Fatty acids in cancer: Metabolic functions and potential treatment
Ao Du and Zhen Wang contributed equally to this study.
Abstract
Lipid metabolic reprogramming is one of the important metabolic characteristics of cancer cells. As major components of lipids, fatty acids provide energy and material basis for cancer cell survival. Abnormal fatty acid metabolism has been found in many cancers. Fatty acid uptake, transport, and synthesis are closely related to the pathogenesis of cancer. Meanwhile, fatty acid changes in the membrane structure of cancer cells and signal transduction mediated by signaling lipids are also helping cancer cells survive in the changing microenvironment. Some of these enzymes and metabolites involved in fatty acid metabolism are emerging as unique cancer biomarkers. Multiple studies have shown that disordered fatty acids can regulate tumor cell proliferation, metastasis, and drug resistance. Therefore, targeting fatty acid metabolism has become a promising treatment strategy. Here, we mainly present metabolic alterations of fatty acids, the basic components of lipids, in cancer. We discuss the cancer treatment based on fatty acid and fatty acid metabolism. These may provide a basis for a better understanding of lipid metabolic reprogramming in cancer, and also provide new ideas for cancer biomarker search, drug development, and combination therapy.
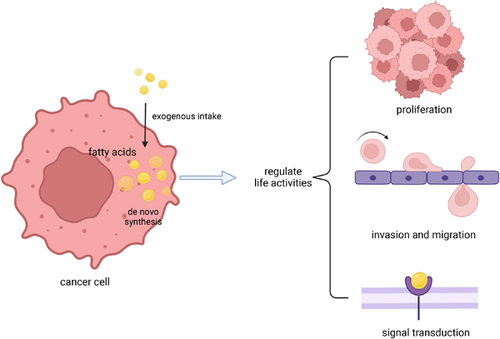
Graphical Abstract
Fatty acids in cancer cells are derived from cellular synthesis and extracellular uptake. Fatty acids are extensively reprogrammed in cancer cells and regulate life activities, including supporting cell growth as energy, influencing cell movement as components of membrane structure, and regulating signal transduction as signaling molecules. Focusing on the role of fatty acids in tumor cells is helpful to find new strategies for tumor treatment.
1 INTRODUCTION
As a noncommunicable disease, cancer ranks second among the leading causes of death worldwide,1 and is expected to be the major obstacle to increase human life expectancy in the 21st century. Cancer development is accompanied by changes in the tumor microenvironment (TME). To adapt to the microenvironment of limited nutrition and hypoxia, cancer cells have formed unique metabolic characteristics.2 Back in the 1920s, Otto Warburg had made pioneering explorations in the field of cancer metabolism. At that time, he discovered that cancer cells had a metabolic preference for converting glucose to lactate even under aerobic conditions.3, 4 With further study of the metabolic phenotype of cancer, the metabolism of nutrients such as glucose, amino acids, and lipids has been demonstrated to play important roles in cancer.5-7 Unlike glucose and amino acids, a lipid is water-insoluble and chemically unstable. Moreover, due to the limitation of chemical technology, lipids cannot be well separated and detected. So, the related studies on lipids have been limited before.8 However, with the development of lipidomics technology, we have gained a deeper understanding of lipid metabolism in cancer.
Lipids are a class of complex biomolecules that mainly include fatty acids (FAs), cholesterol, and phospholipids (PLs). In addition to serving as fuel for energy supply,9 lipids are important substances for cell membranes10 and participate in intracellular signal transduction as signaling molecules.11 FAs are the main components of several lipid species, and FA metabolism is closely related to the development and progression of cancer.12, 13 Several reports have confirmed that the de novo synthesis of FAs is important during the development of cancer.14, 15 In addition to the FA synthesis, the FA uptake and transport also influence the course of cancer.16, 17 Moreover, the expression levels of the key enzymes involved in FA metabolism are frequently altered in cancer, and they have been widely used as biomarkers and therapeutic targets.14, 15, 17 Additionally, FAs are important components of the cellular membrane. Through remodeling of membrane lipids, FAs also affect membrane fluidity and stiffness. There is evidence that this change in membrane structural properties is closely related to the invasion and metastasis of cancer cells.18 What is more, the lipid signaling molecules, such as prostaglandins, leuktrienes and other eicosanoids, can mediate the activation of oncogenic pathways.19, 20
Here, we will describe the research progress of FA metabolism in cancer and the influence of FAs on the membrane structure of cancer cells, as well as the roles of signaling lipids in cancer. By considering the effects of FAs in cancer, we will also discuss the future direction of development of therapeutic strategies targeting FA metabolism.
2 REPROGRAMMING OF FA METABOLISM IN CANCER CELLS
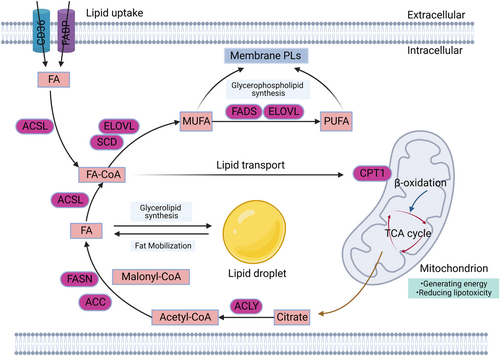
Different from normal cells, extensive metabolic reprogramming occurs within cancer cells to rapidly obtain energy and membrane materials for proliferation. During the proliferation and metastasis of cancer cells, in addition to an increased demand for amino acids and glucose, metabolic reprogramming of FAs is also activated in cancer cells21 (Figure 1). Metabolic reprogramming is affected by multiple factors, including intrinsic and extrinsic factors. One of the main external factors is the TME. The microenvironment can exert many exogenous stresses on cancer cells, including hypoxia, acidification of the extracellular environment, and cell–cell interactions.22 The study found that the acidic microenvironment in solid tumors drives the reprogramming of FA metabolism in cancer cells through changes in mitochondrial and histone acetylation.23 In addition, some noncancer cells can activate the reprogramming of FA metabolism in cancer cells by secreting substances such as cytokines, extracellular vesicles (EVs), and even some FAs. For example, adipocyte-derived lipids can be transferred into breast cancer cells, enhancing intracellular trafficking of FAs and thus contributing to breast cancer progression.24 On the other hand, intrinsic factors are equally important. It mainly includes gene expression and epigenetic information in cancer cells. Here, we will mainly present the changes and effects of various FA metabolism-related enzymes regulated by gene expression in cancer, as well as their mechanisms (Table 1).
| Fatty acid metabolic enzyme | Expression | Regulatory mechanism | Function | Type of cancer | Reference |
|---|---|---|---|---|---|
| ACLY | Upregulated | Wnt/β-catenin signaling pathway | Regulate the stemness, migration and invasion | HCC | [25] |
| ACC1 | Upregulated | NA | Promote proliferation | Non-small cell lung cancer | [26] |
| ACC2 | Downregulated | NA | Inhibit proliferation | Lung adenocarcinoma | [27] |
| FASN | Upregulated | Wnt/β-catenin signaling pathway | Promote epithelial–mesenchymal transition, | Breast cancer | [28] |
| c-Src/AKT/FAK signaling pathway | Promote migration and invasion | Cervical cancer | [29] | ||
| SCD1 | Upregulated | NF-κB | Regulate stemness | Ovarian cancer | [30] |
| NA | Enhance immune resistance | Colon cancer, NSCLC | [31] | ||
| SCD5 | Downregulated | NA | Inhibit proliferation and epithelial–mesenchymal transition | Breast cancer | [32] |
| FADS1 | Upregulated | NA | Promote proliferation | Pancreatic cancer | [33] |
| FADS2 | Upregulated | NA | Promote proliferation | HCC, NSCLC | [34] |
| ELOVL2 | Upregulated | NA | Promote proliferation | RCC | [35] |
| Downregulated | PI3K/Akt signaling pathway | Inhibit proliferation | Prostate cancer | [36] | |
| ELOVL5 | Upregulated | NA | Promote proliferation | Colon cancer | [37] |
| ELOVL6 | Upregulated | AKT | Promote proliferation | HCC | [38] |
| ELOVL7 | Upregulated | NA | Promote proliferation | Prostate cancer | [39] |
| CD36 | Upregulated | AKT/GSK-3β/β-catenin signaling pathway | Promote metastasis | Gastric cancer | [40] |
| FABP1 | Upregulated | VEGFR/Akt/mTOR signaling pathway | Promote angiogenesis | HCC | [41] |
| FABP3 | Upregulated | NA | Promote proliferation | Esophageal squamous cancer | [42] |
| FABP4 | Downregulated | NA | Inhibit proliferation and invasion | HCC | [43] |
| FABP5 | Upregulated | CREB | Promote the proliferation, migration, and invasion | HCC | [44] |
| FABP7 | Downregulated | NA | Promote proliferation | Breast cancer, ccRcc | [45, 46] |
| CPT1A | Upregulated | NA | Promote proliferation | Breast cancer | [47] |
| CPT1C | Upregulated | NA | Promote proliferation | HCC | [48] |
- Abbreviations: ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; ccRcc, clear cell renal cell carcinoma; CPT1, carnitine palmitoyltransferase 1; FABP, fatty acid-binding protein; FADS, FA desaturase; FASN, FA synthase; HCC, hepatocellular carcinoma; NA, not available; NF-κB, nuclear factor-κB; NSCLC, non-lung small cell carcinoma; SCD, stearoyl-CoA desaturase; VEGFR, vascular endothelial growth factor receptor.
2.1 FA de novo synthesis in cancer cells
De novo synthesis is a major part of the way cancer cells acquire FAs.49 The citrate is converted to palmitic acid (PA) in the FA de novo synthesis pathway through the enzymatic reaction catalyzed by the ATP citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), and FA synthase (FASN). In these series of enzymatic reactions, ACLY cleaves citrate in the cytoplasm to produce acetyl-CoA, which is then catalyzed by ACC to produce malonyl-CoA. Next, malonyl-CoA is elongated by FASN. Finally, the completely saturated 16-carbon FA (PA) is obtained.
ACLY, a rate-limiting enzyme in the first step of FA de novo synthesis, is upregulated in colon cancer. What is more, ACLY enhances the migration and invasion of colon cancer cells by promoting CTNNB1 (encoding β-catenin 1) transcriptional activity.50 Moreover, ACLY can regulate the stemness, migration, and invasion of hepatocellular carcinoma (HCC) cells through the Wnt/β-catenin signaling pathway.25 Similarly, ACC1 is expressed abnormally in prostate cancer cells.51 Studies have shown that inhibiting the activity of ACC can reduce cell proliferation and promote apoptosis of non-small cell lung cancer cells.26 However, the expression of ACC2 is inversely correlated with clinical outcomes in lung adenocarcinoma.27 Additionally, FASN, the core enzyme of FA synthesis, has been shown to be highly expressed in breast cancer tissues, suggesting that FASN can act as a biomarker of breast cancer.52 Moreover, FASN was found to promote epithelial–mesenchymal transition by activating the Wnt-1/β-catenin signaling pathway in breast cancer.28 Recent studies have also found that FASN can activate c-Src/AKT/FAK signaling pathway, thereby enhancing cervical cancer cell migration and invasion.29 Furthermore, FASN knockdown can reduce the secretion of proangiogenic factors in colorectal cancer cells and inhibit angiogenesis.53
Overall, FA de novo synthesis, the main source of FA in cancer cells, is abnormally activated during tumor progression to meet the energy required for the rapid proliferation of cancer cells. Meanwhile, in the FA de novo synthesis pathway, the expression of related metabolic enzymes is significantly increased in multiple cancer types. These results suggest that these metabolic enzymes have the potential to be used as markers and targets for cancer therapy.
2.2 Desaturation and prolongation of FAs in cancer cells
In addition to FA de novo synthesis, the aberrant activation of FA desaturation and prolongation are common features of cancer. Furthermore, according to the length of the carbon chain and the degree of saturation, FAs are commonly classified as saturated FA (SFA), monounsaturated FA (MUFA), and polyunsaturated FA (PUFA). When extended to 16 carbon atoms, FA is converted to FA acyl-CoA (FA-CoA) by acyl-CoA synthetase long chain family member (ACSL). Then, FA-CoA can be desaturated by stearoyl-CoA desaturase (SCD) and FA desaturase (FADS) to form unsaturated bonds. On the other hand, FA-CoA can be elongated by long-chain FA elongase (ELOVL), thereby increasing the length of unsaturated FAs (UFAs).54, 55
Desaturation is a process of introducing double bonds into FAs, and FADS has different substrate preferences. Among them, SCD converts FA into MUFA, which increases lipid utilization in the TME. Several studies have reported that SCD is significantly upregulated in a variety of solid tumors, suggesting the protumorigenic role of SCD.56 SCD1 and SCD5 are the SCD subtypes found in humans,57 among which SCD1 is ubiquitously expressed in all tissues.57 Further studies demonstrated that nuclear factor-κB can form a positive feedback loop with SCD1, thereby enhancing the stemness of ovarian cancer cells.30 In addition, Yuki and colleagues recently found that SCD1, which was expressed in cancer cells and immune cells, caused immune resistance, and inhibitors targeting SCD enhance the production of CD8 effector T cells and inhibit the growth of colon and non-lung small cell cancers.31 On the contrary, overexpression of SCD5 can inhibit the metastasis of breast cancer cells.32 As a metabolic enzyme regulating FAs, SCD exerts an antiproliferative effect via regulating lipid accumulation and the ratio between unsaturated and SFAs in cells.58 However, SCD requires energy and oxygen to catalyze the reaction, which also explains that cancer cells rely more on the intake of exogenous UFA to maintain cell survival under a hypoxic microenvironment.59 On the other hand, FADS prefers to convert FA into PUFA. Among them, FADS1 has been reported to be highly expressed in breast cancer and pancreatic cancer cells.33, 60, 61 What is more, FADS1 knockdown not only alleviates tumorigenesis but also increases the antitumour efficacy of chemotherapy drugs.33, 60, 61 Similarly, FADS2 expression was increased in HCC and non-small cell lung cancer patients.34 Further studies showed that inhibition of FADS2 and SCD can inhibit tumor growth.34
The elongation of long-chain FAs requires the participation of the ELOVL. Similarly, the ELOVL family members prolong different types of FA according to the specificity of the substrate. It was shown that ELOVL1, 3, and 6 were involved in the elongation of FA and MUFA. ELOVL2 and 4 elongated PUFA. ELOVL5 elongated MUFA and PUFA, and ELOVL7 was the elongase of SFA and PUFA.62, 63 Some reports have confirmed that ELOVLs are correlated to cancer development.49 It has been found that the expression of ELOVL2 is increased in tumor tissues of renal cell carcinoma patients.35 However, recent studies have found that high expression of ELOVL2 is associated with better prognosis in prostate cancer patients.36 There is a significant difference in the expression of ELOVL5 between tumor and adjacent nontumor tissues of colon cancer patients.37 Meanwhile, high expression of ELOVL6 is associated with shorter overall survival in HCC patients.38 In addition, studies have found that ELOVL7 has promise as a new biomarker for gynecological cancer.64
Briefly, desaturase and elongase catalyze the formation of different kinds of long-chain FAs. Although FA de novo synthesis is a key pathway for endogenous FA acquisition, knockdown of desaturase and elongase also showed significant antitumor efficacy.
2.3 Uptake and transport of FAs in cancer cells
Cancer cells obtained the FAs they need to survive mainly through de novo synthesis. However, the contribution of FA uptake and transport to maintain cellular lipid utilization cannot be ignored. The uptake and transport of FAs are accomplished by the active transport of several transmembrane proteins, such as FA transport proteins and CD36 (also known as FA translocase). In addition, some specialized small proteins, such as FA-binding proteins (FABPs), promote the transport of free FAs within cells.65 In HCC patients, the FA transport is significantly activated in malignant tumor tissues compared with adjacent tissues and normal hepatocytes.66 The upregulation of CD36 is significantly correlated with the epithelial–mesenchymal transition in HCC cells.67 Similarly, in human oral cancer cells, it was found that high expression levels of CD36 promoted cell metastasis by transporting large amounts of FA.68 Mechanistically, CD36 could induce gastric cancer metastasis by activating AKT/GSK-3β/β-catenin signaling pathway.40 Additionally, changes in FABP expression are detected in cancer development. Among the FABP family, both FABP1 is highly expressed in colorectal cancer and HCC, which are associated with the proliferation of tumors.69 Mechanistically, FABP1 activates the Akt/mTOR pathway by phosphorylating vascular endothelial growth factor receptor-2 (VEGFR2) to upregulate VEGF-A and promotes tumor angiogenesis.41 In addition, FABP3 has been found to promote the malignant progression of esophageal squamous cell carcinoma cells.42 Meanwhile, FABP5 is highly expressed in HCC and is associated with tumor proliferation.44 Moreover, inhibitors of FABP5 can reduce the phosphorylation level of peroxisome proliferator-activated receptor-γ (PPARγ) and promote the apoptosis of prostate cancer cells.70 Conversely, the expression of FABP4 is lower in multiple tumor tissues compared to normal tissues and FABP4 inhibits HCC cell proliferation and invasion in vitro.43 Meanwhile, recent studies have found that the expression of FABP7 is decreased in breast cancer and clear cell renal cell carcinoma, which suggests the potential of FABP7 as a cancer biomarker.45, 46 The above-mentioned transport proteins transfer exogenous FA into the cell, whereas carnitine palmitoyltransferase 1 (CPT1) transports activated FA-CoA into the mitochondria for β-oxidation to maintain cellular energy supply. There are three isoforms of CPT1, A, B, and C. CPT1A has been shown to be highly expressed in breast cancer.47 Similarly, in HCC patients, CPT1C is markedly upregulated, and by inhibiting its effects, the ability of cancer cells to proliferate, invade, and metastasize can be significantly alleviated.48
In short, these studies showed that the abnormal expression of enzymes that involved in the uptake and transport of FAs was strongly associated with tumor development, which also suggested that tumor treatment might focus on this aspect.
3 COMPOSITIONAL CHANGES OF FA IN MEMBRANE STRUCTURES OF CANCER CELLS
The FA metabolism provides energy for cancer cells to survive. Additionally, in cancer cells, more FAs are needed for membrane synthesis to proliferate rapidly. Therefore, FAs obtained from TME or from intracellular synthesis will also be incorporated into the lipid membrane and participate in the regulation of membrane function (Figure 2). In this section, we will discuss the effect of compositional changes of FA in the membrane structure of cancer cells.
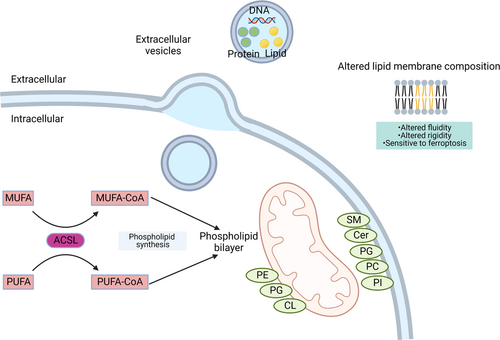
3.1 Compositional changes of FA in cell membranes of cancer cells
Phospholipids (PLs) are essential components of cell membranes. PL is characterized by a hydrophilic head consisting of a phosphoric acid-linked substituent group (containing amino acids or alcohols) and a hydrophobic tail consisting of FA chains. Furthermore, long-chain FAs, such as stearic acids (SAs) and oleic acids (OAs), are the common FAs that constitute the hydrophobic tail. The compositional alternations of FAs in PLs have been found in a variety of cancers.34, 62 For example, changes of saturation degree in the fatty acyl chains are found in lipidomic data of breast cancer tissues, where the levels of monounsaturated forms (phosphatidylcholine, PC 36:1) are higher than those of saturated forms (PC 36:0 and lysoPC 18:0).71 Meanwhile, studies have been found that lipid profiles containing several specific FAs can be used as biomarkers for cancer prediction and prognosis. For instance, the contents of phosphatidylglycerol, ceramide (Cer), sphingomyelin (SM), phosphatidylethanolamine (PE), and lysoPC are significantly different between the plasma of patients with colorectal cancer and that of healthy volunteers, so they can be used as good tumor markers in colorectal cancer detection.72 Similarly, tri-lipid profiles (Cer, SM, and PC) are linked to poor survival of prostate cancer patients.73
In addition to serving as biomarkers for cancer, FAs regulate cellular function by altering membrane fluidity and rigidity.74 Studies have found that PLs containing SFA can pack tightly, thereby reducing cellular membrane fluidity. Meanwhile, tight packing increases the rigidity of the cell membrane. Since the unsaturated bond causes the acyl chain to twist, the high UFAs in the cell membrane decrease the bulk density and increase the membrane fluidity.49 For instance, it was found that cancer cells increase the proportion of SFA on the cell membrane by activating FA metabolism pathways, thereby reducing cell membrane fluidity, and increasing drug resistance (see also Section 5.2). In contrast, high levels of UFAs will stimulate lung cancer metastasis by increasing membrane fluidity75 and are associated with poor prognosis of cancer patients.76 Similarly, studies have found PLs with higher unsaturation degrees in metastatic urothelial carcinoma cells.77 On the other hand, the rigidity of the cell membrane is also important for maintaining cell function. In normal cells, the cell membrane is in a semirigid state, which allows the normal movement of transmembrane proteins and lipid domains across the membrane. Meanwhile, an increased level of PC species with a higher degree of desaturation has been found in cervical and breast cancer cells, which promotes cell invasion and metastasis.78
In general, some specific FA species in the PL membrane of cancer cells can be used as markers of cancer. In addition, cancer cells can alter the fluidity and stiffness of the cell membrane by regulating the saturation and abundance of FA in the cell membrane, thereby affecting the motility of the cell.
3.2 Compositional changes of FA in cancer cell membranes prevent excessive oxidative stress
Regulating the FA composition of the membrane structures can not only promote cancer cell proliferation and metastasis but also prevent death caused by excessive oxidative stress. The imbalance of oxidation–reduction system is one of the most common features in cancer cells. Increased levels of oxidative stress contribute to tumorigenesis. Furthermore, high levels of oxidative stress are accompanied by the production of a large number of active substances, such as reactive oxygen species (ROS), nitrogen-free radicals, and so on. However, excessive ROS levels can cause apoptosis.79, 80 So, how do cancer cells survive in this TME? It has been found that changes in intracellular FAs can protect cancer cells from ROS damage.81 In membrane lipids, PUFA with multiple unsaturated double bonds is more prone to peroxidation, thereby increasing the permeability of cell membrane to promote cell apoptosis.82 Thus, cancer cells can avoid apoptosis by reducing PUFA content in the membrane. In detail, pathways involved in the synthesis of SFA or MUFA are significantly activated (see also Section 2), protecting cancer cells from lipid peroxidation.83 On the other hand, cancer cells can also avoid death by reducing lipid peroxidation levels of PUFA. Glutathione peroxidase 4 (GPX4) is a lipid hydrogen peroxidase that selectively degrades lipid peroxide from membranes. Overexpression of GPX4 in cancer cells reduces cell death caused by ROS via repressing the peroxidation levels of PUFA of membranes.84 Moreover, GPX4 has been found to be a key protein for the resistance of ferroptosis, a recently discovered type of cell death that is closely related to lipid peroxidation, in multiple cancer models.85 These results suggest that cancer cells avoid death by reducing the levels of UFAs in cell membranes.
Overall, compositional changes of FA on the membrane structure of cancer cells show strong associations with tumor progression. As described in Section 3.1, the higher degree of FA unsaturation, the stronger migration of cancer cells. But excessive UFAs can lead to cancer cell death. It is amazing that cancer cells have the ability to precisely regulate the ratio of FAs and the mechanisms behind this are worth exploring.
3.3 Compositional changes of FA in extracellular vesicles of cancer cells
In addition to the cell membrane, other membrane structures within cells have a strong association with cancer development. Extracellular vesicles (EVs) are special vesicles with biofilm structures that are released by cells. According to the size and origin, EVs are classified into three subtypes: microvesicles, apoptotic bodies, and exosomes. With the innovation of detection technology, EVs carrying special molecules, such as messenger RNA (mRNA), proteins and FAs, have gradually been paid more attention as biomarkers of diseases.86-89 Here, we focus on the changes in FAs in tumor-derived EVs. For instance, in the diagnosis of gastrointestinal cancer, the lipid profile of exosomes isolated from cancer patients is significantly different compared to healthy donors.90 Further exploration of the altered lipid profile reveals a change in the unsaturation of FAs in patients’ plasma exosomes and a significant increase in the content of arachidonic acids.91 Collectively, these results suggest that FAs in exosomes have the potential to serve as biomarkers for cancer diagnosis and treatment.87, 88, 92 In addition to serving as biomarkers, EV-carried FAs can act as first or second messengers, activating different signaling pathways.93 For example, lipid signaling molecules activate the arachidonic acid cascade to generate prostaglandins, leukotrienes, and lipoxins, thereby affecting cellular functions94 (see also Section 4). Thus, the special FAs carried by EVs from cancer cells may affect normal signal transduction in target cells. For instance, Yin and colleagues demonstrated that exosomes including FAs hyperactivated the PPAR signal pathway, thereby inducing dysfunction of dendritic cells (DCs) to promote immune evasion.95 On the other hand, FAs are also an important part of the EV membrane structure. When the EV membrane fuses with the target cell membrane, the changes in FAs may alter the fluidity, membrane permeability, and reactivity of receptor proteins on the cell membrane.96 So, EVs derived from cancer cells may also disrupt the normal function of target cells via this pathway.
Currently, among the EV-carried molecules, the FA research is far less in-depth than mRNA and protein. Meanwhile, the current research on EVs in cancer mainly focused on their contents. It remains unknown whether EVs in cancer affect target cells by membrane fusion. Although there is evidence that FAs in EVs can promote tumor development, whether the mechanism of action is the same as FA from synthesis or TME remains to be investigated. Thus, it is worth studying the specific role of FA in the EVs of cancer cells.
3.4 Effects of the compositional changes of FA on mitochondrial membranes in cancer cells
The mitochondrion is a double-membrane-bound organelle and is directly related to the occurrence of diseases such as cancer.97 Here, we will briefly discuss the relationship between mitochondria and cancer from the perspective of the mitochondrial membrane. It has been found that the collapse of mitochondrial membrane potential induced apoptosis in cancer cells.98, 99 Furthermore, exogenous lipids can regulate the mitochondrial membrane function, such as changing membrane permeability and affecting membrane potential.100 For example, a large number of studies related to colon cancer have demonstrated that n-3 PUFA can effectively reduce tumorigenesis by enhancing the unsaturation of mitochondrial PLs.101-103 Further research found that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), known as typical n-3 PUFA, when incorporated into the mitochondrial membrane of colon cells, enhanced the susceptibility of cells to oxidative damage due to a high degree of unsaturation, thereby impairing the mitochondrial function and inducing apoptosis.102 In the meantime, fish oil that contains n-3 PUFA can reduce the abundance of larger mass cardiolipin species, such as C74:7, C76:7, C76:8, and C76:9, in colonic crypts of mice, and promote cell apoptosis and reduce colon cancer risk.104
In conclusion, mitochondrial function is closely related to tumor progression. When exogenous FAs are incorporated into the mitochondrial membrane, they can change the FA composition of the mitochondrial membrane, thereby regulating the progression of cancer. In particular, n-3 PUFA can alter membrane potential and impair mitochondrial function, thereby slowing the progression of cancer.
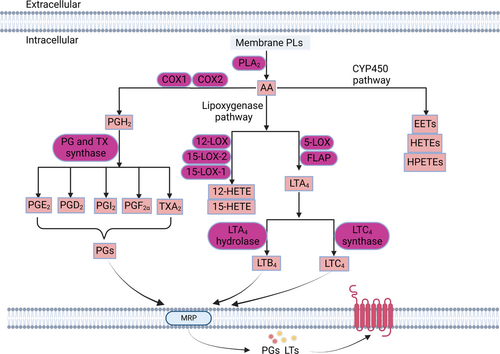
4 EFFECTS OF SIGNALING FAS ON CANCER CELLS
As described above, lipids can not only provide energy for cellular activities and material basis for cell proliferation but also act as messengers to transmit intercellular and intracellular signals. Eicosanoids, sphingolipids, and phosphoinositides are all signaling lipids.105-108 Here, we focus on a class of signaling lipids directly synthesized by FAs, namely eicosanoids. Eicosanoids are a large class of bioactive signaling lipids produced by the metabolism of 20-carbon PUFA. They act as autocrine and paracrine signaling molecules.49 Several studies have found that eicosanoids can regulate a variety of biological processes, including cell proliferation, cell motility, angiogenesis, and inflammatory responses.109, 110 Furthermore, prostaglandin E2 (PGE2) and leukotriene B4 (LTB4), as the common eicosanoids, have been demonstrated to show procancer effects in colorectal cancer.111, 112 On the other hand, abnormal expression of pathways involved in the synthesis of eicosanoids has been found in cancer cells, which synergistically promote tumor development.113-116 In detail, eicosanoids are synthesized by three catalytic enzyme pathways, cyclooxygenase, lipoxygenase (LOX), and cytochrome P450 (CYP450) (Figure 3).117 Here, we will discuss the effects of eicosanoids and three synthesis pathways on cancer in this section (Table 2).
| Eicosanoid/metabolic enzyme | Expression | Function | Type of cancer | Reference |
|---|---|---|---|---|
| PGD2 | Downregulated | Inhibit tumorigenesis | Pancreatic cancer | [118] |
| PGE2 | Upregulated | Promote proliferation | Colorectal cancer | [119] |
| PGF2ɑ | Upregulated | Promote migration and invasion | Colorectal cancer | [120] |
| PGI2 | Downregulated | Inhibit invasion | Ovarian cancer | [118] |
| LTB4 | Upregulated | Promote proliferation | Lung cancer, colon cancer, melanoma | [121-123] |
| LTC4 | Upregulated | Promote migration | Epithelial cancer | [124] |
| 12-HETE | Upregulated | Promote metastasis | Colorectal cancer | [125] |
| 15-HETE | Upregulated | Promote proliferation | Lung adenocarcinoma | [126] |
| 14,15-EET | Upregulated | Promote proliferation and migration | Breast cancer | [127] |
| 20-HETE | Upregulated | Promote metastasis | Prostate cancer | [128] |
| EDP | Downregulated | Inhibit proliferation and invasion | Breast cancer | [129] |
| COX1 | Upregulated | Promote angiogenesis | Renal cancer | [130] |
| COX2 | Upregulated | Promote migration and invasion | Nasopharyngeal carcinoma | [131] |
| 5-LOX | Upregulated | Promote proliferation | Colon cancer | [121] |
| 12-LOX | Upregulated | Promote angiogenesis | Esophageal squamous cell carcinoma | [132] |
| 15-LOX-1 | Downregulated | Inhibit invasion | Colorectal cancer | [133] |
| Upregulated | Promote proliferation | Prostate cancer | [134] | |
| 15-LOX-2 | Downregulated | Inhibit invasion | Prostate cancer | [135] |
| CYP1A1 | Upregulated | Promote proliferation and metastasis | Breast cancer | [136] |
| CYP2J2 | Upregulated | Promote proliferation | Renal cancer | [137] |
| CYP3A4 | Upregulated | Promote proliferation | Breast cancer | [138] |
- Abbreviations: COX, cyclooxygenase; CYP, cytochrome P450; EDP, epoxyeicosapentaenoic acid; EET, epoxyeicosatrienoic acid; HETE, hydroxyeicosapentaenoic acid; LOX, lipoxygenase; LTB4, leukotriene B4; LTC4, leukotriene C4; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; PGI2, prostaglandin I2.
4.1 Cyclooxygenase synthesis pathway
In the cyclooxygenase synthesis pathway, FAs are released from the PL membrane by phospholipase A2. Then, FAs are converted by cyclooxygenase 1 or 2 (COX1 or COX2) to PGs.139 PGE2 is the most abundant PG detected in various tissues. Some studies have found that PGE2 levels are elevated in several human malignancies, including breast, lung, and colon cancer. PGE2 is associated with poorer prognosis in patients.140, 141 Mechanistically, PGE2 promotes the proliferation of colorectal cancer by phosphorylating c-Jun N-terminal kinase.119 Furthermore, PGE2 is also found to promote tumor progression through binding to different e-prostaglandin receptors (EPs).142 Therefore, deficiency of EP2 receptor results in the failure of PGE2 to activate downstream signaling pathways, which delays the development of lung cancer.143 In addition to promoting proliferation, further studies have shown that prostaglandin F2α (PGF2ɑ) can increase the invasion of carcinoma-derived cells in colorectal cancer.120 Additionally, it has been found that PGF2ɑ also has the potential as a breast cancer biomarker.144 However, some PGs are considered to be antitumorigenic. For example, prostaglandin D2 (PGD2) has been reported to reduce pancreatic tumorigenesis.145 Meanwhile, prostaglandin I2 (PGI2) can inhibit the invasion of ovarian cancer cells by downregulating the expression of matrix metallopeptidase-2.118 On the other hand, as the key enzyme in the COX pathway, COX exhibits strong correlations with the expression levels of PGs in cancer. There is evidence that abnormally high expression of COX2 leads to increased downstream PGE2 release, thereby promoting cancer cell growth and migration.131, 143 Meanwhile, high expression of COX1 was found in renal cancer tissues.130 Moreover, the knockdown of COX1 can inhibit the growth and migration of ovarian cancer cells.146
In general, PGs bind to receptors that transmit signals, which affects the proliferation and invasion of cancer cells. Although they play different roles, these PGs all may provide new diagnostic markers. In addition, COX synthesis pathways also have the potential as cancer therapeutic targets.
4.2 LOX synthetic pathway
In the LOX synthetic pathway, FAs are hydrolyzed from membrane PLs and catalyzed by LOX to generate eicosanoids such as LTB4, LTC4, 12-hydroxyeicosatetraenoic acid (12-HETE), and 15-HETE.147 Among these metabolites, LTB4, the most well-studied LTs in cancer, is closely related to cancer cell proliferation.148 Furthermore, LTB4 levels have been found to increase in lung carcinoma patients.121 In the meantime, LTB4 expression is also elevated in colon cancer and melanoma, which promotes tumor growth.122, 123 Mechanistically, LTB4 exerts effects via binding to its receptor. LTB4 receptors have been found to cause KRAS-driven lung tumorigenesis by promoting interleukin-6-mediated inflammation.149 Similarly, as the signaling lipid, LTC4-mediated leukotriene receptor 1 signaling modulates the activation of Rac family small GTPase 1, which increases the expression of metastasis-inducing protein 1 and epithelial cancer migration.124 Furthermore, it has been reported that 12-HETE is involved in colorectal cancer metastasis caused by platelets.125 Meanwhile, 15-HETE is positively correlated with the development of breast cancer.126 On the other hand, as an important LOX isoform, 5-LOX is involved in the formation of various LTs. Moreover, elevated expression of 5-LOX and enhanced signal transduction mediated by its product LTs have been found in prostate, pancreatic, lung, and colon cancer.121 Furthermore, the deficiency of 5-LOX is shown to cause LT synthesis blockade in medulloblastoma, which significantly reduces tumor growth.150 12-LOX is another enzyme in the LOX family. It has been found that the levels of 12-LOX mRNA were elevated in cancer cells and the expression correlated with differentiation and invasiveness of prostate cancer.115 Further studies found that 12-LOX promoted the proliferation of esophageal squamous cell carcinoma by activating PI3K/AKT/mTOR pathway and upregulating the expression of VEGF.132 Meanwhile, the inhibition of 12-LOX can promote the apoptosis of HCC cells.151 However, 15-LOX is thought to have an anticancer effect. The depletion of 15-LOX-1 can promote tumorigenesis in colon cancer.133, 152 Interestingly, the opposite was found in prostate cancer. The results indicated that inhibition of 15-LOX-1 can induce apoptosis.134, 135
Compared with PGs, less is known about the LTs in cancer. Because of the heterogeneity of the tumor, many controversies still exist regarding the effects of some LT synthases. Thus, the roles of LTs and their synthase and the complex interplay among the subsequent intracellular signaling pathways need further investigation.
4.3 CYP450 synthetic pathway
In the CYP450 synthetic pathway, a variety of PUFAs serves as substrates to generate epoxides such as epoxyeicosatrienoic acids (EETs) and HETEs.140 Among epoxides, EETs have been demonstrated to be associated with several cancers.39, 127-129, 136-138, 153-178 It has been reported that 14,15-EET levels are significantly higher in breast cancer than in noncancerous tissue.127 Moreover, EETs promote cancer cell proliferation and tumor angiogenesis, possibly by activating phosphorylation of EGFR and STAT3.153, 154 Additionally, recent studies also found that 20-HETE can promote the metastasis of prostate cancer cells.128 Conversely, as the metabolite of n-3 PUFA, epoxyeicosapentaenoic acid (EDP) combined with the soluble epoxide hydrolase inhibitor will reduce breast cancer growth and metastasis.129 On the other hand, CYP450 is a key enzyme in this metabolic pathway, and some isoforms of CYP450 are shown to be abnormally expressed in cancer. For example, high levels of CYP1A1 can be detected in patients with smoking-induced lung cancer.155 Furthermore, overexpression of CYP1A1 in breast cancer can eliminate the drug effect of tetrahydrocurcumin.136 In the meantime, the expression of CYP2J2 is also found to be upregulated in various forms of cancer cell lines, including lung cancer, prostate cancer, and kidney cancer.137 CYP3A4 shows a significant correlation with the survival of breast cancer patients.138 Moreover, a large number of data have indicated that the activity of CYPs and epoxides can affect tumor angiogenesis.156, 157
In general, PGs, LTs, EETs, and other signaling lipids regulate downstream signaling pathways by binding to receptors, thus affecting the progression of cancer. Furthermore, some signaling lipids have the potential to be biomarkers. Meanwhile, their synthetases have also become drug targets for cancer treatment. Notably, because of the unique way in which signaling lipids act, small molecules targeting their receptors can also be a strategy for cancer therapy.
5 TUMOR THERAPEUTIC STRATEGIES BASED ON FAS AND ALTERED FA METABOLISM
Given the important role of FAs in cancer that has been explored, targeting FA metabolism has become a promising research direction in cancer treatment. Currently, FA metabolic enzymes hold promise as potential anticancer targets. Additionally, FAs themselves have shown good therapeutic prospects in cancer therapy. For example, eating some good FAs can slow tumor progression.104 Here, we briefly outline some of the cancer therapeutic approaches based on FAs and FA metabolism, with drugs that target FA metabolic enzymes detailed in Table 3.
| Target | Compound | Phase of development | Type of cancer | Reference |
|---|---|---|---|---|
| FASN | Orlistat | Preclinical | Melanoma, prostate cancer, ovarian cancer | [159, 179, 180] |
| Cerulenin | Preclinical | Colon cancer, breast cancer | [160, 161] | |
| C75 | Preclinical | Breast cancer, renal cancer | [162, 163] | |
| TVB-2640 | Phase Ι (NCT02223247) | Colon cancer | [164] | |
| ACC | Soraphen A | Preclinical | Prostate cancer, breast cancer | [165, 166] |
| ND-646 | Preclinical | NSCLC | [167] | |
| ND-654 | Preclinical | HCC | [168] | |
| CD36 | ABT-510 | Phase Ι (NCT00586092) | Multiple cancers | [174] |
| FABP | SBFI-102, SBFI-103 | Preclinical | Prostate cancer | [175] |
| CPT1 | Etomoxir | Preclinical | Prostate cancer, breast cancer | [176, 181] |
| Perhexiline | Preclinical | Breast cancer | [177] | |
| Ranolazine | Preclinical | Prostate cancer | [178] | |
| COX2 | Celecoxib | Phase Ⅲ (NCT01150045) | Colon cancer | [182] |
| Phase Ⅲ (NCT02429427) | Breast Cancer | [183] | ||
| Etoricoxib | Phase Ⅱ (NCT04565600) | Breast cancer | [184] | |
| Meloxicam | Phase Ⅲ (NCT00152919) | Prostate cancer | [185] | |
| 5-LOX | MK886 | Preclinical | Melanoma | [186] |
| Zileuton | Phase Ⅱ (NCT00070486) | NSCLC | [187] |
- Abbreviations: ACC, acetyl-CoA carboxylase; COX, cyclooxygenase; CPT1, carnitine palmitoyltransferase 1; FABP, fatty acid-binding protein; FASN, fatty acid synthase; HCC, hepatocellular carcinoma; LOX, lipoxygenase; NSCLC, non-small cell lung carcinoma.
5.1 Targeting FA metabolism to block the FA supply of cancer cells
FA metabolic reprogramming is a common feature of tumors. Thus, targeting FA metabolism may be an effective treatment. It has been found that FA synthesis is significantly increased in cancer cells. Blockers of FA synthesis, such as inhibitors of FASN and ACC, can reduce cancer cell proliferation. FASN-targeted drugs include orlistat, cerulenin, and C75.158 Orlistat, an antiobesity drug that reduces intestinal lipid uptake, is shown to exert great inhibitory effects in preclinical models.159 Similarly, cerulenin, a natural and widely used FASN inhibitor, has antiproliferative effects in multiple cancer cell lines.160, 161 Additionally, C75, the first synthetic anti-FASN molecule, has a more stable chemical property than cerulenin and has been shown to be strongly inhibitory in both kidney and breast cancer.162, 163 Among the new generation of inhibitors targeting FASN, TVB-2640 is in a phase I study to treat colon and other cancers.164 On the other hand, ACC is also a key enzyme in FA metabolism and is upregulated in cancer cells. Likewise, inhibition of ACC has antitumor effects. Among ACC inhibitors, the natural compound sorafen A has been found to attenuate de novo FA synthesis and inhibit cell proliferation in different cancers.165 Moreover, sorafen A can reduce the metastatic potential of breast cancer cells by increasing the saturation degree of membrane lipids.166 In addition, among the synthetic anti-ACC small molecules, the ND-600 series are designed to inhibit the FA synthesis activity of ACC, of which ND-646 and ND-654 are proven to alleviate the proliferation of cancer cells.167, 168
The end product of de novo synthesis is PA, which is an SFA of 16 carbons. In order for cancer cells to obtain long-chain FAs and UFAs, PA needs to be catalyzed by desaturases and elongases. Thus, the process of desaturation and elongation also plays an important role in cancer development. Inhibition of this process may show the anticancer effect. Furthermore, studies have found that inhibition of SCD1 has shown strong efficacy in multiple cancers (including ovarian cancer, bladder cancer, and melanoma).169-171 The FADS inhibitor has also been shown to relieve colon cancer symptoms in preclinical studies.172 On the other hand, inhibiting FA elongation also has anticancer efficacy. For instance, the knockdown of ELOVL7 will alter membrane structural properties, thereby reducing prostate cancer growth.39 However, due to the complex structure of the interacting region of ELOVL, it is difficult to develop small-molecule inhibitors for ELOVL, and the existing inhibitors of ELOVL have not been explored in cancer yet.1
In addition to enhancing FA synthesis, cancer cells also acquire exogenous FAs via FA uptake mechanisms. Among the relevant metabolic enzymes, CD36 acts as a transmembrane protein and several studies have demonstrated that targeting CD36 to block FA uptake by cancer cells is a potentially effective therapeutic approach. For example, ABT-510, a mimetic of TSP-1 (the ligand of CD36), has been reported to inhibit angiogenesis in prostate cancer.173 A phase I study also indicated that the combination of ABT-510 and bevacizumab would be a safe and effective antiangiogenic strategy for the treatment of solid tumors.174 In addition, FABPs also serve as the proteins that promote FA uptake in cancer, suggesting that inhibition of FABPs may show a beneficial effect on cancer treatment. Consistently, SBFI-102 and SBFI-103, inhibitors of FABP5, are shown to inhibit prostate cancer growth.175
Cells get the energy to grow by FA oxidation. This process is more pronounced in tumors. FA oxidation-related enzymes are upregulated in many cancer types. Therefore, inhibition of these metabolic enzymes slows the progression of cancer. As the rate-limiting enzyme of FA β-oxidation, CPT1 is essential for the rapid proliferation of cancer cells. Its inhibitors etomoxir, perhexiline, and ranolazine have all been shown to have antitumor efficacy.176-178 These results not only demonstrate the importance of FAs as an energy source for cancer cell survival but also show that FA oxidation can be used as a key anticancer target.
As mentioned earlier, FAs can also act as signaling molecules to regulate cellular functions. Several studies have reported that eicosanoids, a class of signaling lipids generated by PUFA, affect the development and progression of cancer. Therefore, targeting the eicosanoid pathway also has good antitumor efficacy. In the COX synthesis pathway, the COX2 inhibitor celecoxib has been reported to reduce tumor metastasis in melanoma, prostate cancer, and breast cancer.188-190 In the meantime, a large number of clinical studies have also found that celecoxib has therapeutic effects on different cancers.182, 183 Several other COX2 inhibitors (NSAIDs: etoricoxib, meloxicam) also have anticancer effects in the clinic.184, 185 On the other hand, 5-LOX is one of the key enzymes in the LOX synthesis pathway, and its inhibitor MK886 can inhibit melanoma formation.186 The oral inhibitor of 5-LOX, zileuton, has also been shown to inhibit the development of esophageal adenocarcinoma.191 What is more, the combination of COX2 inhibitor and 5-LOX inhibitor not only reduces tumor growth in a murine model of colon cancer or skin cancer192, 193 but also reduces liver metastasis of pancreatic cancer.194
Overall, drugs targeting FA metabolic enzymes can slow tumor progression. However, some drugs that target FA metabolism still have side effects, such as weight loss with long-term use. Meanwhile, in the treatment of some malignant tumors, targeting a single enzyme in FA metabolism is not effective enough. So, for better efficacy, it may be necessary to use a combinatorial therapy.
5.2 Targeting FA metabolism to improve the sensitivity of cancer cells
Chemotherapy and radiotherapy have been used as the standard treatment for cancer patients. However, patient tolerance greatly limits the effectiveness of the treatment. Notably, targeting FA metabolism can increase cell sensitivity to chemotherapy and radiotherapy.11, 181 It has been demonstrated that decreased sensitivity of cancer cells to chemotherapy has long been associated with increased saturation degree of membrane lipids.195 Specifically, the changes in saturation degree affect PL bilayer fluidity, and reduced fluidity may lead to the destruction of functional domains in cell membranes that mediate drug absorption, thereby resulting in drug resistance.166 Moreover, the activation of de novo FA synthesis increases the incorporation of SFAs into cell membranes and enhances cell resistance to chemotherapy.179, 196, 197 Therefore, blocking de novo synthesis of FA can also improve cell sensitivity. For example, targeting FASN has been proven to reduce the inhibitory concentration of cisplatin and improve efficacy in chemoresistant ovarian cancer cells.179, 197 Furthermore, it was found that increased cell chemotherapy sensitivity by FASN inhibitors (orlistat) may also be associated with decreased expression of multidrug resistance-related proteins.166 Numerous studies have also identified that soraphen A, a drug that targets ACC, can increase chemosensitivity by regulating membrane fluidity and enhancing drug uptake.166 Additionally, in the transcriptome of chemoresistant tumors, CPT1B mRNA is found to be increased compared with primary breast tumors.198 What is more, CPT1 inhibitors can effectively increase the chemosensitivity of tumor cells.195 These data suggest that inhibition of FA synthesis and oxidation can improve patient tolerance and enhance the efficacy of chemotherapy.
On the other hand, the efficacy of radiotherapy also depends on tumor sensitivity. Increased CPT1A expression was also found in radiotherapy-resistant nasopharyngeal carcinoma.199 Furthermore, etomoxir, an inhibitor of CPT1, can improve glioblastoma multiforme by eliminating radioresistance.200 Similarly, it has also been reported that a combination of radiotherapy and a FASN inhibitor (orlistat) can synergistically reduce prostate cancer growth in vivo.201 Notably, combined with the results of targeting FASN described in the previous section, these studies have indicated that FASN inhibitors work well against tumors either alone or in combination with other therapies. Therefore, compared with other FA metabolic enzymes, molecules targeting FASN may be more effective in cancer. In addition, a recent study also unravels that elevated ACSL4 levels can increase tumor susceptibility to radiotherapy in vitro.180 ACSL4 not only participated in the activation of long-chain FAs and lipid peroxidation but also induced cell ferroptosis. It has been found that ferroptosis inducers can increase the efficacy of radiotherapy in cancer treatment via increasing intracellular lipid peroxidation levels.202, 203
Generally, in addition to directly blocking the FA supply of cancer cells, targeting FA metabolism can increase the sensitivity of tumors to chemotherapy and radiotherapy and improve the killing effect of cancer cells. In particular, drugs targeting FASN have shown promising efficacy either alone or in combination with radiotherapy or chemotherapy. Therefore, the development of a new generation of inhibitors targeting FASN may be a promising therapeutic strategy.
5.3 Dietary therapies based on FA metabolism
In recent years, more and more attention has been paid to the influence of nutrient choices on certain malignancies.204 Proteins, vitamins, and lipids have all been reported to regulate cancer progression.205 Furthermore, clinical studies have shown that excessive intake of dietary FAs such as SFA may increase the risk of breast cancer, while intake of UFAs may reduce the risk of digestive system cancer.206 With the economy developing, dietary recommendations encourage reducing SFAs in the diet and replacing it with PUFAs. However, high n-6/n-3 PUFA ratios in Western diets have been found to increase the risk of cancer.207 These conclusions have been confirmed in animal studies. The result showed that the high-fat diets that were rich in MUFA and n-6 PUFA promoted tumor growth and induced liver metastasis.59 Conversely, administration of n-3 PUFA, such as EPA or DHA, has been reported to reduce cancer-related symptoms.208, 209 Further research found that n-3 PUFA induces ferroptosis in cells through lipid peroxidation and further inhibits tumor growth in combination with ferroptosis inducers.210 Meanwhile, several studies have shown that dietary supplementation of DHA and EPA amplifies antitumor efficacy of chemotherapy.211 In addition, some dietary regimens have also been reported to slow the development of cancer.204, 212 Among these dietary regimens, the ketogenic diet, a low-carb and high-fat diet, can significantly attenuate tumor growth.212 Furthermore, a calorie-restricted diet, reducing carbohydrate and fat intake, can impair SCD activity to cause an imbalance between saturated and UFAs and ultimately inhibits tumor growth.212 In addition, periodic fasting also increases the chemosensitivity of cancer cells and delays tumor progression.204
In conclusion, both clinical data and animal experiments have confirmed the efficacy of dietary intervention on cancer.187 Both direct administrations of dietary FAs and FA-related dietary pattern modification have shown improvement in cancer. However, there are still no clear dietary guidelines or recommendations for cancer treatment. There are no specific recommendations on the type of FAs to take, especially the other nutritional deficiencies and increased risk of disease due to the special diet should also be considered when patients are given dietary treatment. It is necessary to give possible individualized dietary advice according to the age, sex, and health status of the patients.
6 CONCLUSION AND PERSPECTIVE
During the course of tumor development, cancer cells are challenged by various harsh factors, such as nutrient deprivation, hypoxia, oxidative stress, and inflammation. In such a microenvironment, the metabolism of substances in cancer cells is altered to support their continued growth and metastasis.6, 7 As one of the main nutrients, the metabolic pathway of FA is reprogrammed in cancer cells, and a series of related metabolic enzymes are activated. Currently, with the development of metabolomics technology and the innovation of analytical technology, FA reprogramming has been more deeply interpreted. As mentioned above, FAs can be used as a source of energy for cells. Studies have found that FA anabolic, transport and oxidation pathways are significantly activated in cancer cells.49, 56, 66 We elaborate on the changes of relevant metabolic enzymes in various biological processes of FAs and highlight the oncogenic role of aberrant activation of FA de novo synthesis in cancer development. As described above, metabolic enzymes involved in FA de novo synthesis, such as ACLY, ACC, and FASN, are all highly expressed in cancer cells and have a strong positive correlation with tumor development (see also Table 1). For example, ACLY can enhance the stemness of liver cancer and FASN can regulate the migration of cervical cancer.25, 29 In addition, aberrant activation of FA de novo synthesis leads to the accumulation of SFAs, which not only provide sufficient energy for cancer cell proliferation but also increase the saturation of the cell membrane, thereby avoiding death caused by excessive ROS.83 Meanwhile, excessive incorporation of SFA into the cell membrane can also reduce the fluidity of the cell membrane, which is closely related to the drug resistance of cells.75 On the other hand, excessive intake of SFAs also increases the risk of cancer.207 However, current studies have focused on de novo synthesis and oxidation of FAs. The changes in desaturation and elongation of FAs cannot be ignored, especially the products of these processes, MUFA and PUFA. Although their content is less than SFA, they can also affect the survival and death of cancer cells.76, 101 In addition, FAs are also important components of the PL membrane, particularly long-chain UFAs (LCUFAs), which can change the fluidity and stiffness of the membrane and regulate the function of cancer cells.78 For example, the increase of LCUFAs in the membrane of cancer cells will enhance fluidity and promote cell invasion.77 However, it has been found that decreased membrane fluidity in resistant cells led to a decrease in drug uptake, which was related to the reduction of LCUFAs on the cell membrane.75 It can be seen that the amount of LCUFAs in the membrane can affect different functions, which also raises the important question of how cancer cells control the amount of LCUFAs to favor survival. Further studies on the mechanisms by which cancer cells regulate the contents of membrane lipids to adapt to different microenvironments are needed for a better explanation. At last, FAs also act as signaling molecules that modulate various biological processes. Eicosanoids are the major signaling lipids, and most of them have procancer effects, such as PGE2, LTB4, 20-HETE, and 14,15-EET.119, 121, 127, 128 Meanwhile, some eicosanoids can also play an antitumor role, such as PGD2, PGI2, and EDP.107, 108 In addition, the expression levels of metabolic enzymes that synthesize eicosanoids are also inconsistent in different tumors. For instance, 15-LOX-1, an enzyme involved in LTs synthesis, has been reported as a cancer suppressor in colon cancer, but a cancer promoter in prostate cancer.118, 129 Therefore, it is necessary to investigate the specific roles of FA metabolic enzymes and their metabolites (such as eicosanoids) in different cancers.
Given the prominent role played by FA in cancer, existing drugs and designed small-molecule inhibitors that target FA metabolic enzymes have shown good preclinical effects in cancer. For example, inhibitors targeting FA synthetase can inhibit proliferation and improve the efficacy of chemotherapy drugs.159, 165 In the meantime, targeting FA transport or oxidation also shows anticancer effect by blocking the supply of FA.173, 176 However, in the development of lipid metabolism inhibitors, many small molecules remain in the preclinical experimental phase due to drug safety, metabolic plasticity of cancer cells, and heterogeneity of tumors. Therefore, overcoming these barriers is one of the key points in the future development of inhibitors. At present, some drugs have poor efficacy when used alone. Combination therapies have been found to better performance. For example, combined treatment with both 5-LOX and COX2 inhibitors further reduced the tumor growth compared to treatment with each inhibitor alone.192 More importantly, these drugs can increase the sensitivity of cancer cells to chemotherapy or radiotherapy and enhance their efficacy.166, 197 Thus, the combination of these agents warrants further investigation. In addition to these direct effects on FA metabolism, FA-based dietary therapy provides new ideas for cancer treatment. Clinical studies have shown that the intake of specific types of FAs is effective in reducing cancer risk.211, 212 In particular, some n-3 PUFAs can induce apoptosis in cancer cells, thereby slowing the progression of cancer. Additionally, regulating the content of FAs in the diet can also inhibit tumor growth, such as in the high-fat ketogenic diet.204 Moreover, considering the individual differences of patients, it is also important to further propose personalized diet programs.
Overall, both drugs targeting FA metabolism and dietary treatment based on FAs have been shown to work well in cancer. What is more, combinations of the drugs or dietary treatment together with other antitumor therapies may hold promise as new treatment strategies for cancer.
AUTHOR CONTRIBUTIONS
Ao Du: Investigation (equal); visualization (equal); writing—original draft (equal). Zhen Wang: Investigation (equal); visualization (equal); writing—original draft (equal). Tengda Huang: Resources (equal). Shuai Xue: Resources (equal). Chuang Jiang: Writing—review and editing (equal). Guoteng Qiu: Writing—review and editing (equal). Kefei Yuan: Conceptualization (lead); methodology (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The illustrations are created with BioRender.com. This work was supported by the Science and Technology Major Program of Sichuan Province, Grant/Award Number: 2022ZDZX0019; the National multidisciplinary collaborative diagnosis and treatment capacity building project for major diseases, Grant/Award Number: TJZ202104; the Natural Science Foundation of China, Grant/Award Numbers: 82272685, 82202260, and 82173124; the fellowship of China National Postdoctoral Program for Innative Talents, Grant/Award Numbers: BX20200225 and BX20200227; the Project funded by China Postdoctoral Science Foundation, Grant/Award Numbers: 2022TQ0221 and 2021M692278; the Science and Technology Support Program of Sichuan Province, Grant/Award Number: 2021YJ0436.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study did not involve human participants and/or animals or informed consent. Thus, ethical clearance is not applicable to this article.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.