A multiparameter radiomic model for accurate prognostic prediction of glioma
Yan Li, Li Bao, and Caiwei Yang contributed equally to this study.
Abstract
An accurate prediction of prognosis is important for clinical treatments of glioma. In this study, a multiparameter radiomic model is proposed for accurate prognostic prediction of glioma. Three kinds of region of interest were extracted from preoperative postcontrast T1-weighted images and T2 fluid-attenuated inversion recovery images acquired from 140 glioma patients. Radiomics score (Radscore) was calculated and the conventional image features and clinical molecular characteristics that may be related to progression-free survival (PFS) were evaluated. Five uniparameter and various combinations of biparameter and multiparameter models based on above characteristics were built. The performance of these models was evaluated by concordance index (C index), and the nomogram of the multiparameter radiomic model was constructed. The results show that the proposed multiparameter radiomic model has a better prediction performance than other models. In the training and validation sets, the calibration curves of the multiparameter radiomic model for the 1-, 2-, and 3-year PFS probability demonstrate a high consistence between predictions and observations. In conclusion, this study demonstrates that the multiparameter radiomic model based on Radscore, conventional image features and clinical molecular characteristics can improve the prediction accuracy of glioma prognosis, which could be informative for individualized treatments.
1 INTRODUCTION
Glioma is the most common primary intracranial tumor, which is derived from glial cells and neurons of the nervous system. The treatment strategies for glioma usually depend on many factors, such as Karnofsky performance status (KPS), the location of the tumor in the brain, the World Health Organization (WHO) grade of the glioma, and so on.1
Adult diffuse gliomas show different therapeutic responses and prognosis due to the heterogeneity of tumor tissues.2 The prognosis of glioma patients relies on multiple clinical and molecular characteristics, such as age, KPS, the status of isocitrate dehydrogenase (IDH) mutation, 1p/19q co-deletion, and the status of O6-methylguanine-DNA methyltransferase (MGMT) methylation.3-7 In addition, some studies have shown that some image features (i.e., peritumoral edema, necrosis, and enhancement) can also be used as reliable prognostic factors, which indicates that the collection of image features and clinical molecular characteristics of glioma patients is of great value in evaluating the prognosis of the patients.8-13
Recently, radiomics are increasingly applied to traditional and advanced magnetic resonance imaging (MRI) data in a variety of tumors for evaluating treatment response, classifying tumor subtype, and predicting progression-free survival (PFS) and overall survival (OS).14-18 Many novel prognostic radiomic models have been proposed for predicting the prognosis of glioma.19-22 Kickingereder et al. extracted quantitative image features from multiparameter MRI data of glioblastoma (GBM) patients before treatments to construct a model for predicting PFS and OS. It was found that adding radiomic features to a comprehensive model that includes clinical indicators, molecular features, and standard imaging parameters could improve the prognosis prediction.23 In addition, for the radiomic model based on advanced MRI data and combined with clinical molecular factors, the performance of the prognostic model can be substantially improved.24, 25 Summarily, radiomic can provide diverse features of the tumor and peritumoral tissues, extend the evaluation of tumor characteristics beyond the ability of human visual interpretation, and then supplement and support the prognostic information of glioma patients, which could improve the accuracy of prognosis prediction and the personalized treatments.
In this study, by evaluating the preoperative MRI features of glioma patients and extracting radiomic features, a multiparameter radiomic model is constructed based on radiomics score (Radscore), conventional image features, and clinical molecular characteristics. In addition, four other prediction models were built based on uniparameter or various combinations of biparameter for comparisons with the multiparameter radiomic model. The aim of this study is to prove that the multiparameter radiomic model can improve the accuracy of prognostic prediction for patients of glioma.
2 RESULTS
2.1 Information of patients
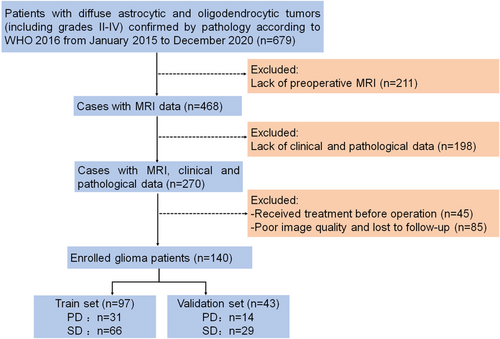
A total of 140 glioma patients were randomly divided into a training set (n = 97) and a validation set (n = 43) by a ratio of 7:3. All patients were followed up for at least 1 year, and 45 of 140 (32%) patients have a tumor progression at the last evaluation (Figure 1). The median PFS of glioma patients in the training set is 19 months (interquartile range, IQR: 12–32 months) and that in the validation set is 22 months (IQR: 14–35 months). The image features and clinical molecular characteristics of patients are shown in Table 1, and there are no differences in all factors between the training set and the validation set (p > 0.05).
| Characteristic | n | Validation set | Training set | p-value |
|---|---|---|---|---|
| Gender | 0.404 | |||
| Male | 74 | 25 (58.14%) | 49 (50.52%) | |
| Female | 66 | 18 (41.86%) | 48 (49.48%) | |
| Age [mean ± SD] | 42.22 ± 13.11 | 41.37 ± 10.43 | 42.60 ± 14.16 | 0.569 |
| PD | 0.944 | |||
| Yes | 45 | 14 (32.56%) | 31 (31.96%) | |
| No | 95 | 29 (67.44%) | 66 (68.04%) | |
| PFS [median (IQR), months] | 21.00 (13.00, 34.75) | 19.00 (12.00, 32.00) | 22.00 (14.00, 35.00) | 0.321 |
| Tumor location | 0.358 | |||
| Frontal | 46 | 18 (41.86%) | 28 (28.87%) | |
| Temporal | 40 | 10 (23.26%) | 30 (30.93%) | |
| Insular | 9 | 1 (2.33%) | 8 (8.25%) | |
| Parietal | 37 | 13 (30.23%) | 24 (24.74%) | |
| Occipital | 3 | 0 (0.00%) | 3 (3.09%) | |
| Brainstem | 2 | 1 (2.33%) | 1 (1.03%) | |
| Cerebellum | 3 | 0 (0.00%) | 3 (3.09%) | |
| Side of tumor epicenter | 0.928 | |||
| Right | 62 | 18 (41.86%) | 44 (45.36%) | |
| Center/bilateral | 3 | 1 (2.33%) | 2 (2.06%) | |
| Left | 75 | 24 (55.81%) | 51 (52.58%) | |
| Tumor crosses midline | 0.143 | |||
| No | 116 | 31 (72.09%) | 85 (87.63%) | |
| Yes | 24 | 12 (27.91%) | 12 (12.37%) | |
| Peritumoral edema | 0.389 | |||
| <1 cm | 36 | 9 (20.93%) | 27 (27.84%) | |
| ≥1 cm | 104 | 34 (79.07%) | 70 (72.16%) | |
| Edema crosses midline | 0.125 | |||
| No | 109 | 30 (69.77%) | 79 (81.44%) | |
| Yes | 31 | 13 (30.23%) | 18 (18.56%) | |
| Proportion enhancing | 0.888 | |||
| None | 57 | 19 (44.19%) | 38 (39.18%) | |
| Ring | 4 | 1 (2.33%) | 3 (3.09%) | |
| Part | 73 | 22 (51.16%) | 51 (52.58%) | |
| All | 6 | 1 (2.33%) | 5 (5.15%) | |
| nCET | 0.35 | |||
| Absent | 26 | 6 (13.95%) | 20 (20.62%) | |
| Present | 114 | 37 (86.05%) | 77 (79.38%) | |
| Proportion necrosis | 0.191 | |||
| None | 89 | 24 (55.81%) | 65 (67.01%) | |
| Mild | 19 | 9 (20.93%) | 10 (10.31%) | |
| Moderate | 17 | 7 (16.28%) | 10 (10.31%) | |
| Severe | 15 | 3 (6.98%) | 12 (12.37%) | |
| Cyst | 0.276 | |||
| Absent | 65 | 17 (39.53%) | 48 (49.48%) | |
| Present | 75 | 26 (60.47%) | 49 (50.52%) | |
| Multifocal | 0.756 | |||
| Absent | 134 | 42 (97.67%) | 92 (94.85%) | |
| Present | 6 | 1 (2.33%) | 5 (5.15%) | |
| Satellites | 0.96 | |||
| Absent | 94 | 29 (67.44%) | 65 (67.01%) | |
| Present | 46 | 14 (32.56%) | 32 (32.99%) | |
| Midline displacement | 0.654 | |||
| <1 cm | 120 | 36 (83.72%) | 84 (86.60%) | |
| ≥1 cm | 20 | 7 (16.28%) | 13 (13.40%) | |
| WHO grades | 0.507 | |||
| 2 | 95 | 31 (72.09%) | 64 (65.98%) | |
| 3 | 23 | 7 (16.28%) | 16 (16.49%) | |
| 4 | 22 | 5 (11.63%) | 17 (17.53%) | |
| Ki67 index | 0.321 | |||
| <20% | 107 | 36 (83.72%) | 71 (73.20%) | |
| ≥20% | 33 | 7 (16.28%) | 26 (26.80%) | |
| IDH mutation | 0.069 | |||
| Negative | 48 | 9 (20.93%) | 39 (40.21%) | |
| Positive | 92 | 34 (79.07%) | 58 (59.79%) | |
| MGMT methylation | 0.655 | |||
| - | 52 | 17 (39.53%) | 35 (36.08%) | |
| Negative | 25 | 8 (18.60%) | 17 (17.53%) | |
| Positive | 63 | 18 (41.86%) | 45 (46.39%) | |
| 1p/19q co-deletion | 0.312 | |||
| - | 56 | 15 (34.88%) | 41 (42.27%) | |
| Negative | 26 | 7 (16.28%) | 19 (19.59%) | |
| Positive | 58 | 21 (48.84%) | 37 (38.14%) |
- Note: Mann–Whitney U test and t-test were used for the continuous variable with abnormal and normal distribution respectively. Chi-square test or Fisher's exact test was used for the nominal variable.
- Abbreviations: IDH, isocitrate dehydrogenase; IQR, interquartile range; MGMT, O6-methylguanine-DNA methyltransferase; nCET, noncontrast-enhancing tumor; PD, progressive disease; PFS, progression-free survival; SD, standard deviation; WHO, World Health Organization.
2.2 Development of radiomic model
Three thousand sixty-nine radiomic features were extracted from the three kinds of region of interest (ROI). The least absolute shrinkage and selection operator (LASSO) cox regression model was used for features selection. The optimal parameter λ of 0.039 was determined by using the LASSO L1 regularization technique (Supporting Information: Figure S1). Among all the radiomic features, the nine most predictive features were screened and retained, and the corresponding Radscore was calculated in a linear combination of selected features and their respective nonzero coefficients (Supporting Information: Table S1). The developed radiomic model has good prediction performance. The concordance index (C index) of radiomic model in the training set is 0.786 (95% confidence interval [CI]: 0.716–0.857), and that in the validation set is 0.741 (95% CI: 0.622–0.860).
The univariate analysis for all factors shows that age, peritumoral edema, proportion necrosis, Satellite Lesion, Midline Displacement, WHO grades, IDH mutation, 1p/19q co-deletion, and Radscore are correlated with PFS (p < 0.05) (Table 2).
| Characteristics | Univariate analysis | |
|---|---|---|
| HR (95% CI) | p-value | |
| Gender | 0.900 (0.444–1.825) | 0.770 |
| Age | 1.043 (1.015–1.071) | 0.001 |
| Tumor location | 1.111 (0.892–1.385) | 0.356 |
| Side of tumor epicenter | 0.994 (0.694–1.422) | 0.972 |
| Tumor crosses midline | 1.251 (0.479–3.272) | 0.656 |
| Peritumoral edema | 4.574 (1.389–15.063) | 0.002 |
| Edema crosses midline | 1.434 (0.616–3.336) | 0.419 |
| Proportion enhancing | 1.985 (1.316–2.995) | <0.001 |
| nCET | 1.566 (0.599–4.094) | 0.337 |
| Proportion necrosis | 1.979 (1.495–2.619) | <0.001 |
| Cyst | 0.818 (0.403–1.659) | 0.577 |
| Multifocal | 2.184 (0.658–7.242) | 0.248 |
| Satellites | 2.324 (1.145–4.719) | 0.021 |
| Midline displacement | 2.892 (1.281–6.529) | 0.020 |
| WHO grades | 2.660 (1.774–3.990) | <0.001 |
| Ki67 index | 3.894 (1.897–7.991) | <0.001 |
| IDH mutation | 0.166 (0.076–0.365) | <0.001 |
| MGMT methylation | 1.299 (0.880–1.917) | 0.180 |
| 1p/19q co-deletion | 0.355 (0.212–0.595) | <0.001 |
| Radscore | 4.729 (2.703–8.275) | <0.001 |
- Note: p < 0.05. Bold values indicate that these characteristics are statistically significant in univariate analysis.
- Abbreviations: CI, confidence interval; HR, hazard ratio; Radscore, Radiomics score.
2.3 Performance of prediction models
Factors with statistical significance in univariate analysis were included in multivariate Cox regression analysis (Table 3), and four other models were established to determine the model with best prediction performance, including imaging-radiomic model, imaging-clinical model, molecular model, and the combined model.
| Models | Multivariate analysis | |
|---|---|---|
| HR (95% CI) | p-value | |
| Imaging-clinical model | ||
| Age | 1.024 (0.997–1.051) | 0.0841 |
| Peritumoral edema | 2.504 (0.730–0.860) | 0.1445 |
| Proportion enhancing | 1.643 (0.994–2.717) | 0.0529 |
| Proportion necrosis | 1.496 (1.094–2.045) | 0.0116 |
| Imaging-radiomic model | ||
| Peritumoral edema | 4.02 (1.14–14.178) | 0.03049 |
| Proportion enhancing | 1.66 (1.006–2.740) | 0.04726 |
| Proportion necrosis | 1.522 (1.118–2.073) | 0.00765 |
| Radscore | 5.221 (2.626–10.38) | <0.001 |
| Molecular model | ||
| Ki67 index | 2.4688 (1.159–5.261) | 0.0192 |
| IDH mutation | 0.3495 (0.144–0.847) | 0.0198 |
| 1p/19q co-deletion | 0.5077 (0.297–0.868) | 0.0133 |
| Combined model | ||
| Age | 1.03 (1.000–1.061) | 0.05 |
| Peritumoral edema | 8.500 (1.986–36.37) | 0.004 |
| Proportion necrosis | 1.792 (1.156–2.780) | 0.009 |
| Satellites | 5.362 (2.005–14.338) | <0.001 |
| Midline displacement | 2.302 (0.867–6.113) | 0.094 |
| WHO grades | 0.306 (0.137–0.683) | 0.004 |
| IDH mutation | 0.142 (0.037–0.539) | 0.004 |
| 1p/19q co-deletion | 0.258 (0.106–0.629) | 0.003 |
| Radscore | 6.772 (3.228–14.208) | <0.001 |
- Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
The results of the prediction performance of these models are listed in Table 4. Molecular model and imaging-radiomic model have good prediction performance. The C index of molecular model in the training and validation sets are 0.852 (95% CI: 0.786–0.918) and 0.844 (95% CI: 0.714–0.974), respectively. The factors correlated with PFS in this model include Ki67 index, IDH mutation and 1p/19q co-deletion. The C index of imaging-radiomic model in the training and validation sets are 0.851 (95% CI: 0.796–0.906) and 0.801 (95% CI: 0.702–0.900), respectively. The factors correlated with PFS in this model include peritumoral edema, proportion necrosis, proportion enhancing, and Radscore. The prediction performance of imaging-clinical model in the training and validation sets is relatively low. The C index of imaging-clinical model was 0.796 (95% CI: 0.817–0.874) and 0.754 (95% CI: 0.609–0.898), respectively. The factors correlated with PFS in this model include age, peritumoral edema, proportion necrosis, and proportion enhancing. In all the models, the combined model with multiparameter has the best prediction performance in the training set (C index, 0.923; 95% CI: 0.892–0.954) and the validation (C index, 0.861; 95% CI: 0.760–0.963) set. The predictive indicators in the combined model include age, peritumoral edema, proportion necrosis, Satellites, Midline Displacement, WHO grades, IDH mutation, 1p/19q co-deletion, and Radscore.
| Models | Concordance index (C index) (95% CI) | |
|---|---|---|
| Training set (n = 97) | Validation set (n = 43) | |
| Radiomic model | 0.786 (0.716–0.857) | 0.741 (0.622–0.860) |
| Imaging-clinical model | 0.796 (0.817–0.874) | 0.754 (0.609–0.898) |
| Imaging-radiomic model | 0.851 (0.796–0.906) | 0.801 (0.702–0.900) |
| Molecular model | 0.852 (0.786–0.918) | 0.844 (0.714–0.974) |
| Combined model | 0.923 (0.892–0.954) | 0.861 (0.760–0.963) |
- Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
2.4 Validation of the combined model

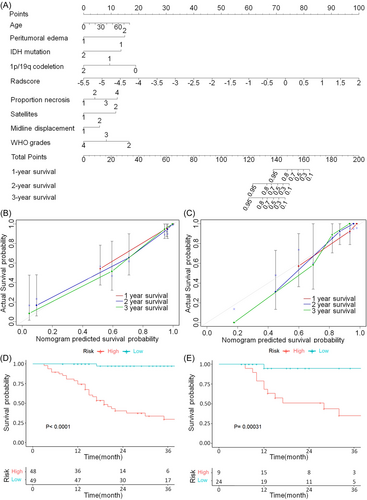
According to the cut-off value, the glioma patients in the training and validation sets were classified into a high-risk group and a low-risk group, respectively. In the training set, there are 48 patients in the high-risk group and 49 patients in the low-risk group (Figure 2D), and that in the validation set are 19 and 24, respectively (Figure 2E). The median PFS of high-risk and low-risk group is 17 (IQR: 11.75–28 months) and 26 months (IQR: 18–40 months) in the training set, and that is 15 (IQR: 12–30 months) and 22 months (IQR: 12–32.75 months) in the validation set. The 1-, 2-, and 3-year PFS probability in the high-risk and low-risk group are 75.0%, 29.2%, 12.5% and 95.9%, 61.2%, 34.7% for the training set, respectively (p < 0.0001). In the validation set, the 1-, 2-, and 3-year PFS probability in the high-risk and low-risk group are 78.9%, 42.1%, 15.8%, and 79.2%, 45.8%, 20.8%, respectively (p = 0.00031). The nomogram of combined model is presented in Figure 2A, and the calibration curves of nomogram for the 1-, 2-, and 3-year PFS probability demonstrate a high consistence between predictions and observations (Figure 2B,C). summarily, the combined model shows good results in model validation.
3 DISCUSSION
In this study, we constructed five prediction models based on Radscore, conventional image features, and clinical molecular characteristics to predict the risk of glioma recurrence. The results show that the combined model with multiparameter has the best performance in predicting the risk of glioma recurrence, and glioma patients are successfully classified into the high-risk group and the low-risk group. The results not only validate that radiomic features can be used as potential biomarkers for predicting the prognosis of gliomas, but also demonstrate that the prediction performance of the model can be improved by combining radiomic features with conventional image features and clinical molecular characteristics. The multiparameter radiomic model may help clinicians to evaluate the risk of glioma recurrence and implement individualized treatments for patients.
Our study reveals that several image features in the nomogram are independent factors for predicting PFS of glioma, including peritumoral edema, proportion necrosis, midline displacement, and the satellites. Previous studies have demonstrated that the degree of peritumoral edema is correlated with the PFS of glioma patients. The severe peritumoral predict a worse prognosis, which may be explained by the increased tumor cell infiltration in the edema area of glioma patients with severe edema.9, 11, 26, 27 In addition, the proportion of tumor necrosis could also reflect the prognosis of glioma. For the tumor in a state of high proliferative activity, the imbalance between the supply of nutrition and the demand of the tumor metabolism could lead to the necrosis of tissues. Higher degree of necrosis associated with more active of the tumor cells and more aggressive of the patient's prognosis.28-30 In this study, in addition to the combined model, the degrees of peritumoral edema and proportion necrosis are also demonstrated to be effective predictors in imaging-clinical model and imaging-radiomic model, which is consistent with previous studies. To some extent, the midline displacement also reflects the tumor size or the degree of peritumoral edema. Therefore, it is reasonable to use the midline displacement as an independent prognostic factor, but the sample size needs to be expanded to verify its robustness of being an independent prognostic factor. A satellite lesion is defined as a lesion in areas of the signal abnormality but not contiguous to any part of the major tumor mass. Some studies have shown that glioma patients with multifocal and satellite lesions have a poor prognosis.11 These two image features may manifest an invasive growth of glioma. Our results show that satellite lesion is one of the independent factors of PFS in the multivariate analysis of the combined model, while multifocal is not correlated with PFS, which may be due to that the number of patients with multifocal in our sample is too small.
The glioma grading system proposed by WHO can reflect the different morphology and structure, growth potential, and invasiveness of glioma. Low-grade gliomas usually have a good prognosis with a high differentiation, a slow growth, and a low invasiveness, while the prognosis of high-grade gliomas is poor.31 And high-grade gliomas mainly occur in the middle-aged and elderly.32 Previous studies have confirmed that age plays an important role in the prognosis of glioma.33, 34 In addition to WHO grade and age, our study also shows that IDH mutation and 1p/19q co-deletion are independent prognostic factors. Many previous studies have confirmed that IDH mutation indicates prolonged survival. The mutation occurs in about 70% of grade II-III gliomas and 12% of GBM, and is rarely seen in the elderly.35 1p/19q co-deletion is a kind of chromosome variation, which is the whole arm deletion of 1p and 19q caused by unbalanced translocation between chromosomes 1 and 19. It is most common in oligodendroglial tumors.36 Almost all tumors with 1p/19q co-deletion also have IDH mutation,37 so the detection of 1p/19q co-deletion is important in diagnosing oligodendroglial tumors and evaluating the prognosis of patients. Another molecular indicator, MGMT methylation, is also a favorable prognostic factor for glioma,38, 39 but, in our study, it is not an independent factor. In our data set, the information about MGMT methylation status in 1/3 patients is missing, which may explain that our results are inconsistent with previous studies.
Radiomic features can reflect the heterogeneity of tumor and extract molecular information that is invisible to the naked eye. The techniques of artificial intelligence and big data for fully mining the potential information of images can address the limitations of traditional evaluation methods. In this study, we used multimodel fusion technology to extract features from three kinds of ROI and constructed several models, in which the complementarity of a variety of heterogeneous information could improve the performance of model.40 The performance of the combined model (training set: C index 0.923, validation set: C index 0.861) which built on Radscore, image features, and clinical molecular characteristics is better than any other uniparameter or biparameter models. The superiority of our study is that the constructed multiparameter radiomic model combines a variety of conventional image and molecular features. These features can be used as potential markers for the prognosis of glioma. Because of the heterogeneity of glioma, a comprehensive analysis of various information could improve the prognosis of the tumor. Second, five prediction models were established by taking different combinations of Radscore, conventional image features, and clinical molecular characteristics. The results show that the multiparameter combined model can improve the accuracy of prediction. It is emphasized that integrating radiomic into the multilevel decision-making framework with key image and clinical molecular features can improve the risk stratification of glioma recurrence and may improve personalized treatment.
The limitations of this study are analyzed as below. First, this is a retrospective study from a single institution with inevitable potential deviations, such as the varying MRI acquisition parameters. However, as mentioned above, we preprocessed the images to minimize the influences of these differences on the results. Second, due to the incomplete clinical data of most patients, some critical prognostic factors of clinical characteristics are not considered in this study, such as the KPS score, the extent of resection and the treatment options. Third, our sample size is relatively small, and the number of glioma patients with different WHO grades is unevenly distributed, which may affect the stability and repeatability of the multiparameter radiomic model that we constructed. Hence, the results in this study still need to be externally verified by multicenter institutions in the future.
In conclusion, our research demonstrates that the multiparameter radiomic model based on Radscore, conventional image features, and clinical molecular characteristics can improve the prognostic prediction in glioma, which could be helpful for individualized treatments.
4 MATERIALS AND METHODS
4.1 Patients and evaluation criteria
This retrospective study was ethically approved by the West China Hospital Institutional Review Board (Ethic number: 2019.200), and the requirement for informed consent was waived.
In all, 140 glioma patients who had received MRI scans from January 2015 to December 2020 in the West China Hospital, Sichuan University (Chengdu, China), were enrolled in this study. All patients meet the following criteria: (a) according to the 2016 WHO classification of central nervous system (CNS) tumors,41 patients with diffuse astrocytic and oligodendroglial tumors (including grades II–IV) were histopathologically diagnosed; (b) MRI images with the same imaging scheme were obtained before surgery, including pre- and postcontrast T1-weighted (T1WI), T2WI and T2 fluid-attenuated inversion recovery (FLAIR) imaging. (c) patients who had received preoperative treatment such as radiotherapy and chemotherapy, lack of clinical and pathological data, image quality problems, and lost to follow-up were excluded.
The study endpoint was PFS, which was determined by calculating the date of operation to the date of tumor progression. The disease progression was assessed according to the histopathological diagnosis after tumor recurrence or the response assessment in neuro-oncology (RANO).42 Patients with any of the following conditions were considered to be progressive disease (PD): (a) the size of enhancing lesions increased more than 25% at stable or increasing doses of steroids; (b) the nonenhancing lesions in T2 FLAIR are increased significantly; (c) the appearance of new lesions; and (d) persistent deterioration of clinical symptoms, not attributed to a decrease in steroids. If there is no evidence of disease progression during the last follow-up, the interval between the operation's date and the last follow-up is calculated.
4.2 Image acquisition and evaluation
All images were acquired by 3T MRI or 1.5T MRI scanner in West China Hospital. All image features of the study set were independently evaluated by two neuroradiologists (reader1: Yan Li, with 4 years of experience, reader2: Zhenglong Deng, with 10 years of experience). They knew nothing about the clinical and pathological data of all patients except imaging information. All differences were agreed upon after discussion.
4.3 Collection of image features and clinical molecular characteristics
The image features include the tumor location, side of tumor epicenter, tumor crosses the midline, peritumoral edema, edema acrosses midline, the proportion enhancing, noncontrast-enhancing tumor (nCET), the proportion necrosis, cyst, multifocal, satellites, and midline displacement. The evaluation of the above image features refer to these standards.9, 11, 43, 44 Clinical molecular characteristics include sex, age, WHO grades, Ki67 index, IDH mutation status, 1p/19q co-deletion, and MGMT methylation status.
4.4 Tumor segmentation and radiomic feature extraction
We delineated three kinds of ROI using ITK-SNAP (version 3.8.0; www.itksnap.org): (1) the tumor region in postcontrast T1WI; (2) the tumor region in T2 FLAIR imaging; (3) the peritumoral edema region in T2 FLAIR imaging (Figure 3A). To evaluate the interobserver agreement, all ROIs were manually segmented by two neuroradiologists (Yan Li and Zhenglong Deng). The obtained ROIs were used for radiomic features extraction. And image standardization was used to eliminate the potential differences of MRI images obtained by different parameters and reduce the impact of these differences on the prediction model.45
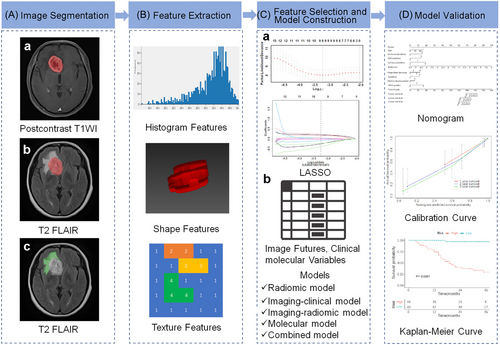
Radiomic features extracted by using Pyradiomics, the wavelet transform, and Laplacian of Gaussian (LoG) filtering were implemented for image fusion. Three thousand sixty-nine radiomic features were extracted from the three kinds of ROI by using multimodal fusion technique for each patient. The types of radiomic features include shape-based (2D), shape-based (3D), first-order statistics, gray-level co-occurrence matrix (GLCM), gray-level dependence matrix (GLDM), gray-level run-length matrix (GLRLM), gray-level size zone matrix (GLSZM), and neighboring gray-tone difference matrix (NGTDM) (Figure 3B). The radiomic features of first-order statistics extracted from two patients with high- and low-grade glioma is shown in Supporting Information: Figure S2.
4.5 Radiomic feature selection
Before feature selection, the data were preprocessed, the median interpolation method was used to fill the missing values, and the dimensional influence was eliminated by using standardization method. Five hundred thirty-nine features were retained after spearman correlation analysis (threshold > 0.9). Then, the features with p < 0.05 were retained by using univariate COX analysis. The best subset of features was selected by the least absolute shrinkage and selection operator (LASSO) for the Cox regression model. To avoid overfitting of the model, the 10-fold cross validation was carried out, then the LASSO L1 regularization technique was used to shrink the feature coefficient estimates toward zero to determine the optimal parameter λ (Figure 3C). At last, nine features with nonzero coefficient were retained finally.
4.6 Development of PFS predictive models
The radiomic features selected from postcontrast T1WI and T2 FLAIR imaging were used to build radiomic model for PFS prediction in glioma patients, and the Radscore was calculated in a linear combination of selected features and their respective nonzero coefficients. Then, a variety of PFS prediction models were built with conventional image features and clinical molecular characteristics.
Five models were constructed by using multivariate Cox regression, including a model based on radiomic features (radiomic model), a model based on clinical and image features (imaging-clinical model), a model based on image and radiomic features (imaging-radiomic model), a model based on molecular features (molecular model) and a multiparameter radiomic model based on Radscore, image features and clinical molecular characteristics (combined model) (Figure 3C).
Glioma patients were classified into a high-risk group and a low-risk group according to the cut-off value of the combined model in the training and validation sets. Kaplan-Meier (KM) survival curves were drawn, and a nomogram was constructed to predict the 1-, 2-, and 3-year PFS probability. Calibration curves were plotted to evaluate the prediction performance of the model (Figure 3D).
4.7 Statistical analysis
In this study, R (version 3.5.1) and Python (version 3.5.6) were used to perform all statistical analyses. The difference was considered to be statistically significant with a two-tailed p < 0.05. Mann–Whitney U test and t-test were used for the continuous variable with abnormal and normal distributions, respectively. Chi-square test or Fisher's exact test was used for the nominal variable. Interobserver reliability was evaluated by intraclass correlation coefficients, and values greater than 0.85 were considered for subsequent investigation. C index was used to quantify the performance of each prediction model.
AUTHOR CONTRIBUTIONS
Yan Li: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing—original draft (lead). Li Bao: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing—original draft (supporting). Caiwei Yang: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); writing—original draft (supporting). Zhenglong Deng: Data curation (equal); investigation (equal); methodology (equal). Xin Zhang: Investigation (equal); methodology (equal); software (equal). Pin Xu: Investigation (equal); methodology (equal). Xiaorui Su: Writing—review and editing (equal). Fanxin Zeng: Formal analysis (equal); investigation (equal); supervision (equal); writing—review and editing (equal). Mir Q. U. Mehrabi: Investigation (equal); methodology (equal); software (equal). Qiang Yue: Funding acquisition (equal); supervision (equal); writing—review and editing (equal). Bin Song: Formal analysis (equal); supervision (equal); writing—review and editing (equal). Qiyong Gong: Formal analysis (equal); validation (equal); writing—review and editing (equal). Su Lui: Formal analysis (equal); supervision (equal); writing—review and editing (equal). Min Wu: Formal analysis (equal); funding acquisition (lead); resources (equal); validation (equal); writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the Sichuan Foundation for Distinguished Young Scholars (2021YFS0242); the Chengdu International Science and Technology Cooperation Funding (Grant 2019-GH02-00074-HZ); the Chengdu Science and Technology Bureau (Grant 2021-YF05-00698-SN); the 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant 2021HXFH035); the Scientific and Technological Achievements Transformation Fund of West China Hospital, Sichuan University (Grant CGZH21002); the fund for Fostering Talents in Interdisciplinary Convergence of Medical Engineering of West China Hospital, Sichuan University and University of Electronic Science and Technology of China (Grant ZYGX2022YGRH019).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This retrospective study was ethically approved by the institutional review board of West China Hospital (Ethic number: 2019.200), Sichuan University, China, and the requirement for informed consent was waived.
Open Research
DATA AVAILABILITY STATEMENT
The data in this study are available and can be obtained from the corresponding author with a reasonable request.




