TME compartments, the activity of T-cells can be either enhanced or suppressed (Figure 1).
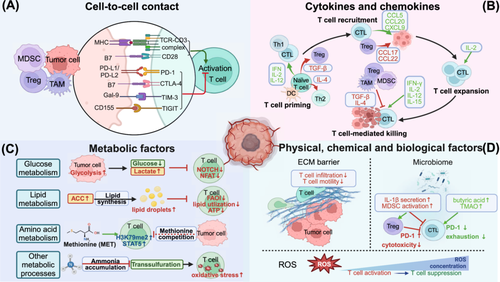
2.1 Impact of Cellular Signals on T-Cell Function
T-cell function in the TME is intricately regulated by cellular signals, which mainly include direct -cell-to-cell contact and soluble factors such as cytokines and chemokines. These signals dynamically shape T-cell-mediated immune responses, ultimately determining the balance between tumor elimination and immune escape.
2.1.1 Cell-to-Cell Contact
Direct communication between cells primarily depends on ligand‒receptor interactions at the cell surface (Figure 1A). As is well known, the interplay between the T-cell receptor (TCR) and the antigen-major histocompatibility complex (MHC) complex triggers T-cell activation [15, 16]. However, tumor cells often downregulate the expression of MHC class I molecules to achieve immune escape [17]. Costimulatory receptors also play pivotal roles in T-cell activation. CD28 is the main costimulatory receptor and potently enhances the T-cell-mediated immune response by inducing the expression of antiapoptotic proteins such as Bcl-XL and stimulating the synthesis of cytokines such as interleukin-2 (IL-2) [18].
Conversely, the activation of inhibitory receptors such as programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) by their ligands (PD-L1, PD-L2, and B7-1, B7-2, respectively) present on the surfaces of various cells (such as tumor cells, macrophages, dendritic cells and CAFs) significantly suppresses T-cell cytotoxicity [19-22]. Mechanically, PD-1 recruits Shp2 phosphatase upon activation by its ligand PD-L1, which further leads to the dephosphorylation of CD28 and immunosuppression [23]. CTLA-4 can inhibit T-cell activation by competing with CD28 for binding B7-1 and B7-2. Other immune checkpoint molecules, such as T-cell immunoglobulin (Ig) and mucin domain-containing protein 3 (TIM-3), and T-cell immunoreceptor with Ig and ITIM domains (TIGIT), have also been identified as immunosuppressive receptors [24]. For instance, TIM-3, expressed on most immune and some tumor cells, inhibits CD4+ T-cell activation by suppressing the IL-6-signal transducer and activator of transcription 3 (STAT3) pathway, blocking Th1 polarization, and impairing T-cell responses to tumor antigens [25]. Recent publications have uncovered novel ligand‒receptor interactions involved in T-cell regulation. Intriguingly, sialic acid binding Ig like lectin 15 (Siglec-15), which is upregulated on tumor cells and myeloid cells, appears to bind a putative receptor on T-cells rather than currently known receptors to transmit a suppressive signal for T-cell-mediated response [26]. Further studies are required to identify specific ligands in greater detail.
2.1.2 Cytokines and Chemokines
Cells within the TME produce a variety of cytokines, including ILs, transforming growth factor-beta (TGF-β), interferons (IFNs), and chemokines such as C–C motif chemokine ligand 5 (CCL5) and receptor 4 (CCR4), all of which are essential for T-cell function [27] (Figure 1B).
IL-2 was the first interleukin identified as capable of driving activated T-cell proliferation, thereby enhancing the cytotoxic activity of CD8+ T-cells [28]. Meanwhile, IL-2-mediated STAT5 activation is essential for the immunosuppressive activity of Tregs, which are critical for immune homeostasis in tumors [29]. IL-12 enhances T-cell priming and promotes the differentiation of naïve T-cells into Th1 cells by activating the Janus kinase (JAK)-STAT signaling pathway, particularly through the phosphorylation of STAT4, which induces the expression of the Th1-specific transcription factor T-bet [30]. Other interleukins, such as IL-1 and IL-15, also contribute significantly by promoting T-cell survival and activation [31, 32]. Conversely, IL-4 promotes Th2 differentiation in CD4+ T-cells and reduces perforin and granzyme expression in CD8+ T-cells, suppressing antitumor immunity [33].
TGF-β is recognized as an immune suppressor and can inhibit T-cell function through multiple mechanisms. First, TGF-β promotes FOXP3 expression through SMAD family member 3 (SMAD3) and nuclear factor of activated T-cells (NFAT) binding to the FOXP3 enhancer region, thereby directing naïve T-cells to convert into the Treg population [34]. Moreover, TGF-β dampens CD8+ TEFF cell function through the suppression of key genes, including granzyme-B and IFN-γ, which is mediated by TGF-β-activated SMADs and the transcription factor ATF1 [35]. TGF-β also indirectly inhibits T-cell activation by compromising the antigen-presenting function of antigen-presenting cells (APCs) [36].
Sufficient early production of IFN in the TME enhances the priming and cytotoxicity of CD8+ T-cells via diverse pathways. For example, type I IFNs facilitate the cross-priming of CD8+ T-cells by promoting dendritic cell maturation and boosting their cytotoxic activity by upregulating perforin and granzyme expression [37]. Additionally, IFNs protect T-cells from NK cell-mediated elimination through modulating the expression of NK cell receptor ligands on T-cells [38, 39]. Notably, IFN-γ has been shown to promote T-cell recruitment into the TME through regulating the transcription of chemokines such as C-X-C motif chemokine ligand 9 (CXCL9) and its receptor CXCR3 on T-cells [40]. Studies indicate that IFN-γ may upregulate PD-L1 expression on Tregs, potentially reducing their immunosuppressive function and enhancing antitumor immunity [41]. However, prolonged IFN signaling induces STAT1-related epigenomic alterations and increases the expression of multiple ligands for T-cell inhibitory receptors [42]. Therefore, the nature of cytokine signaling plays a crucial role in determining the balance between immune-mediated tumor elimination and immune escape.
Chemokines, such as CCL5, CCL20, and CXCL9, are responsible for T-cell recruitment at the tumor site [43-45]. Among these, CXCL12 enhances the adhesion of T-cells to dendritic cells through regulating the affinity of integrins, thereby promoting T-cell priming. Additionally, CCR5 and CXCR4 can be recruited to the immunological synapses by APC-secreted chemokines, strengthening T-cell-APC attraction and promoting T-cell proliferation as well as cytokine production [46]. However, multiple chemokine systems, such as CCR4-CCL17/CCL22, CCR10-CCL27, and CXCR3-CXCL9/CXCL10/CXCL11, can also recruit Tregs to the TME, thereby interfering with the host's antitumor immunity [47, 48].
In summary, cellular signals within the TME play crucial roles in regulating T-cell activity. Future research should explore how these signals interact‒whether synergistically or antagonistically—to shape T-cell responses, paving the way for the development of more effective combination therapies. Moreover, the discovery of novel immune checkpoints and cytokine-related targets offers significant potential for enhancing T-cell-mediated antitumor immunity while reducing the risk of adverse effects.
2.2 Impact of Metabolic Factors on T-Cell Function
The intense nutrient competition and unique metabolic characteristics of the TME play critical roles in regulating T-cell function, either by inducing metabolic reprogramming of T-cells or directly modulating their immune activity [49]. The following sections elaborate on this topic from the perspectives of glucose metabolism, lipid metabolism, amino acid metabolism and other metabolic processes (Figure 1C).
2.2.1 Glucose Metabolism
Tumor cells preferentially rely on glycolysis for energy production even under aerobic conditions, known as the Warburg effect, which leads to excessive glucose consumption and lactate accumulation. This metabolic characteristic restricts the availability of glucose for T-cells and thereby impacts their immune function. For example, glycolysis restriction in the TME, such as through glucose competition with cancer cells, impairs CD8+ TEFF cell function [50]. Mechanistically, tumor-induced glucose restriction leads to miRNA-mediated suppression of the methyltransferase EZH2, an important activator of the NOTCH signaling pathway, thereby inhibiting cytokine expression in TEFF cells [51]. Glucose deprivation can also impair T-cell function by reducing phosphoenolpyruvate, a downstream metabolite of glycolysis that is essential for the Ca2+-NFAT signaling in T-cells [52]. Elevated extracellular lactate levels, another consequence of the high glycolytic activity of tumor cells, can also impair T-cell cytotoxicity by diminishing NFAT levels and reducing succinate secretion, which has been shown to promote the synthesis of inflammatory cytokines and T-cell cytotoxicity [53, 54]. Notably, Tregs can extensively remodel their metabolism to adapt to the low-glucose and high-lactate microenvironment. The transcription factor FOXP3 plays a central role in this process by suppressing Myc and glycolysis while enhancing oxidative phosphorylation (OXPHOS) and NAD+ oxidation [55]. These metabolic adaptations provide Tregs a distinct survival advantage, enabling them to resist lactate-induced suppression of T-cell activity and maintain their immunosuppressive function even under metabolically challenging conditions.
2.2.2 Lipid Metabolism
Lipid metabolism plays a pivotal role in regulating the function of tumor-infiltrating T-cells. Linoleic acid (LA) has been proven to protect CD8+ T-cells from exhaustion and promote a memory phenotype with superior cytotoxic functions [56]. This effect is attributed to LA's ability to foster the formation of ER‒mitochondria contacts (MERCs), which enhances the mitochondrial energetics and effector functions of T-cells. Furthermore, fatty acid binding protein 5 (FABP5) regulates Treg suppressive function by preserving lipid metabolism and maintaining mitochondrial integrity [57]. Inhibition of FABP5, either genetically or pharmacologically, disrupts lipid metabolism and further leads to mitochondrial DNA release and activation of cGAS-STING-dependent type I IFN signaling, which in turn enhances IL-10 production and promotes the immunosuppressive function of Tregs. Nutrient stress in the TME also drives lipid metabolic reprogramming in T-cells. For example, T-cells may catabolize lipids through mitochondrial fatty acid oxidation (FAO), a process initiated by the enzyme carnitine palmitoyltransferase 1A (CPT1A), to maintain effective energy synthesis for effector functions and survival [58, 59]. However, the activity of acetyl-coenzyme A carboxylase (ACC), a key enzyme in fatty acid synthesis, is increased in the solid TME, leading to lipid synthesis and accumulation in T-cells, which is opposite to the process of FAO [60]. As a result, most tumor-infiltrating T-cells in the solid TME struggle to sustain long-term bioenergetics. Additionally, the uptake of fatty acids mediated by CD36 can exacerbate lipid accumulation in CD8+ T-cells, leading to lipid peroxidation and ferroptosis, which further compromises T-cell function [61].
2.2.3 Amino Acid Metabolism
Various amino acids can regulate the antitumor immunity of T-cells. For instance, methionine enhances T-cell function by upregulating the expression of H3K79me2 and STAT5. However, tumor cells can compete with T-cells for methionine through expressing high levels of the methionine transporter SLC43A2, resulting in T-cell dysfunction [61, 62]. Moreover, L-arginine metabolism undergoes significant changes following T-cell activation. Although T-cells exhibit increased l-arginine uptake, arginase rapidly converts it into downstream metabolites and thereby leads to a decline in intracellular L-arginine levels, which could impair T-cell immune responsiveness by reducing the expression of the CD3ζ chain [63, 64]. In contrast, higher intracellular L-arginine levels can enhance T-cell antitumor responses and facilitate the generation of central memory-like T-cells by inducing a metabolic shift from glycolysis to OXPHOS [64]. Additionally, kynurenine released by tumor-repopulating cells in the TME can upregulate PD-1 expression when transferred into CD8+ T-cells via the transporters SLC7A8 and PAT4, thereby inhibiting their antitumor effects [39].
2.2.4 Other Metabolic Processes
Various metabolic pathways, beyond those previously mentioned, also play crucial roles in T-cell regulation. A representative example is elevated ammonia levels in the TME of colorectal cancer due to dysregulation of the urea cycle. High ammonia levels could induce oxidative stress in T-cells by inhibiting the transsulfuration pathway, thereby leading to their exhaustion [65]. A recent study illustrated that tumor cells could upregulate N-acetyltransferase 8-like (NAT8L) and its metabolite N-acetylaspartate (NAA) in the TME of HER2+ breast cancer, mimicking anti-inflammatory mechanisms in the central nervous system to inhibit the cytotoxicity of CD8+ T-cells through disrupting the formation of immunological synapses [66]. In addition, metabolites produced by the gut microbiota can also exert complex effects on T-cell immune function within the TME, and the underlying mechanisms are currently under further investigation [67, 68].
To summarize, metabolic factors within the TME exert profound effects on T-cell function. Future studies are needed to further elucidate the dynamic metabolic crosstalk between tumor cells and immune cells. A deeper understanding of how specific metabolites influence distinct T-cell subsets and their antitumor functions could provide valuable insights for therapeutic strategies. Additionally, developing approaches to modulate T-cell metabolism to overcome the suppressive effects of the TME holds promise for enhancing the efficacy of cancer immunotherapy.
2.3 Impact of Physical, Chemical and Biological Factors on T-Cell Function
In addition to cellular signals and metabolic factors, TME also encompasses a complex network of physical, chemical, and biological factors that profoundly influence T-cell function. Key elements such as the extracellular matrix, ROS, and the intratumoral microbiota have emerged as critical modulators of T-cell activity in recent years (Figure 1D).
2.3.1 Extracellular Matrix
Tumor progression is accompanied by significant remodeling of the extracellular matrix (ECM), which is typically characterized by increased collagen content and increased stiffness [69]. Studies have shown that T-cell proliferation and cytotoxic functions are significantly suppressed in high-density matrix environments, potentially due to the regulatory effects of autocrine TGF-β signaling [70]. Moreover, increased matrix stiffness resulting from collagen deposition in the ECM architecture can reduce T-cell migration and hinder their infiltration into the tumor [71]. Mechanistically, the binding of the extracellular domain of discoidin domain receptor 1 to collagen can align the fibers into a highly-organized structure that acts as an immune-exclusion barrier, thus blocking the infiltration of T-cells and other immune cells [72, 73].
2.3.2 Reactive Oxygen Species
ROS, commonly generated by cells within the TME, play a key role in tumor progression. With respect to T-cell regulation, ROS exhibits a concentration-dependent effect on immune functions. Low to moderate levels of ROS, generated by complex III of the mitochondrial electron transport chain, are necessary for NFAT activation and subsequent IL-2 production, which promotes T-cell activation and proliferation [74]. Conversely, excessive ROS accumulation may inhibit the DNA-binding ability of transcription factors such as NFAT and NF-κB, thereby reducing IL-2 production and negatively impacting T-cell functions [75, 76]. Moreover, large amounts of ROS produced by macrophages and neutrophils at the tumor site can impair T-cell function through the inhibition of DNA synthesis. Studies have also shown that excessive ROS generated by dysfunctional mitochondria could contribute to exhausted-like states of T-cells by inhibiting phosphatase activity and driving continuous NFAT signaling [77].
2.3.3 Intratumoral Microbiota
Recent evidence has revealed an association between antitumor immunity and the intratumoral microbiota. Microbiota exhibit bidirectional roles in antitumor immunity through a variety of mechanisms. On the one hand, the commensal microbiota may restrain T-cell activity via specific signaling activation, metabolic reprogramming and interactions with other immune cells [78-80]. For example, the microbiome in pancreatic ductal adenocarcinoma drives the differentiation of suppressive monocytes by activating specific Toll-like receptors, thereby leading to T-cell dysfunction [78]. In patients with lung tumors, A. sydowii colonization in the TME contributes to the suppression of TEFF cell activity and the accumulation of PD-1+ CD8+ T-cells mediated by IL-1β signaling [81]. On the other hand, a distinct community of microbiota may increase T-cell activation via multiple pathways [82, 83]. Specifically, intratumoral Fusobacterium nucleatum inhibits PD-1 expression in a manner dependent on its production of butyric acid, which helps alleviate CD8+ T-cell exhaustion and enhances their cytotoxic function through increasing H3K27ac levels at the Tbx21 promoter [84]. Similarly, the microbial metabolite trimethylamine N-oxide (TMAO) promotes CD8+ T-cell-mediated antitumor immunity by inducing pyroptosis in tumor cells in triple-negative breast cancer (TNBC) [85].
This section highlights the regulatory effects of physical, chemical, and biological factors in the TME—such as the ECM, ROS, and intratumoral microbiome—on T-cell function. A deeper understanding of the intricate interplay between these factors and T-cell biology is essential to uncover novel mechanisms driving immune evasion. Furthermore, exploring therapeutic strategies that modulate these factors may provide new avenues for enhancing T-cell-mediated antitumor immunity.
3 T-Cell Plasticity in Spatial Organization Within the TME
The spatial architecture of T-cells within the TME is a key determinant of antitumor immunity. Understanding the spatial architecture of T-cells is crucial for comprehending how immune responses are regulated and how tumors may evade immune surveillance. In this section, we explore how T-cells are distributed within tumors and delve into the barriers they face in infiltrating tumor tissues.
3.1 T-Cell Localization in Tumors
With the advent of high-resolution sequencing technologies, the topological gradient of diverse T-cell subsets has been comprehensively characterized. On the basis of their spatial proximity to the tumor, T-cells can be divided into three distinct subgroups: those predominantly located at the tumor core (TC), those at the invasive margin (IM), and those within the tumor stroma (TS) [86] (Figure 2).
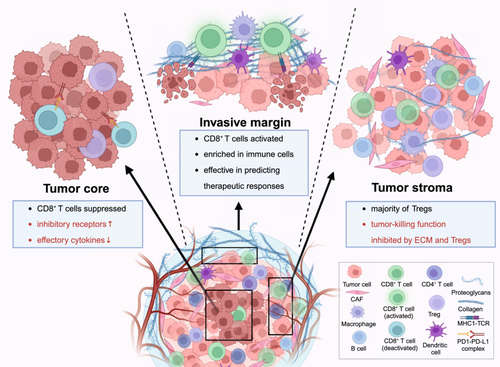
Importantly, the unique architecture of different regions within the TME orchestrates T-cell immunologic effects in patients with various solid tumors. TC is typically characterized by hypoxia and a dense population of tumor cells [87]. Within this region, TEFF cells are crucial for directly targeting and destroying tumor cells. However, the effectiveness of these T-cells can be largely compromised by surrounding immunosuppressive components, including increased expression levels of inhibitory receptors and decreased secretion of effector cytokines [88, 89]. The IM represents the interface between the tumor and surrounding normal tissue. This area is rich in activated T-cells, dendritic cells, and many other immune cells. Thus, these T-cells serve as the primary force in tumor cell elimination and can effectively predict therapeutic responses [90, 91]. In contrast, TS regions, composed of various nonmalignant-cells, restrict the infiltration and functionality of CD8+ T-cells [92]. Meanwhile, Tregs, a major subset of CD4+ T-cells which are predominantly located in this compartment, exert an immunosuppressive effect and inhibit the cytotoxicity of surrounding TEFF cells [93, 94].
3.2 T-Cell Zones and Tumor Progression
T-cell zones within the TME, primarily T-cell-enriched zones and immunosuppressive regions, play pivotal roles in shaping the immune response and influencing tumor progression. T-cell-enriched zones are typically found within the TC or IM regions, where TEFF cells exert cytotoxic activity against tumor cells [95]. However, T-cell plasticity allows for phenotypic transitions under TME-derived pressures, leading to exhausted or regulatory states that dampen antitumor responses. For example, exhausted CD8+ T-cells accumulate in dysfunctional T-cell niches, characterized by high expression of inhibitory receptors (e.g., PD-1, TIM-3) and impaired effector function [89].
Specifically, tertiary lymphoid structures (TLSs), ectopic aggregates that reflect lymphoid neogenesis during tumor progression, may form within the TME across several solid tumors [96]. TLSs are characterized by a T-cell-rich zone, the presence of mature dendritic cells, and B-cell follicles containing a germinal center. These organized structures play critical roles in the effective immune responses through interactions between T-cells, dendritic cells, and B cells [97]. Recent studies have demonstrated that TLSs are heterogeneously distributed in the TC, IM, and TS regions in a significant fraction of cancers [98]. Notably, tumors with infiltrative TLSs have more CD8+ T-cells, indicating that TLSs may play a key role in CD8+ T-cell activation to fight against tumor cells [99]. For example, TLSs in lung adenocarcinoma have been reported to be spatially correlated with CD8+ T-cells and facilitate their interactions with tumor-antigen presenting dendritic cells [100]. Consequently, the presence of TLSs is strongly correlated with superior overall survival and can be considered a novel marker to predict the clinical outcome of cancer patients, regardless of tumor type and tumor stage [101-103].
Moreover, T-cells are distributed in a spatially organized manner in the pre-metastatic niches (PMNs). In the early stages of PMN formation, TEFF cells lead to immune surveillance by detecting and eliminating disseminated tumor cells. As the PMN evolves, environmental factors such as cytokine signals, immune checkpoint pathways, and hypoxia can drive TEFF cells toward exhaustion or functional suppression, limiting their ability to mount effective antitumor responses [104, 105]. Additionally, Tregs are polarized within the PMNs, which contributes to immune tolerance by dampening the activation of TEFF cells and inhibiting the immune response against circulating tumor cells [106]. Consequently, there is a decline in the proliferation and activation of TEFF cells, accompanied by an increase in Tregs, which collectively contribute to the establishment of an immunosuppressive environment that fosters tumor growth and metastasis [106].
3.3 Barriers to T-Cell Infiltration
In addition to the generation and accumulation, the efficient migration of T-cells is indispensable for their ability to combat tumors. Indeed, both physical barriers and biochemical signals mediate T-cell exclusion in the TME.
The fundamental role of blood vessels in initiating T-cell migration has been explicitly demonstrated. The interaction between homing molecules, such as E-selectin and P-selectin, and their ligands enables the rolling of T-cells along the vessel walls, followed by adhesion and extravasation through the blood vessel wall to the tumor site [107]. However, the abnormal vasculature in tumors can impede T-cell movement. Besides, tumor-secreted galectin-1 (Gal1) on the tumor endothelium can upregulate the expression of PD-L1 and thereby drive immune evasion [108]. Notably, a dense ECM creates physical barriers that restrict the migration of T-cells into tumors. Collagen fibers can form a rigid scaffold that accelerates T-cell movements through contact guidance. Within low-density collagen matrixes, T-cells exhibit active migration, enabling efficient surveillance and interaction with other immune cells [109].
In addition, cytokines secreted by ECM components and other surrounding cells also lead to the T-cell-excluded phenotype [110]. Importantly, TGF-β produced by tumor-associated macrophages (TAMs) and other stromal components contributes to the dense extracellular matrix production, thereby preventing T-cell infiltration [111-113]. Furthermore, several malignant cell-autonomous programs have been identified to impair T-cell migration ability. As an example, the tumor-intrinsic active Wnt/β-catenin pathway is associated with a paucity of T-cell infiltration within the TME in melanoma, colorectal cancer, and many other cancer types, resulting in the exclusion of the host immune response [114-117]. In addition, Wnt1 leads to the transcriptional silencing of CC/CXC chemokines and consequently T-cell exclusion.
4 T-Cell Plasticity During Conventional Therapies
Over the past decade, traditional chemotherapy and radiotherapy have been thought to provide therapeutic benefits through the disruption of DNA replication and blockade of tumor cell division. With the advent of high-throughput technologies, multiple novel targets have been identified for therapeutic intervention. Emerging studies suggest that these therapies may result in a series of immunological effects [118]. Focusing on these cancer approaches, we summarized how T-cells acquire functional plasticity during therapies and how they affect therapeutic effectiveness (Figure 3).
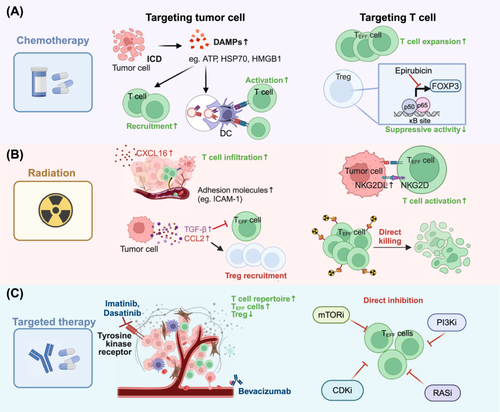
4.1 Chemotherapy
Chemotherapeutic agents tend to exhibit direct immunosuppressive effects due to their cytotoxic effects on T-cells and other immune cells [119]. However, the efficacy of various chemotherapies used at doses below the maximum tolerated dose, including anthracyclines, oxaliplatin, and paclitaxel, is confirmed to be much greater in immunocompetent mouse models than in their immunodeficient counterparts [120]. As demonstrated in previous preclinical and clinical studies, several chemotherapeutic agents significantly increase the ratio of TEFF to Treg cells [118, 121]. These observations were validated in a single-cell transcriptomics study on paired pretreatment and on-treatment samples of gastric cancer patients treated with fluoropyrimidine and platinum agents. In responders, the proportion of effector CD8+ T-cells tended to increase, whereas the percentage of Treg cells within the TME tended to decrease compared with that in pretreatment samples. In contrast, the fraction of TTEX cells in non-responders increased during chemotherapy [122]. Therefore, accumulating evidence clearly indicates that conventional chemotherapy agents have the potential ability to stimulate antitumor activity through a range of immunological mechanisms [123]. On the one hand, chemical agents can trigger immunogenic cell death (ICD) to initiate an adaptive antitumor immune response. However, these agents may directly target specific immune cell subsets.
ICD is a form of regulated cell death that can promote the release or exposure of damage-associated molecular patterns (DAMPs) from dying tumor cells, involving the secretion of ATP from lysosomes and the translocation of endoplasmic reticulum chaperones (e.g., HSP70, HSP90). In addition, numerous cytoplasmic and nuclear proteins, including ANXA1 and HMGB1, are released into the TME [124]. Under chemotherapy-induced stress, these DAMPs drive the cross-presentation of tumor antigens to TEFF cells to support the initiation of anticancer immunity (Figure 3A). For example, docetaxel shows the potential to recruit CD8+ T-cells by increasing the secretion of HMGB1 and CXCL11, thereby increasing antitumor immune activity [125].
More importantly, chemotherapeutic agents have direct effects on immune cells, including the regulation of TEFF cells and the suppression of Treg cells (Figure 3A). Oligoclonal expansion of T-cells is detected in high-grade serous ovarian cancer following neoadjuvant chemotherapy with paclitaxel and cisplatin, suggesting local immune activation within the TME [126]. Several chemotherapies (including anthracyclines, camptothecin, and gemcitabine) have been shown to selectively decrease Treg numbers and restrain their inhibitory function [127-129]. Mechanistically, epirubicin, for example, disrupts the interaction between FOXP3 and the NF-κB subunit p65 [130].
In summary, chemotherapies play a key role in modulating the immune response. Different cytotoxic mechanisms and delivered doses of chemotherapeutic agents may result in various effects of chemotherapy on T-cell plasticity. Understanding these immunological changes mediated by chemotherapies can provide more mechanistic and clinically applicable insights, facilitating the development of approaches combining with immune checkpoint inhibitors (ICIs) for a wide range of malignancies.
4.2 Radiotherapy
Radiotherapy, a standard locoregional treatment using ionizing radiation, has a multifaceted role in the management of malignant tumors. While primarily aimed at cancer cell killing via damage to the cellular DNA, it also induces immunological changes within the TME [131] (Figure 3B).
Notably, radiation appears to increase the infiltration of CD8+ T-cells within the TME. Mechanically, radiation can directly stimulate the expression of CXCL16 in cancer cells, a chemokine that binds to CXCR6 on CD8+ TEFF cells, thereby promoting the migration of CXCR6+ activated T-cells to tumors [132]. Additionally, radiation-induced NF-κB activation upregulates various cell adhesion molecules on endothelial cells, such as VCAM-1, ICAM-1, and E-selectin, facilitating T-cell to migrate across endothelial barriers and infiltrate into the TME [133-135]. Furthermore, radiotherapy-induced DNA damage may increase the mutational burden and increase the variety of peptides [136, 137]. Therefore, MHC-I expression on the tumor cell surface is upregulated in a radiation dose-dependent manner, enhancing the presentation of tumor-specific peptides to T-cells [138]. Such perturbation induced by radiotherapy strongly promotes CD8+ T-cell-mediated identification and killing of tumor cells. Aside from its effect on tumor antigen presentation, radiation upregulates the expression of NKG2DL on tumor cells, which can bind to NKG2D on the activated T-cell surface to facilitate tumor cell destruction [139, 140].
Nonetheless, cellular and molecular adaptive changes within the TME in response to radiation may contribute to tumor radioresistance via multiple mechanisms, including alterations in cytokine signaling and the recruitment of locally suppressive immune cells [141]. One of the most important cytokines induced by radiation is TGF-β, which supports the immunosuppressive1 phenotype by reducing CD8+ T-cell cytotoxicity, inhibiting CD4+ T-cell differentiation, and fostering Treg transformation [138]. Moreover, radiotherapy increases CCL2 production in tumor cells and recruits CCR2+ Tregs and monocytes, further leading to an immunosuppressive environment [142]. Of note, T-cells are inevitably exposed to radiation over prolonged treatment courses. The conventional 2 Gy once-daily schedule has been confirmed to be sufficient to induce T-cells functional plasticity [143]. Studies have shown that Tregs exhibit greater resistance to radiation-induced cell death than conventional CD4+ T-cells and CD8+ T-cells [144, 145]. Therefore, radiation can shift T-cells to an immunosuppressive phenotype and reduce the therapeutic efficacy of radiation therapy.
In summary, radiotherapy can reprogram the TME to an immunostimulatory phenotype while paradoxically having the potential to induce immunosuppression and, in turn, weaken the effectiveness of radiotherapy.
4.3 Targeted Therapy
Over the past few decades, agents and monoclonal antibodies (mAbs) that specifically target membrane antigens or growth factor receptors have emerged as promising strategies for cancer patients. Owing to the nature of antibodies, mAbs participate in immune response mechanisms, including antibody-dependent-cellular cytotoxicity and complement-dependent cytotoxicity [146]. Here, we summarized the relatively unexpected T-cell plasticity mediated by several targeted therapies (Figure 3C).
Imatinib, the first targeted agent for the treatment of chronic myeloid leukemia (CML), has been shown to promote the infiltration of CD3+ T-cells, suggesting its role in T-cell recruitment [147]. Similarly, dasatinib, a broad-spectrum tyrosine kinase inhibitor approved for CML, has been confirmed to trigger the expansion of a therapeutically superior T-cell repertoire while reducing the population of CD4+ FOXP3+ Tregs [148]. Bevacizumab, an anti-vascular endothelial growth factor (VEGF) antibody, has been shown to promote vascular normalization and facilitate the entry of T-cells into tumors, thus enhancing the effectiveness of adoptive cell transfer-based immunotherapy [149]. In another study, bevacizumab increased the number of intratumoral CD8+ T-cells and promoted the migration of antigen-specific T-cells in the companion of atezolizumab [150].
Other targeted agents also exhibit T-cell modulatory effects. For instance, the administration of olaparib (a PARP inhibitor) to BRCA-deficient TNBCs increases CD8+ T-cell abundance and activates antitumor immunity [151]. Additionally, MEK inhibitors increase the number of effector-phenotype antigen-specific CD8+ T-cells within the tumor and protect antitumor T-cells from apoptosis driven by chronic TCR stimulation while sparing their cytotoxic activity [152, 153]. In addition, BRAF inhibition has been demonstrated to increase CD8+ T-cell infiltration [154].
Together, multiple targeted anticancer agents appear to stimulate T-cell-mediated immune responses. However, the proliferation and activation of T-cells rely on multiple signaling pathways, such as the RAS-MAPK, PI3K-mTOR, and CDK pathways, which are also targeted by antitumor small-molecule inhibitors [155]. As a result, the cytotoxic activity of T-cells might be directly influenced by targeted drugs. The variable effects need to be further confirmed in certain clinical scenarios. The molecular mechanisms underlying radiotherapy-induced T-cell plasticity also remain to be explored in depth.
5 Potential Therapies Harnessing the Plasticity of T-Cells in the TME
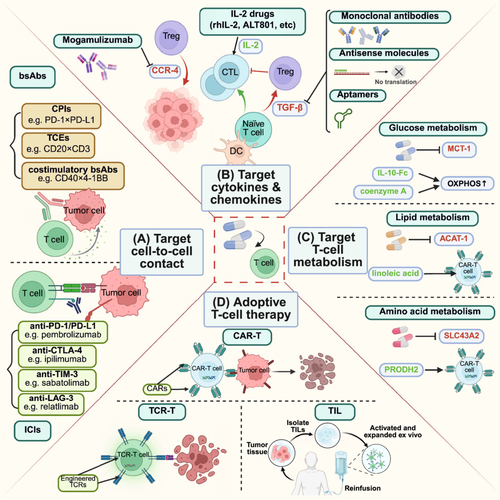
Over the past decade, considerable efforts have been devoted to the development of potential therapies based on the plasticity of T-cells within the TME. By leveraging T-cell functional plasticity, immune responses can be more effectively activated to enhance the efficacy of immunotherapy. In this section, we summarize key clinical trials and preclinical studies that illustrate these findings (Figure 4 and Table 2).
| Therapy | Compound | Target | Clinical trial | Phase | Conditions | Efficacy | Ref. |
|---|---|---|---|---|---|---|---|
| Targeting cell-to-cell contact | |||||||
| ICIs | Ipilimumab | CTLA-4 | NCT00289640 | Ⅱ | Advanced melanoma | Dose-dependent effect; ORR: 11.1%for 10 mg/kg, 4.2% for 3 mg/kg, and 0% for 0.3 mg/kg | [157] |
| Pembrolizumab | PD-1 | NCT01866319 | Ⅲ | Advanced melanoma | Pembrolizumab (Q2W/Q3W) versus ipilimumab: improved PFS [47.3% (Q2W), 46.4% (Q3W) vs. 26.5%] | [158] | |
| NCT03036488 | Ⅲ | Early TNBC | Neoadjuvant pembrolizumab vs. placebo: improved EFS (84.5% vs. 76.8%) | [159] | |||
| NCT03036488 | Ⅲ | Early TNBC | Neoadjuvant pembrolizumab vs. placebo: improved pCR (64.8% vs. 51.2%) | [162] | |||
| Nivolumab | PD-1 | NCT03838159 | Ⅱ | Stage III NSCLC | Neoadjuvant pivolumab plus chemotherapy versus chemotherapy: improved pCR (37% vs. 7%) and PFS (67.2% vs. 40.9%) | [160] | |
| Atezolizumab | PD-L1 | NCT03191786 | Ⅲ | NSCLC | Atezolizumab versus chemotherapy: improved OS (10.3 vs. 9.2 months) | [161] | |
| Relatlimab | LAG-3 | NCT03470922 | Ⅱ/Ⅲ | Advanced melanoma | Relatlimab + nivolumab versus nivolumab: improved PFS (10.1 vs. 4.6 months) | [163] | |
| Sabatolimab | TIM-3 | NCT02608268 | Ⅰ/Ⅱ | Advanced solid tumors | Sabatolimab + spartalizumab versus sabatolimab: responses (6% vs. 0%) | [164] | |
| Ociperlimab | TIGIT | NCT04047862 | Ⅰ/Ⅰb | Advanced solid tumors | Ociperlimab plus tislelizumab: 10% ORR | [165] | |
| BsAbs | PD-L1×EGFR | PD-L1×EGFR | — | Preclinical | PD-L1×EGFR vs. PD-L1×Mock in mice: 140 times lower IC50 and higher tumor uptake | [167] | |
| LY3434172 | PD-1×PD-L1 | NCT03936959 | Ⅰ | LY3434172 vs. anti-PD-1 and anti-PD-L1 antibody or respective monotherapies: improved antitumor immune response. Data from clinical trials are not yet available. | [168] | ||
| Mosunetuzumab | CD20×CD3 | NCT02500407 | Ⅰ/Ⅱ | Advanced follicular lymphoma | Mosunetuzumab versus historical control copanlisib: increased complete response rate (60.0% vs. 14%) | [169] | |
| CD40×4-1BB | CD40×4-1BB | NCT04083599 | Ⅰ/Ⅱ | Advanced solid tumors | CD40×4-1BB mediates clear immune modulation of peripheral APCs and T-cells in patients. Data from clinical trials are not yet available. | [170] | |
| Targeting cytokines and chemokines | |||||||
| TGF-β inhibitors | Trabederson | TGF-β2 | — | Ⅱb | High-grade glioma | Trabederson versus chemotherapy: did not improve DCR at 6 months | [173] |
| Fresolimumab | TGF-β | NCT01401062 | Ⅱ | Metastatic breast cancer | Fresolimumab in combination with radiotherapy: improved OS | [176] | |
| NCT00356460 | Ⅰ | Melanoma; RCC | 1/28 patients with achieved PR and 6/28 had stable disease | [177] | |||
| Galunisertib | TGF-βR1 | NCT02688712 | Ⅱ | Advanced rectal cancer | galunisertib to neoadjuvant chemoradiotherapy: achieved CR (12/38) | [174] | |
| SHR-1701 | PD-L1×TGF-β | NCT04580498 | Ⅱ | Stage III NSCLC | Neoadjuvant SHR-1701 with chemotherapy: achieved an ORR of 58% and an 18-month EFS of 56.6% | [178] | |
| Recombinant IL-2 | Aldesleukin | IL-2R | NCT00026312 | Ⅲ | Neuroblastoma | Ch14.18 (dinutuximab) + Aldesleukin + isotretinoin vs. isotretinoin alone: improved EFS at 2 years (66% vs. 46%) and OS at 2 years (86% vs. 75%) | [182] |
| ALT801 | NCT00496860 | Ⅰ | Advanced tumors | ALT-801: 38% patients achieved SD | [185] | ||
| ALKS 4230 | NCT03861793 | Ⅰ/Ⅱ | ALKS 4230 versus rhIL-2 in mice: superior antitumor activity and lower toxicity. Data from clinical trials are not yet available. | [186] | |||
| Recombinant IFNγ | IFNγ | IFN-γR1,2 | NCT04628338 | Ⅰ | AML | Data from clinical trials are not yet available. | [192] |
| CCR4 inhibitors | Mogamulizumab | CCR4 | NCT01728805 | Ⅲ | Cutaneous T-cell lymphoma | Mogamulizumab vs. vorinostat: improved PFS (7.7 versus 3.1 months) | [189] |
| NCT02476123 | Ⅰ | Advanced or metastatic tumors | Mogamulizumab in combination with nivolumab: show responses in 4/15 patients with hepatocellular carcinoma and 1/15 patients with pancreatic adenocarcinoma. | [190] | |||
| Targeting T-cell metabolism | |||||||
|---|---|---|---|---|---|---|---|
| As this section covers only preclinical studies, column Clinical trial and Phase are omitted. Instead, the drugs’ mechanisms are provided. | |||||||
| Compound | Target | Mechanism | Efficacy | Ref | |||
| Targeting glucose metabolism | AR-C155858 | MCT1 | Reduce PD-1 expression on Tregs by blocking their lactate uptake | AR-C155858 in combination with anti-PD-1 mAbs in C57BL/6 mice bearing MC38Myc tumors: inhibit tumor growth | [196] | ||
| Interleukin-10-Fc | IL-10R | Enhance the effector functions of terminally exhausted CD8+ T-cells by promoting mitochondrial pyruvate carrier-dependent OXPHOS | Interleukin-10-Fc in combination with ACT vs. ACT treatment alone: induced a more durable and significant tumor regression in C57BL/6 mice bearing B16F10 melanoma | [197] | |||
| Coenzyme A | Unknown | Reprogram T-cell metabolism through the transcription factors HIF-1α and the aryl hydrocarbon receptor, enhancing OXPHOS and thereby promoting T-cell antitumor effects | CoA-treated CD8+ T-cells vs. vehicle-treated CD8+ T-cells: enhanced tumor control and prolonged survival in C57BL/6 mice bearing B16-gp33 melanoma | [198] | |||
| Targeting lipid metabolism | Avasimibe | ACAT1 | Inhibit cholesterol esterification in T-cells and subsequently increase membrane cholesterol levels of CD8+ T-cells, leading to more effective immunological synapse and enhancing the effector function of CD8+ T-cells | Avasimibe in combination with anti-PD-1 antibody versus monotherapies: demonstrated stronger tumor control and prolonged survival in mice bearing B16F10 tumors and Lewis lung carcinoma | [200] | ||
| LA-redirected CAR-T cells | CD30 | Enhance the formation of ER-mitochondria contacts, thereby promoting Ca2+ signaling, improving mitochondrial energetics, and boosting antitumor effector function of T-cells | LA-redirected CD30. CAR-T versus nontreated CD30. CAR-T: stronger tumor control and prolonged survival in mice bearing Karpas-299 tumors | [56] | |||
| Cerulenin | FASN | Restore dendritic cell activation and promote effector T-cell infiltration through inhibiting FASN | Cerulenin achieved at least partial tumor control in preclinical models of ovarian cancer | [199] | |||
| Targeting amino acid metabolism | BCH | SLC43A2 | Alleviate the competition for methionine between tumor cells and T-cells, which restores H3K79me2 and STAT5 in T-cells, thereby enhancing antitumor immunity | BCH in combination with anti-PD-L1 antibody vs. monotherapies: stronger T-cell infiltration and produced a more potent inhibitory effect on tumor growth | [62] | ||
| PRODH2-engineered CAR-T cells | BCMA | Reprogram proline metabolism in T-cells, thereby upregulating OXPHOS and the expression of various T-cell effector molecules such as IFNγ and TNF-α | PRODH2-engineered BCMA CAR-T exhibited significantly stronger efficacy and improved OS compared to control BCMA CAR-T | [201] | |||
| Epacadostat | IDO1 | Inhibit IDO1-initiated tryptophan catabolism, thereby reducing the accumulation of kynurenine and alleviating its immunosuppressive effects on T-cells. | Epacadostat plus pembrolizumab did not improve PFS or OS compared with placebo plus pembrolizumab in patients with advanced melanoma. | [206] | |||
| Adoptive T-cell therapy | |||||||
| CAR-T | CTL019 | CD19 | NCT01626495; NCT01029366 | Ⅰ/ⅡA | ALL | 90% CR: 90%; 6-month EFS: 67%; 6-month OS: 78% | [210] |
| PD1-CD19-CART | NCT04213469 | — | B-cell lymphoma | CR: 87.5% | [212] | ||
| Bb2121 | BCMA | NCT02658929 | Ⅰ | Multiple myeloma | ORR: 85%; PFS: 11.8 months | [211] | |
| GCC19CART | CD19×GCC | ChiCTR2000040645 | Ⅰ | Metastatic colorectal cancer | ORR: 40%; OS: 22.8 months | [213] | |
| HER2-CAR-T | HER2 | NCT00902044 | Ⅰ/Ⅱ | HER2-positive sarcoma | 4/17 patients had stable disease for 12 weeks to 14 months; the median OS: 10.3 months | [214] | |
| TCR-T | Afami-cel | MAGE-A4 | NCT04044768 | Ⅱ | Advanced synovial sarcoma and myxoid round cell liposarcoma | ORR: 37% | [215] |
| SCG101 | HBsAg | NCT05339321 | Ⅰ | Advanced HBV-HCC | Partial response; the transferred T-cells were still detectable after 6 months | [217] | |
| CEA-TCR-T | CEA | NCT00923806 | Ⅰ | Metastatic colorectal cancer | Objective regression of lung and liver metastases in one patient | [218] | |
| E6-TCR-T | HPV16-E6 | NCT02280811 | Ⅰ/Ⅱ | Metastatic HPV16-positive cancer | 2/12 patients achieved objective tumor responses | [219] | |
| TIL | Lifileucel | — | NCT02360579 | Ⅱ | Advanced melanoma | ORR: 31.4%; OS: 13.9 months | [220] |
| NCT03645928 | Ⅱ | Metastatic NSCLC | ORR: 21.4% (6/28) | [222] | |||
- Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CR, complete remission; DCR, disease control rate; EFS, event-free survival; HCC, hepatocellular carcinoma; HR, hazard ratio; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; OXPHOS, oxidative phosphorylation; pCR, pathological complete response; PFS, progression-free survival; PR, partial remission; RCC, renal cell carcinoma; TNBC, triple-negative breast cancer.
- Data sources: MY CANCER GENOME (https://www.mycancergenome.org/content/clinical_trials/); ClinicalTrials.gov (https://clinicaltrials.gov/).
5.1 Targeting Cell-to-Cell Contact
Intercellular interactions, such as those between PD-1 and its ligands, significantly influence T-cell function and form the basis for therapies targeting cell-to-cell contacts between T-cells and other cells in the TME. Currently, drugs targeting cell-to-cell contact are categorized into ICIs and bispecific antibodies (bsAbs).
5.1.1 Immune Checkpoint Inhibitors (ICIs)
Considering the immune suppression of T-cells through interactions between immune checkpoint molecules, ICIs constitute a novel strategy to strengthen antitumor immunity [156] (Figure 4A). Since the approval of ipilimumab (an anti-CTLA-4 monoclonal antibody) by the U.S. Food and Drug Administration (FDA) in 2011 for melanoma treatment [157], an increasing number of ICIs have entered clinical trials. Currently, multiple PD-1/PD-L1 antibodies have demonstrated significant antitumor activity in various clinical trials and have been approved for several malignancies, including melanoma [158], TNBC [159], and non-small cell lung cancer (NSCLC) [160, 161]. For instance, perioperative treatment with nivolumab (an anti-PD-1 monoclonal antibody) plus chemotherapy achieved a higher pathological complete response (pCR) rate and longer survival than chemotherapy alone in patients with stage III NSCLC [160]. Similarly, in a phase III clinical trial, the addition of pembrolizumab to neoadjuvant chemotherapy led to a pCR rate of 64.8% in patients with early TNBC, significantly higher than that in patients who received placebo plus neoadjuvant chemotherapy [162]. These findings underscore the promise of integrating ICIs with other treatments, such as chemotherapy, to maximize antitumor efficacy across different malignancies. In addition to the well-established immune checkpoints PD-1 and CTLA-4, inhibitors targeting other checkpoints, such as lymphocyte activation gene-3 (LAG-3), TIM-3, and TIGIT, are also undergoing extensive research and clinical trials, some of which have shown promising antitumor effects [163-165].
5.1.2 Bispecific Antibodies (BsAbs)
BsAbs can promote T-cell antitumor immunity through binding to different antigens on T-cells and other cells. Depending on the types of epitopes they target, these bsAbs can be categorized into dual checkpoint inhibitors (CPIs), T-cell engagers (TCEs), and costimulatory bsAbs [166]. Compared with monospecific antibodies, dual CPIs reduce side effects and improve treatment efficacy because of their greater specificity or stronger inhibitory effect. For example, PD-L1×EGFR (an anti-PD-L1 and anti-EGFR bsAb) blocks PD-L1 in an EGFR-specific manner, resulting in enhanced activity against EGFR-expressing tumor cells [167]. Additionally, the bsAb LY3434172 (a bsAb targeting both PD-1 and PD-L1) induces dramatic and potent antitumor activity by completely inhibiting the PD-1/PD-L1 pathway, outperforming both the parent antibody or their combination [168]. TCEs can bypass MHC molecules to trigger T-cell activation through binding directly to tumor antigens and TCRs. A notable example is mosunetuzumab, a CD20×CD3 TCE that redirects T-cells to eliminate malignant B cells. In a phase Ⅰ/II clinical trial involving patients with relapsed and refractory lymphoma, mosunetuzumab achieved a 60% complete response rate, significantly higher than that of historical controls [169]. The costimulatory bsAbs used for activating T-cells can be classified into tumor-targeting costimulatory bsAbs and dual costimulation bsAbs. The former provides tumor-specific costimulatory signals for T-cells, whereas the latter not only activates T-cells but also stimulates other immune cells [166]. As an example, BNT312 (a CD40×4-1BB bsAb targeting T-cells and APCs) upregulated costimulatory molecules and immunostimulatory cytokines through immune modulation of peripheral APCs and T-cells, thus leading to a stronger antitumor immune response in patients with advanced solid tumors [170].
Owing to the versatile combinatorial strategies of bsAbs, the field has made rapid progress in recent years, with several bsAbs being evaluated in clinical trials. Future research should fully harness the potential of bsAbs' diverse combinatorial approaches, focusing on tumor heterogeneity to develop more precise and personalized therapeutic strategies that improve treatment efficacy and address resistance challenges.
5.2 Targeting Cytokines and Chemokines
As mentioned above, a variety of cytokines and chemokines in the TME can influence the antitumor effects of T-cells directly or indirectly. On the basis of their different effects on T-cell immune function, several therapies have been developed to target immunosuppressive factors or supplement immune-boosting factors, among which TGF-β inhibitors, recombinant IL-2, and CCR4 inhibitors have been studied extensively (Figure 4B).
5.2.1 Transforming Growth Factor-β (TGF-β) Inhibitors
Neutralizing systemic TGF-β in mice enabled tumor clearance by restoring cytotoxic gene expression in antigen-specific CTLs (cytotoxic T lymphocytes) [171], highlighting the potential of TGF-β inhibitors in enhancing immunotherapy. The most effective TGF-β inhibitors currently work through three key mechanisms: (1) directly inhibiting TGF-β synthesis via antisense molecules, such as Trabederson; (2) blocking TGF-β and its receptor interactions with mAbs or soluble TGF-β decoy receptors, such as fresolimumab; and (3) disrupting the TGF-β signaling pathway with kinase inhibitors or aptamers that interfere with downstream Smad signaling proteins, such as galunisertib [172-175]. Among these drugs, fresolimumab has shown promising safety and preliminary antitumor activity, with higher doses demonstrating robust systemic immune responses and longer median survival [175-177]. Moreover, the combination of galunisertib with neoadjuvant chemoradiotherapy increased the complete response rate to 32% in patients with locally advanced rectal cancer and was well tolerated [174]. It is worth mentioning that the development of bsAbs targeting both PD-(L)1 and TGF-β is also a hotspot in cancer immunotherapy. For instance, in a phase II trial, SHR-1701 (a PD-L1/TGF-β bsAb) plus chemotherapy achieved a post-induction objective response rate of 58% among patients with unresectable stage III NSCLC, demonstrating promising efficacy and tolerable safety [178].
5.2.2 Recombinant Interleukin-2 (IL-2)
Another important cytokine in the TME, IL-2, plays a critical role in the expansion and differentiation of CTLs and the reinvigoration of exhausted T-cells, particularly when combined with ICIs [179]. In fact, research into the use of recombinant IL-2 for cancer treatment dates back several decades. Aldesleukin was first approved by the FDA for treating metastatic renal cell carcinoma in 1992 and melanoma in 1998 and was later approved as part of a combination therapy for neuroblastoma in 2015 [180-183]. Along with breakthroughs in ICIs, the potential of IL-2 therapy to render poorly immunogenic tumors more immunogenic has further fueled research into IL-2-based therapies [184]. Several IL-2 drug candidates with a wide variety of additional functions are currently in clinical trials. For example, ALT-801 targets tumor cells and has demonstrated good safety in humans, inducing immunogenic changes associated with antitumor effects [185]. Additionally, compared with rhIL-2, CD122-biased IL-2 formulations such as ALKS4230 exhibit enhanced pharmacokinetic and selective pharmacodynamic properties, leading to improved antitumor efficacy and reduced toxicity in mice [186].
5.2.3 C-C Motif Chemokine Receptor 4 (CCR4) Inhibitors
The recruitment role of CCR4 in Tregs makes it another potential therapeutic target [48]. Mogamulizumab, the first approved glycoengineered therapeutic antibody that targets CCR4, is currently used in Japan for the treatment of relapsed adult T-cell leukemia [187, 188]. In a phase III clinical trial for cutaneous T-cell lymphoma, mogamulizumab achieved a progression-free survival of 7.7 months, significantly higher than that of patients who received standard therapy [189]. Moreover, this anti-CCR4 antibody can be used in combination with nivolumab for the treatment of advanced or metastatic solid malignancies, demonstrating an acceptable safety profile and antitumor activity [190].
While other cytokines, such as IFN-γ, also affect T-cell function [191], the development of drugs targeting these factors is still in the early stages [192, 193] and will not be elaborated here.
Targeting cytokines and chemokines offers a promising strategy to modulate T-cell function and overcome immunosuppression in the TME. With deeper insights into the immune regulatory mechanisms of cytokines and chemokines, these therapies hold the potential to transform immune-cold tumors into immune-hot ones, broaden the range of patients benefiting from immunotherapy, and overcome current limitations of immunotherapy, such as limited response rates and resistance.
5.3 Targeting T-Cell Metabolism
As researchers gain deeper insights into their understanding of T-cell metabolism within the TME, targeting T-cell metabolism to increase their antitumor activity has garnered increasing attention in recent years. The following section briefly outlines key metabolic targets in T-cells, focusing on glucose metabolism, lipid metabolism, and amino acid metabolism (Figure 4C).
5.3.1 Targeting Glucose Metabolism
Previous studies have indicated that elevated glycolysis in the TME is associated with poor T-cell infiltration and resistance to ICIs and adoptive cell therapies (ACTs) [194-196]. Mechanistically, Tregs utilize monocarboxylate transporter 1 (MCT1) to absorb lactate and subsequently upregulate PD-1 expression. Pharmacological inhibition of MCT1 can reduce PD-1 expression in Tregs and increase the number of activated CD8+ T-cells in the TME, thus enhancing the antitumor efficacy of ICIs [196]. Studies have also shown that interleukin-10-Fc, a potent metabolic intervention agent, can restore the function of exhausted T-cells and improve their response to cancer immunotherapy through upregulating mitochondrial pyruvate carrier-dependent OXPHOS [197]. Additionally, exogenous supplementation with coenzyme A (CoA) can reprogram T-cell metabolism to increase OXPHOS, thereby boosting the antitumor immunity of T-cells [198].
5.3.2 Targeting Lipid Metabolism
Fatty acids have been shown to impair T-cell function when they are absorbed by CD8+ T-cells in a CD36-dependent manner. Therefore, targeting CD36 represents an effective method for enhancing the effector functions of CD8+ T-cells through reducing lipid uptake [61]. Inhibiting fatty acid synthase with cerulenin could also restore dendritic cell activation and promote effector T-cell infiltration, leading to at least partial tumor control in preclinical models of ovarian cancer [199]. Additionally, pharmacological inhibition of ACAT1, a key enzyme involved in cholesterol esterification, has been shown to improve the effector functions of CD8+ T-cells, facilitating more effective immune synapse formation [200]. Furthermore, linoleic acid (LA)-redirected chimeric antigen receptor-T (CAR-T) cells demonstrated improved tumor control in preclinical models, based on LA's role in protecting T-cells from exhaustion [56].
5.3.3 Targeting Amino Acid Metabolism
The regulatory effect of amino acid metabolism on T-cell function also presents several potential therapeutic targets. For instance, directly inhibiting the methionine transporter SLC43A2 in tumor cells enhances T-cell antitumor immunity by alleviating methionine competition between tumor cells and T-cells. Exogenous methionine supplementation can yield similar effects [62]. Moreover, overexpressing PRODH2 in CAR-T cells significantly increases their antitumor efficacy in vivo by reprogramming proline metabolism and subsequently facilitating OXPHOS [201]. However, although studies have shown that tryptophan catabolism initiated by indoleamine 2,3-dioxygenase 1 (IDO1) can suppress CD8+ T-cells and promote Treg differentiation [202-204], and preliminary research has demonstrated that blocking IDO1 can enhance robust immunotherapeutic effects [205], IDO1 inhibitor epacadostat failed to show therapeutic benefit in a phase III clinical trial [206]. This highlights the complexity of metabolism within the TME, which requires further in-depth investigation. It is also worth mentioning that amino acid metabolism has also been identified as a key biomarker for predicting immunotherapy efficacy, with histidine associated with better outcomes and homocysteine and phenylalanine associated with worse outcomes [207, 208].
Therapeutic strategies targeting T-cell metabolism, particularly ICIs, hold promise for improving the efficacy of immunotherapies. However, many metabolic regulators lack tumor specificity and may induce toxicity in healthy tissues. Additionally, T-cell metabolism within the TME is likely influenced by the host's systemic macroenvironment, including metabolic conditions such as obesity and diabetes. Future efforts should focus on the development of metabolic regulators with improved tumor specificity while deepening our understanding of the intricate interplay between the systemic macroenvironment and the metabolic dynamics of the TME. Such advances could pave the way for more effective and safer immunotherapeutic strategies targeting T-cell metabolism.
5.4 Adoptive T-Cell Therapy
Adoptive T-cell therapy enhances the antitumor activity of T-cells by extracting them from patients or donors, expanding or genetically modifying them in vitro, and reinfusing them to strengthen immune responses. The most extensively studied ACT approaches include CAR-T cell therapy, T-cell receptor-engineered T (TCR-T) cell therapy, and tumor-infiltrating lymphocyte (TIL) therapy (Figure 4D).
5.4.1 Chimeric Antigen Receptor-T (CAR-T) Cell Therapy
CAR-T cell therapy involves genetically engineering T cell to express CARs that target specific antigens on tumor cells [209]. This approach has shown significant efficacy in patients with hematological malignancies, such as leukemia, myeloma, and non-Hodgkin B-cell lymphoma, particularly in those who are refractory to chemotherapy [210-212]. For instance, CTL019 (autologous T-cells transduced with a CD19-directed CAR) therapy achieved a 90% complete remission rate in patients with relapsed and refractory acute lymphoblastic leukemia (ALL), including those who had previously failed stem-cell transplantation. Remarkably, durable remissions lasting up to 24 months were observed [210]. CAR-T cell therapy targeting B-cell maturation antigen (BCMA) also achieved an ORR of 85% and a PFS of 11.8 months in patients with relapsed or refractory multiple myeloma [211]. BCMA-CAR-T cells persisted durably in the body, with 86% and 57% of the patients having detectable CAR-T cells at 3 and 6 months, respectively. The promising results of CAR-T cell therapy in hematological malignancies have raised hopes for its application in solid tumors. Although challenges, such as the difficulty of T-cells infiltrating tumors, may impede their application in solid tumors, several clinical trials have reported promising results. For example, CAR-T cell therapy targeting CD19 and guanylyl cyclase C (GCC) achieved an objective response rate of 40% in patients with metastatic colorectal cancer, making it the first CAR-T cell therapy to demonstrate objective clinical activity in refractory solid tumors [213]. Another CAR-T cell therapy targeting human epidermal growth factor receptor 2 (HER2) also showed encouraging results in a phase I/II clinical study involving 19 patients with HER2-positive sarcoma [214]. Notably, long-term persistence of HER2-CAR-T cells was demonstrated after infusion, and these cells remained in the body for at least 6 weeks and up to 18 months. Although the efficacy of CAR-T cell therapy in solid tumors has not yet reached the same level as that in hematological malignancies, these clinical trials indicate the potential application of CAR-T therapy in various tumors.
5.4.2 T-Cell Receptor-Engineered T (TCR-T) Cell Therapy
Compared with CAR-T cell therapy, TCR-T cell therapy involves a broader range of targetable antigens and requires a lower epitope density for T-cell activation [214]. These advantages point to a promising future for TCR-T cell therapy. Afamilolipid gene autoleucel (Afami-cel) is the first FDA-approved TCR-T cell therapy. It is an autologous T-cell product that includes both CD4+ and CD8+ T-cells, and is genetically modified to express an affinity-enhanced TCR specific to MAGE-A4. In a phase II clinical trial, Afami-cel demonstrated durable responses in heavily pretreated patients with HLA-A*02- and MAGE-A4-expressing synovial sarcoma, and the researchers observed a positive correlation between Afami-cel cellular persistence and overall survival [215]. The success of Afami-cel provides a strong rationale for expanding TCR-T cell therapy to other solid malignancies. On the basis of findings that T-cells expressing HBV-specific TCRs can selectively eliminate HBV-positive hepatoma cells in preclinical models [216], the TCR-T cell therapy SCG101 has exhibited reliable antitumor and antiviral effects in patients with HBV-related hepatocellular carcinoma (HCC). SCG101 effectively eliminates HBsAg+ cells and achieves sustained tumor control after a single dose [217]. Additionally, numerous TCR-T clinical trials targeting tumor-associated antigens such as carcinoembryonic antigen and tumor-specific antigens such as human papillomavirus 16-E6 are currently underway [218, 219].
5.4.3 Tumor-Infiltrating Lymphocyte (TIL) Therapy
TIL therapy involves the infusion of autologous T lymphocytes that have been activated and expanded ex vivo in the presence of IL-2. This approach was first utilized in 1988 to treat metastatic melanoma [220]. Among the numerous clinical trials on TIL therapy for melanoma, Lifileucel demonstrated durable responses (the objective response rate was 31.4%, and the median duration of response was not reached at a median follow-up of 27.6 months [220]) and ultimately became the first FDA-approved TIL therapy for unresectable or metastatic melanoma, particularly in patients who had previously received other therapies such as PD-1 inhibitors or BRAF inhibitors [221]. While TIL therapy has shown high effectiveness in melanoma, its response rates are notably lower in non-melanoma solid tumors such as NSCLC [222]. Therefore, further research is needed to improve the efficacy of TIL therapy and expand its application to other solid tumors.
Antigen heterogeneity and low T-cell infiltration in solid tumors are key challenges limiting the application of adoptive T-cell therapies. Developing adoptive T-cells with multi-antigen recognition capabilities offers a potential solution to overcome antigen heterogeneity, while a thorough understanding of T-cell chemotactic responses and the mechanisms of immune barriers within the TME is critical for improving T-cell infiltration. Off-tumor toxicity is another significant concern, and strategies such as modifying scFv to modulate CAR-T cell affinity and designing logic-gated CAR-T cells activated specifically within the TME have been proposed to address this issue [223].
6 Conclusions
In summary, T-cells exhibit extraordinary plasticity in response to the dynamic alterations in the TME. Notably, the effectiveness of current therapeutic regimens is closely connected to the spatial distribution and activation states of T-cells. Hence, improving T-cell-mediated antitumor immunity by leveraging their plasticity offers a promising clinical strategy for the treatment of malignancies.
Currently, therapies targeting immunosuppressive signals, such as anti-PD-L1, have erupted into clinical practice and shown durable benefits. In addition, approaches such as ACT aimed at expanding cytotoxic T-cells have yielded favorable patient outcomes. The majority of regimens can promote T-cell activation and thereby improve the therapeutic response. Hence, combining immunotherapy with existing therapeutic approaches could yield synergistic effects. Nevertheless, a significant challenge remains: the functional shift of T-cells to an immunosuppressive phenotype during treatment. Research efforts should focus on elucidating the precise molecular mechanisms that drive T-cell exhaustion and identifying strategies to counteract these processes effectively.
Recent advancements in technology have opened new avenues for understanding and enhancing T-cell responses within the TME. Techniques such as single-cell RNA sequencing and spatial transcriptomics enable the identification of specific molecular pathways that regulate T-cell activation and differentiation, providing insights into how T-cell can be reprogrammed to enhance their antitumor activity. Furthermore, real-time imaging techniques facilitate the observation of T-cell dynamics within tumors, revealing how these cells interact with cancer cells and other immune components. By integrating these advanced methodologies, the underlying mechanisms driving T-cell plasticity can be further decoded to facilitate the development of novel therapeutic strategies.
Author Contributions
Xiao-Hong Ding: conceptualization, writing – original draft preparation, writing – review and editing. Xue-Pei Li: writing – original draft preparation, writing – review and editing. Fenfang Chen: writing – review and editing, funding acquisition. Han Wang: conceptualization, writing – review and editing, Yi-Zhou Jiang: conceptualization, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This study was supported by grants from the Shanghai Anticancer Association EYAS PROJECT (SACA-CY22A05) and the National Natural Science Foundation of China (82103451). The figures are created with BioRender.com.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.