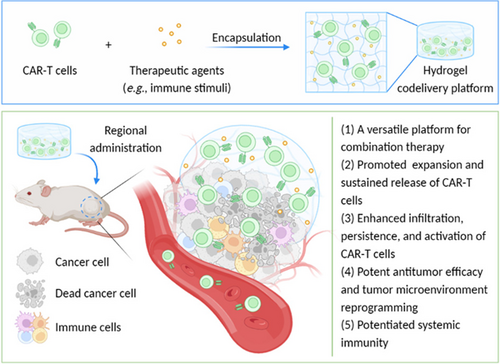
Delivering CAR-T cells into solid tumors via hydrogels
Abstract
Chimeric antigen receptor (CAR)-T cell therapy is a promising form of cancer immunotherapy that genetically modifies a patient's own T cells to express CARs for the specific recognition and eradication of cancer cells. Unfortunately, unlike the impressive advancements it achieves in hematologic cancer treatment, CAR-T cell therapy has encountered obstacles in treating solid tumors such as high cost, inadequate tumor infiltration, and immunosuppressive tumor microenvironment. Recently, the regional administration of CAR-T cells via hydrogel platforms has been investigated as a potential method to not only promote tumor infiltration, cell expansion, and anticancer efficacy of the CAR-T cells but also provide a multifunctional platform to introduce additional therapeutic agents for achieving potentiated cancer therapy. In this perspective, different design strategies of CAR-T cell delivery hydrogels are introduced. Besides, various types of therapeutic agents incorporated in the hydrogel platforms and diverse hydrogel formulations have been discussed. The current challenges and future research directions on CAR-T cell delivery hydrogels are also proposed. It is hoped that this perspective can help future researchers develop new CAR-T cell delivery hydrogels that can effectively fight against solid tumors.
Graphical Abstract
This perspective comments on the regional codelivery of chimeric antigen receptor (CAR)-T cells and therapeutic agents via hydrogels for solid tumor treatment. Diverse design strategies of CAR-T cell delivery hydrogel depots are introduced and discussed, with an emphasis on their applications for cancer immunotherapy. The current limitations and future research directions regarding the use of hydrogels for CAR-T cell therapy are also proposed.
1 INTRODUCTION
Chimeric antigen receptor (CAR)-T cell therapy, as a promising form of cancer immunotherapy, is based on the genetical modification of a patient's own T cells to express CARs permitting the redirection of T cells to specifically recognize and eradicate cancer cells.1 Since the US Food and Drug Administration (FDA) approved the first CAR-T cell product Tisagenlecleucel for the treatment of acute lymphoblastic leukemia in 2017,2, 1 CAR-T cell therapy has been considered as a potent approach for the treatment of certain hematological malignancies.3 Unfortunately, unlike the impressive advancements it achieves in hematologic cancer treatment, CAR-T cell therapy has encountered additional obstacles in treating solid tumors such as inadequate tumor infiltration and T cell dysfunction and exhaustion within the immunosuppressive tumor microenvironment (TME).4
To date, numerous efforts have been made to realize effective CAR-T cell treatments against solid tumors. For example, various combinations of CAR-T cells and therapeutics (such as immune checkpoint inhibitors5 and nanoparticles6) have been investigated to elevate the CAR-T cell activity and remodel the immunosuppressive TME for achieving enhanced anticancer efficacy. Note that the codelivery of therapeutic agents and CAR-T cells can be realized by coadministration or engineering strategies that allow CAR-T cells to express diverse “armor” proteins (e.g., inflammatory cytokines including interleukin [IL]-12,7 IL-15,8 and IL-18,9 and immune checkpoint inhibitors like antiprogrammed death-ligand 1 antibody [aPDL1]10). Additionally, the regional administration of CAR-T cells into body cavities provides a method to enhance the infiltration of CAR-T cells in solid tumors.11-13 Moreover, owing to their outstanding biocompatibility and biodegradability, various types of hydrogels have been developed as cell delivery platforms that permit the regional delivery of CAR-T cells to enhance the tumor infiltration and persistence of the CAR-T cells for the treatment of solid tumors. Especially, serving as versatile delivery platforms, hydrogels also exhibit the attractive capacities of coencapsulating and codelivering diverse therapeutic agents with CAR-T cells, to further promote the activity and proliferation of CAR-T cells and/or reprogram the immunosuppressive TME for realizing potentiated cancer immunotherapy or combined therapy (Figure 1). Therefore, in this perspective, emerging regional codelivery strategies based on injectable or in situ forming hydrogels for CAR-T cell therapy against solid tumors will be introduced and discussed. It is hoped that this perspective may help researchers in the related field to develop new CAR-T cell delivery hydrogels and facilitate their clinical translation against solid tumors in the near future.
2 HYDROGEL PLATFORMS FOR THE CODELIVERY OF CAR-T CELLS AND THERAPEUTIC AGENTS
2.1 Different therapeutic agents for promoted CAR-T cell activity
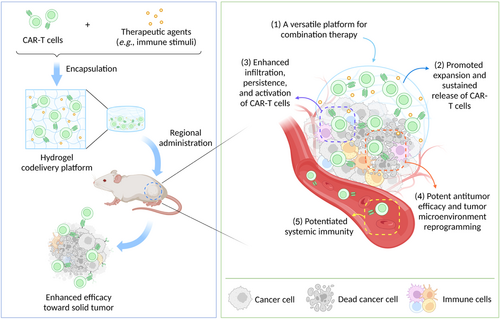
Since the cytokines have been frequently applied to enhance the function of CAR-T cells and can be easily incorporated into hydrogels,14 codelivery hydrogel platforms comprising CAR-T cells and cytokines have been explored as a universal strategy for achieving promoted anticancer efficacy. Recently, Grosskopf et al.15 developed a simple-to-implement injectable hydrogel for the locoregional delivery and controlled release of CAR-T cells and the stimulatory cytokine IL-15 to enhance the therapeutic efficacy against solid tumor (Figure 2A). Dodecyl-modified hydroxypropyl methylcellulose (HPMC-C12) polymers were mixed with biodegradable poly(ethylene glycol)-b-poly(lactic acid) nanoparticles (PEG-PLA NPs) to form the robust polymer–nanoparticle (PNP) hydrogels via physical crosslinking. Leveraging its unique encapsulation property, the PNP hydrogel could serve as an injectable depot of both CAR-T cells and IL-15 that simultaneously inhibited the passive diffusion of the entrapped IL-15 and allowed the active movement of encapsulated CAR-T cells, to enable the long-term retention, sustained exposure, and promoted expansion and activation of CAR-T cells. The codelivery of CAR-T cells and IL-15 via the peritumoral administration of PNP hydrogel in human medulloblastoma solid tumor-bearing non–obese diabetic (NOD) scid gamma (NSG) mice (Figure 2B) successfully created a transient inflammatory niche where CAR-T cells could exhibit a more tumor-reactive CAR-T phenotype and effectively expand. Results demonstrated that the hydrogel-based codelivery strategy realized enhanced T cell expansion and promoted anticancer efficacy without any adverse immune response. Interestingly, the transient inflammatory niche could also be formed in the tumor-bearing NSG mouse who received a distal subcutaneous administration of the PNP hydrogel depot (Figure 2C). The formation of the transient inflammatory niche within the PNP hydrogel realized the promoted expansion and maintained antitumor activity of CAR-T cells, thereby eliciting a potent abscopal effect. Utilizing a similar strategy, Zhou et al.16 constructed a gelatin methacryloyl (GelMA)-based injectable and photocurable hydrogel depot of CAR-T cells and cytokines (IL-2, IL-7, and IL-15) to build a suitable living environment for the activation, proliferation, and infiltration of CAR-T cells at the tumor site, achieving promoted efficacy in a melanoma-bearing mouse model (Figure 2D). Besides cytokines, Chao et al.17 discovered that metformin (Met), a drug prescribed for patients with type 2 diabetes, displayed dual modulation ability of cancer cells and CAR-T cells. Met could suppress the oxidative and glycolytic metabolism of cancer cells, leading to the relief of tumor hypoxia. Meanwhile, Met could upregulate the oxidative metabolism of CAR-T cells and make them transform toward a long-lived memory-like phenotype with enhanced anticancer responses. To realize a potent yet safe anticancer therapy, the authors constructed a sodium alginate (ALG)-based hydrogel scaffold (termed CAR-T@Met/gel) for the regional codelivery of CAR-T cells and Met (Figure 2E). In several postsurgical tumor models, the CAR-T@Met/gel implanted in the tumor resection cavity remarkably increased the tumor infiltration and proliferation of CAR-T cells and displayed enhanced efficacy against both local tumors and distant abscopal tumors with reduced systemic immune-related adverse effects. Furthermore, Luo et al.18 incorporated IL-15 and oxygen carriers (HEMOXCell, a marine extracellular hemoglobin with a strong capacity of oxygen storage) into an ALG-based CAR-T cell delivery hydrogel to elevate the oxygen tension throughout the solid tumor, contributing to the survival of infiltrated CAR-T cells and the downregulation of the expression of hypoxia-inducible factor-1α (HIF-1α) in the tumor site.
Besides the incorporation of extra immunostimulatory molecules, “armor” proteins such as cytokines and antibodies can be secreted by modified CAR-T cells. In a recent study, Wang et al.19 encapsulated ganglioside 2 (GD2)-specific CAR-T cells (termed GD2.CAR-Ts) with the ability to secret IL-15 (termed GD2.CAR.15-Ts) within a chitosan-PEG injectable hydrogel. In a retinoblastoma (RB) tumor-bearing nude mouse model, GD2.CAR.15-Ts intratumorally delivered by hydrogels enabled complete tumor eradication with minimized inflammation and retinal detachment. The authors suggested that the increased persistence of CAR-T cells and/or the enhanced retention of IL-15 within the tumor site might underlie the robust anticancer effect of this local delivery strategy.
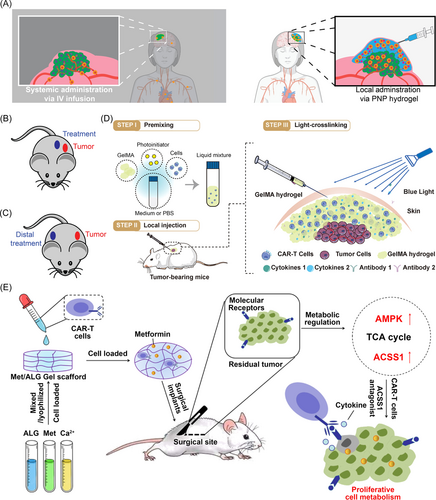
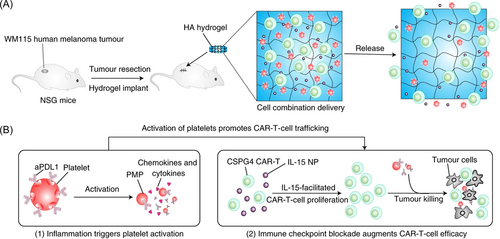
Recent studies also reveal the possibility of constructing hydrogel-based multifunctional CAR-T cell delivery platforms to compatibly incorporate more than one type of therapeutic agents and/or functional cells by utilizing the flexibility of hydrogels, thus realizing combined cancer therapy. For instance, Hu et al.20 constructed a hyaluronic acid (HA)-based hydrogel reservoir to encapsulate CAR-T cells, IL-15-loaded polymeric NPs (IL-15 NPs), and aPDL1-conjugated platelets (P–aPDL1) to fight against cancer recurrence after surgery via combination immunotherapy (Figure 3A). The encapsulated platelets could be activated by the inflammation occurring after tumor surgery and these activated platelets could not only form microplatelets to promote the local release of aPDL1 but also be considered as a source of ligands and chemokines. Cooperating with the IL-15 NPs, the P–aPDL1 could further boost CAR-T cell immunity, preserve the T cells from exhaustion, and recruit other immune cells, thus creating a favorable milieu for CAR-T cells (Figure 3B). Results confirmed that this HA hydrogel reservoir could promote the persistence and expansion of CAR-T cells, and therefore prevent the recurrence of local tumors as well as inhibit the growth of distant tumors by the abscopal effect in WM115 melanoma tumor resection models.
2.2 Diverse hydrogel formulations utilized in the construction of codelivery platforms
In addition to hydrogels based on polymer chains (e.g., chitosan and ALG), an increasing number of biomolecular hydrogels that contain peptides or proteins have been designed and applied as delivery platforms.21, 22 Peptide molecules can form secondary structural motifs such as β-sheet and α-helix and the resultant secondary structures can further self-assemble into nanofibers.23 Then, the elongation of these nanofibers can generate thicker and longer fibers to spontaneously assemble into a fibrous hydrogel network. The peptide-based hydrogels possess nanofiber structures that are similar to the extracellular matrix (ECM) for the growth and differentiation of cells, as demonstrated by Jie et al.24 In this study, the authors engineered CAR-T cells to secrete antiprogrammed death-1 single-chain variable fragments (anti-PD-1 scFv) and loaded these engineered CAR-T cells and cytokines within a peptide-based hydrogel scaffold for effective treatment against breast cancer. The anti-PD-1 scFv could realize intrinsic checkpoint blockade of CAR-T cells in the hydrogel scaffold to reverse the depletion of CAR-T cells and promote T cell proliferation and cytotoxicity. Furthermore, as an important protein from ECM, fibrin has also been considered as a promising candidate for the construction of protein-based hydrogels due to its good biocompatibility and cell adhesion ability.25 The formation of the fibrin hydrogel is based on the natural polymerization process of fibrin after vascular injury. With the help of thrombin, the polymerization sites of the fibrin precursor fibrinogen can be exposed to make fibrinogen molecules be polymerized into a three-dimensional fibrin network. In 2021, Ogunnaike et al.26 utilized a fibrin hydrogel to regionally deliver CAR-T cells in a subtotal glioblastoma resection model and demonstrated that the hydrogel-based delivery of CAR-T cells exhibited better control over the growth of partially resected tumor compared with the direct injection of CAR-T cells in the resection cavity. The authors also proposed the possibility of loading cytokines and biological agents in fibrin gels to sustain CAR-T cell growth and reprogram the immunosuppressive TME in solid tumors. Recently, Uslu et al.27 constructed an in-situ forming fibrin hydrogel as a cell delivery carrier by simply mixing the mesothelin-specific CAR-T cells, cytokines, fibrinogen, and thrombin in the tumor resection cavity and realized effective clearance of residual cancer cells in adenocarcinoma and triple-negative breast cancer xenograft models.
Overall, diverse types of hydrogels based on hydroxypropyl methylcellulose,15 GelMA,16 ALG,17, 19 chitosan,18 HA,20 peptide,24 and fibrin27, 26 have been introduced in this perspective. Similar to ECM, these hydrogels have a porous structure, which endows them with good encapsulation ability of cells (e.g., around 1–2 million CAR-T cells per 100 μL of hydrogel). Also, the biochemical and biophysical properties of hydrogels can be easily tailored and optimized to mimic ECM, further supporting the proliferation and activity of the loaded cells.28 Furthermore, the shear-thinning property of injectable hydrogels can realize in vivo implantation of cell-laden hydrogels without invasive procedures.
3 CONCLUSION AND PERSPECTIVES
To sum up, we have discussed different hydrogel-based regional delivery strategies for effective CAR-T cell therapy against solid tumors, as summarized in Table 1. First, compared with the traditional intravenous administration, the regional delivery of CAR-T cells can avoid the potential systemic toxicity and promote their utilization efficiency (which may reduce the high cost of CAR-T cell therapy by lowering the number of CAR-T cells required in the treatment). However, the intratumoral delivery of free CAR-T cells may be unsuitable for prolonging the time of the intralesional persistence of CAR-T cells due to the fast diffusion and exhaustion of CAR-T cells. Chao et al.17 stressed that free CAR-T cells delivered by intratumoral injection only achieved the moderate therapeutic effect in a solid tumor-bearing mouse model. Therefore, regionally administered hydrogels have provided a possible solution to realize the prolonged retention, sustained release, and improved biodistribution of CAR-T cells in solid tumor sites with no adverse effects on the functions of loaded CAR-T cells, as revealed in the above examples. Meanwhile, hydrogels permit the encapsulation and diffusion of diverse cargoes (e.g., cytokines, antibodies, and NPs) to maintain the activity and proliferation capacity of CAR-T cells within the tumor site. Remarkably, results have demonstrated that these regional hydrogel reservoirs can elicit both local and abscopal effects in different mouse models, suggesting the potential of codelivery hydrogels for not only fighting against accessible solid tumors but also eliciting systemic efficacy to treat metastatic cancers or inaccessible solid tumors.
| Hydrogel formulation | Model | Target | Therapeutic agent | Engineered armor protein | Administration type | References |
|---|---|---|---|---|---|---|
| HPMC-C12 polymer and PEG-PLA NP | A human medulloblastoma solid tumor-bearing NSG mouse model | B7H3 | IL-15 | – | Peritumoral administration | [15] |
| GelMA | A mouse melanoma-bearing C57BL/6N mouse model | HER2 | IL-2, IL-7, and IL-15 | – | Intratumoral administration | [16] |
| ALG | Postsurgical human gastric and pancreatic tumor-bearing NSG mouse models | CEA | Met | – | Local implantation in the surgical cavity | [17] |
| ALG | A human ovarian adenocarcinoma-bearing male NPG/Vst mouse model | Meso | IL-15 and the oxygen carrier HEMOXCell | – | Intratumoral administration | [18] |
| Chitosan-PEG | A human retinoblastoma-bearing nude mouse model | GD2 | – | IL-15 | Intratumoral administration | [19] |
| HA | A postsurgical human melanoma-bearing NSG model | CSPG4 | IL-15 NP and P–aPDL1 | – | Local implantation in the surgical cavity | [20] |
| FEFEFKFK peptide | A human breast tumor-bearing NSG mouse model | HER2 | – | Anti-PD-1 scFv | Peritumoral administration | [24] |
| Fibrin | Postsurgical adenocarcinoma and triple-negative breast cancer NSG xenograft models | Meso | IL-7 and IL-15 | – | Local implantation in the surgical cavity | [27] |
- Abbreviations: ALG, sodium alginate; anti-PD-1 scFv, antiprogrammed death-1 single-chain variable fragment; CEA, carcino-embryonic antigen; CSPG4, chondroitin sulfate proteoglycan 4; GD2, ganglioside 2; GelMA, gelatin methacryloyl; HER2, human epidermal growth factor receptor 2; HPMC-C12, dodecyl-modified hydroxypropyl methylcellulose; IL, interleukin; IL-15 NP, IL-15-loaded polymeric nanoparticle; Meso, mesothelin; Met, metformin; NPG/Vst mouse, a member of non–obese diabetic (NOD)-Prkdcscid Il2rgnull family; NSG, non–obese diabetic (NOD) scid gamma; P–aPDL1, antiprogrammed death-ligand 1 antibody (aPDL1)-conjugated platelet; PEG-PLA NP, poly(ethylene glycol)-b-poly(lactic acid) nanoparticle.
Overall, the hydrogel-based regional delivery strategy is well worth considering as a promising approach to enabling effective CAR-T cell therapy against solid tumors. Nevertheless, more studies are needed to optimize the design of these CAR-T cell delivery hydrogels and investigate their in vivo performance in the treatment of different solid tumors to facilitate their clinical translation. First, as the ex vivo expansion of CAR-T cells remains a costly and time-consuming process, the possibility of in vivo CAR-T cell manufacturing within implantable hydrogels can be investigated in solid tumor models, which may save the time and cost during the manufacturing process and broaden the scope of CAR-T cell treatments. Besides, the hydrogel formulations should be investigated and optimized to create a favorable environment for CAR-T cell proliferation and achieve desired release behaviors of the loaded CAR-T cells and therapeutic agents. Furthermore, as a simple yet versatile platform, hydrogels are compatible with various types of payloads. Since the feasibility of combining the CAR-T cell therapy with the immune checkpoint blockade therapy has already been verified by Hu et al.,20 different combinations of CAR-T cells and functional components, such as cytokines, antibodies, small-molecule drugs, and multifunctional NPs, within the local hydrogel depots can be further explored to realize reprogram the TME, elicit systemic immunity, prevent tumor recurrence, and improved therapeutic effect. In addition to the regional delivery of both CAR-T cells and functional components, other administration strategies (e.g., intramuscular injection, intraperitoneal injection, and intraspinal injection) can be tested to deliver drugs or vaccines for realizing combinational anticancer treatments. The efficiencies of different administration methods also remain to be evaluated and compared. In addition, since the TME is one of the major obstacles to the infiltration and function of CAR-T cells, TME modulation (such as ECM viscosity reprogramming, tumor hypoxia relief, change of tumoral acidity, and regulation of tumor immunosuppressive microenvironment) before CAR-T cell therapy may create a suitable environment for CAR-T cells to further boost their therapeutic efficacy.29 Moreover, with the advancements of engineering technologies, various CARs have been designed and constructed for the production of next-generation CAR-T cells (such as bispecific CAR-T cells, cytokine-secreting CAR-T cells, and chemokine receptor-expressing CAR-T cells).30 Furthermore, as a major obstacle for all targeted cancer therapies, tumor heterogeneity can cause heterogeneous antigen expression. Diverse expression levels of tumor-associated antigens in solid tumor sites make it difficult for CAR-T cells to recognize target cancer cells. Also, the treatment of CAR-T cells targeting only one type of antigen may result in the outgrowth of the tumor cell subpopulations with loss or downregulation of the target antigen.31 Therefore, incorporating several types of CAR-T cells with different targets, multispecific CAR-T cells, multivalent CAR-T cells, or other CAR-engineered cells (such as CAR macrophages and CAR natural killer cells) into the hydrogel depots may provide a promising solution to overcome the antigen expression heterogeneity of solid tumors. Last but not the least, additional studies are needed to carefully evaluate any beneficial or adverse effects of the proposed hydrogel codelivery strategy-based CAR-T cell therapies in both preclinical and clinical solid tumor models, especially metastatic and inoperable tumor models.
AUTHOR CONTRIBUTIONS
Shun-Yu Wu: Conceptualization (supporting); investigation (lead); writing—original draft (lead). Feng Ji: Writing—review and editing (supporting). Bin Xu: Supervision (supporting); writing—review and editing (supporting). Fu-Gen Wu: Conceptualization (lead); supervision (lead); writing—review and editing (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the Fundamental Research Funds for the Central Universities (2242023K5007), the Natural Science Foundation of Jiangsu Province (BK20211510), Jiangsu Provincial Medical Key Discipline (Laboratory) Cultivation Unit (ZDXK202220), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23_0311). Graphical abstract and figure 1 in this article were created with the help of BioRender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.