A prospective, randomized, multicenter, open-label trial comparing survival in subjects receiving peritoneal dialysis or conventional in-center hemodialysis
Abstract
Background
Peritoneal dialysis (PD) and conventional in-center hemodialysis (HD) are treatment options for patients with end-stage kidney disease (ESKD). However, their impact on all-cause mortality is unclear.
Methods
We conducted a multicenter, open-label, randomized, non-inferiority trial to determine the effect of dialysis modality on mortality in patients with ESKD. Eligible patients were recruited from 30 sites across China and assigned to receive either PD or HD in a ratio of 1:1. The primary outcome was all-cause mortality. Non-inferiority was defined as the upper bound of the one-sided 95% confidence interval (CI) for the hazard ratio (HR) being ≤1.25.
Results
A total of 414 patients with incident ESKD were randomly assigned to PD (n = 213) or HD (n = 201). During a median follow-up of 1.7 years, 37 patients in the PD group and 31 in the HD group died, giving respective event rates per patient-year of 0.061 and 0.071. The HR for mortality on PD in comparison with HD was 0.76 (95% CI 0.47–1.24) after adjustment for age, sex, and diabetes status, achieving the limit for non-inferiority. There were more adverse events (p = 0.003), serious adverse events (p = 0.009), and adverse events leading to hospitalization (p = 0.003) in the PD group than in the HD group; however, there was no significant between-group difference in adverse events leading to death or discontinuation of treatment.
Conclusions
PD was non-inferior to conventional in-center HD in terms of survival in patients with ESKD. Our findings underscore the need for shared decision-making between physicians and patients regarding the selection of dialysis modality.
Trial registration
Registered at ClinicalTrials.gov (NCT01413074).
Abbreviations
-
- CI
-
- confidence interval
-
- DSMC
-
- Data and Safety Monitoring Committee
-
- ESKD
-
- end-stage kidney disease
-
- HD
-
- hemodialysis
-
- HR
-
- hazard ratio
-
- ITT
-
- intention-to-treat
-
- PD
-
- peritoneal dialysis
-
- PP
-
- per-protocol
-
- RCT
-
- randomized controlled trial
-
- RKF
-
- residual kidney function
1 INTRODUCTION
Chronic kidney disease is an increasing global public health problem and is associated with high morbidity, mortality, and treatment costs [1, 2]. Mortality increases rapidly when chronic kidney disease progresses to end-stage kidney disease (ESKD), with an unadjusted 5-year survival of 41%–60% reported for patients with onset of ESKD in 2004–2008 [3]. Kidney replacement therapy (KRT) is a life-saving treatment for patients with ESKD. In 2010, it was estimated that at least 4.9 million patients with ESKD needed KRT but only 2.6 million received it, leaving 2.3 million patients at risk of premature death because of lack of access to this treatment [4].
Hemodialysis (HD) is the most common modality of KRT globally and is used by approximately 89% of patients on dialysis [5, 6]. Peritoneal dialysis (PD) is a home-based KRT modality and is used by 11% of patients on dialysis [7, 8], with prevalence varying from 11% in the US to 69% in Hong Kong, China. PD is simpler, more convenient, and more flexible, places fewer demands on dialysis facilities and staff, and in most countries is less expensive than HD [9-11]. Therefore, PD may be a good treatment option for ESKD by providing more patients with access to life-saving dialysis, especially in rural and remote areas and during epidemics of infectious diseases. However, the usefulness of PD is limited in some countries because of poor outcomes, including a high incidence of peritonitis and poor patient survival, although the peritonitis rate has decreased and patient survival has improved with advances in dialysis technology [12].
To date, the impact of dialysis modality on patient survival has been controversial. Survival of patients on PD and those on HD is best compared using a randomized controlled trial (RCT) design, but only two RCTs have been reported. One trial that included 114 patients with one year of follow-up was reported about 38 years ago, and its results may no longer be applicable in contemporary dialysis practice [13]. The other trial was terminated prematurely as a result of poor recruitment and patient adherence, with only 38 patients randomized over 3 years [14]. Most of the other studies comparing patient survival between PD and HD have been observational with inconsistent findings [15-17].
Therefore, we conducted this large-scale trial to compare the impact of PD on survival with that of conventional in-center HD in patients with ESKD.
2 METHODS
2.1 Study design
This prospective, randomized, multicenter, open-label, non-inferiority trial was conducted at 30 sites across China. The study protocol was approved by all participating sites and conducted in compliance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. All patients provided written informed consent before undergoing any trial procedures. The trial was overseen by an external data and safety monitoring board and a steering committee.
2.2 Patients
Patients were eligible for participation in the study if they met the following inclusion criteria: age 18 years or older; newly diagnosed with kidney failure (defined as an estimated glomerular filtration rate (eGFR) ≤15 mL/min/1.73 m2); anticipated to initiate maintenance dialysis within 10 weeks after enrollment; expected to remain on dialysis for at least 48 weeks; able to complete the standardized pre-dialysis education program and home-based PD training program; able to attend HD clinics as required by the protocol; able to adhere to the study visits and other protocol requirements; normal liver function; a negative pregnancy test result; and able to understand and voluntarily sign the informed consent form. Key exclusion criteria were as follows: positive for human immunodeficiency virus; not eligible for either PD or HD in the opinion of the investigator; receiving maintenance dialysis for more than 4 weeks; diagnosed with active infection or another condition determined by the investigators to have jeopardized the ability to receive either dialysis modality; previous kidney transplantation and ongoing immunosuppressive therapy; and an anticipated life expectancy of <48 weeks.
2.3 Randomization and masking
Randomization was achieved using a central randomization method via an interactive voice response system. Eligible patients were randomly assigned to PD or conventional in-center HD in a ratio of 1:1. The randomization code was generated by a trial statistician who was blinded to trial implementation. Enrolled patients were informed of their treatment allocation by the investigators at each site. Neither the patients nor the investigators could be masked to group allocation because of the nature of the treatment.
2.4 Procedures
After randomization, the patients underwent 8 weeks of preparation for starting dialysis, including implantation of a PD catheter for those allocated to PD and placement of an autogenous arteriovenous fistula, arteriovenous graft, or other permanent vascular access for those allocated to HD. Patients were followed up every 4 weeks at the first 3 visits and every 12 weeks thereafter.
In the PD group, 97.8% of patients received continuous ambulatory PD (3–5 manual PD exchanges [1.0–2.5 L of dialysate at each exchange]) and 2.2% received automated PD (3–5 automated PD cycles [1.0–2.5 L at each cycle]) per night with a dwell volume of 2 L per day at home. Patients in the HD group received 3–4 sessions of dialysis (4–4.5 h per session) of conventional in-center HD per week. Dialysis prescriptions were adjusted to maintain a total Kt/Vurea of ≥1.7 per week in the PD group and a single-pool Kt/Vurea ≥1.2 per dialysis session in the HD group.
2.5 Outcomes
The primary study outcome was patient survival. The safety outcomes included adverse events, serious adverse events, and abnormal laboratory test findings with clinical significance.
2.6 Statistical analysis
The original design required a total of 1370 patients (685 patients in each group), which would provide 80% power under a one-side type I error of 2.5% to test the hypothesis that patient survival on PD was non-inferior to that on HD. The total trial duration was 5 years, and a total of 554 death events would be required based on the assumption of a mortality rate of 0.15 deaths per patient-year. Non-inferiority would be claimed if the upper bound of the one-sided 95% confidence interval (CI) for the hazard ratio (HR) was ≤1.25. A planned interim analysis by the Data and Safety Monitoring Committee (DSMC) found that the actual mortality rate was only 0.03 per patient-year, which was much lower than the rate of 0.15 per patient-year used for the calculation of the sample size. Since the DSMC was concerned that the design of the SURIND trial may be futile because of a much lower event rate, the steering committee decided to stop patient recruitment until December 2013.
The continuous variables were presented as the mean ± standard deviation or median (interquartile range) as appropriate, and the categorical variables were presented as number (percentage). The primary efficacy analysis was performed following the intention-to-treat (ITT) principle. Kaplan-Meier curves and the log-rank test were used to compare survival between patients on PD and those on conventional in-center HD. The HR and 95% CI for all-cause mortality were calculated and then compared between the PD and HD groups in a Cox proportional hazards model with adjustment for age, sex, and diabetes status. The adjusted HRs were presented with their 95% CIs. The sensitivity analyses were performed in the per-protocol (PP) population, which included only patients who received randomly allocated treatment (Supplemental Figure S1). All statistical analyses were performed using R for Windows (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 indicated statistical significance.
3 RESULTS
3.1 Study participants
A total of 2946 patients with newly diagnosed ESKD from 30 sites in China were screened between June 1, 2011 and December 27, 2013; 2532 patients were excluded, of whom 2327 patients refused to participate in the trial because they already had a preferred dialysis modality. A total of 414 patients were finally enrolled in the study and randomly assigned to receive PD (n = 213) or conventional in-center HD (n = 201). One patient in the PD group and two in the HD group withdrew consent after randomization and were not included in the ITT population (Figure 1).
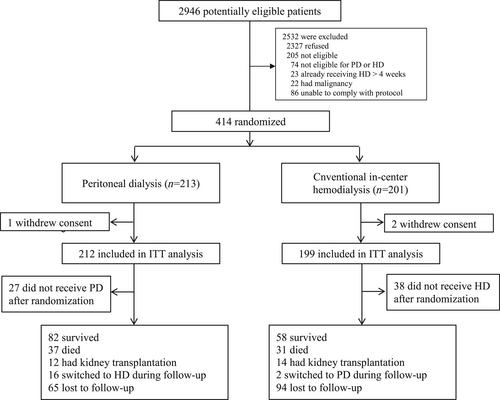
Trial profile. ESKD, end-stage kidney disease; HD, hemodialysis; ITT, intention-to-treat; PD, peritoneal dialysis.
In the ITT population, the mean age was (51.8 ± 15.4) years, 178 patients (43.3%) were female, 53 (12.9%) had diabetes, and 41 (10.0%) had a history of cardiovascular disease. The mean hemoglobin level was (87.2 ± 18.2) g/L and the eGFR was (5.9 ± 3.1) mL/min/1.73 m2 at the time of screening. The baseline patient characteristics were balanced between the PD and HD groups (Table 1).
| All (n = 411) | Peritoneal dialysis (n = 212) | Hemodialysis (n = 199) | |
|---|---|---|---|
| Age (yr) | 51.8 ± 15.4 | 52.8 ± 14.5 | 50.6 ± 16.2 |
| Female, n (%) | 178 (43.3) | 93 (43.9) | 85 (42.7) |
| Height (cm) | 164.4 ± 7.9 | 164.2 ± 7.9 | 164.6 ± 7.8 |
| Weight (kg) | 62.4 ± 11.1 | 61.3 ± 10.6 | 63.6 ± 11.5 |
| Body mass index (kg/m2) | 23.0 ± 3.5 | 22.7 ± 3.4 | 23.4 ± 3.7 |
| Systolic blood pressure (mmHg) | 150.1 ± 20.0 | 149.6 ± 20.1 | 150.6 ± 20.0 |
| Diastolic blood pressure (mmHg) | 86.7 ± 13.8 | 85.7 ± 12.5 | 87.8 ± 15.0 |
| Diabetes, n (%) | 53 (12.9) | 26 (12.3) | 27 (13.6) |
| History of CVD, n (%) | 41 (10.0) | 19 (9.0) | 22 (11.1) |
| Dialysis access for HD, n (%) | |||
| AVF | 144 (35.0) | NA | 144 (72.4) |
| AVG | 4 (1.0) | NA | 4 (2.0) |
| Permanent venous catheter | 13 (3.2) | NA | 13 (6.5) |
| Not availablea | 250 (60.8) | 212 (100.0) | 38 (19.1) |
| Hemoglobin (g/L) | 87.2 ± 18.2 | 85.4 ± 18.2 | 89.1 ± 18.0 |
| Serum albumin (g/L) | 34.5 ± 6.0 | 33.2 ± 6.1 | 35.9 ± 5.6 |
| Phosphate (mmol/L) | 1.6 (1.4, 2.0) | 1.6 (1.4, 2.0) | 1.6 (1.3, 1.9) |
| hsCRP (mg/L) | 3.4 (1.0, 8.1) | 4.4 (1.4, 8.8) | 2.7 (0.8, 6.7) |
| 24 h urine volume (mL) | 1000 (600, 1400) | 1000 (800, 1500) | 900.0 (550, 1400) |
| eGFR (mL/min/1.73 m2) | 5.9 ± 3.1 | 6.0 ± 3.1 | 5.8 ± 3.2 |
- Note: Data were presented as the mean ± standard deviation, median (interquartile range), or number (percentage) as appropriate.
- Abbreviations: AVF, autogenous arteriovenous fistula; AVG, arteriovenous graft; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; NA, not applicable.
- a Data were not available because patients received PD.
3.2 Patient survival
During a median follow-up of 1.7 years (interquartile range: 0.9–5.0), 37 patients (17.5%) in the PD group and 31 (15.6%) in the HD group died. For all-cause mortality, the event rate per patient-year was 0.061 in the PD group and 0.071 in the HD group. The Kaplan-Meier survival curves showed no difference in overall survival between the HD and PD groups (p = 0.5, log-rank test; Figure 2a). After adjustment for age, sex and diabetes status, the HR of mortality between PD and HD was 0.76 (95% CI 0.47–1.24) in the ITT analysis, achieving the limit for non-inferiority (Table 2, Supplemental Table S2). However, in the PP analysis, the adjusted HR for mortality between PD and HD was 0.83 (95% CI 0.48–1.41), failing to show non-inferiority of PD compared to HD (Table 2, Figure 2b, Supplemental Table S1).
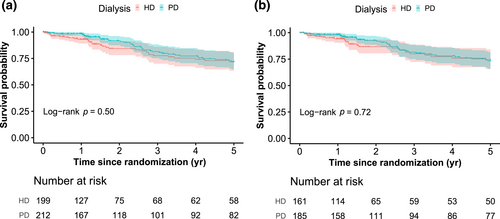
Kaplan-Meier curves for patient survival according to randomized dialysis modality. (a) Curve for the intention-to-treat population. (b) Curve for the per-protocol population. p-values were calculated using the log-rank test. HD, hemodialysis; PD, peritoneal dialysis.
| Peritoneal dialysis | Hemodialysis | HR (95% CI)a | p-value | |||
|---|---|---|---|---|---|---|
| Events/number of patients (%) | Events per patient-year | Events/number of patients (%) | Events per patient-year | |||
| ITT population | ||||||
| All-cause mortality | 37/212 (17.5) | 0.061 | 31/199 (15.6) | 0.071 | 0.76 (0.47, 1.24) | 0.3 |
| PP population | ||||||
| All-cause mortality | 33/185 (17.8) | 0.058 | 24/161 (14.9) | 0.063 | 0.83 (0.48, 1.41) | 0.5 |
- Note: Non-inferiority was defined as the upper bound of the 95% CI for an HR of ≤1.25.
- Abbreviations: CI, confidence interval; HR, hazard ratio; ITT, intention-to-treat; PP, per-protocol.
- a Adjusted for age, sex, and diabetes status.
3.3 Safety
Adverse events were reported in 108 (50.9%) of the 212 patients in the PD group and in 71 (35.7%) of the 199 patients in the HD group (p = 0.003). Serious adverse events were more common in the PD group than in the HD group (76 (35.8%) vs. 47 (23.6%), p = 0.009), as were adverse events leading to hospitalization (64 (30.2%) vs. 34 (17.1%), p = 0.003). However, there was no significant between-group difference in the frequency of adverse events leading to death or discontinuation of participation in the trial. There were significantly more infection-related adverse events in the PD group (p = 0.001) but no significant between-group differences in the frequency of cardiac, cerebral, or gastrointestinal diseases, disorders of potassium metabolism, or secondary hyperparathyroidism (Table 3).
| PD (n = 212) | HD (n = 199) | p-value | |
|---|---|---|---|
| Patients, n (%) | |||
| All events | |||
| Any adverse event | 108 (50.9) | 71 (35.7) | 0.003 |
| Any serious adverse event | 76 (35.8) | 47 (23.6) | 0.009 |
| Any adverse event leading to hospitalization | 64 (30.2) | 34 (17.1) | 0.003 |
| Any adverse event leading to discontinuation | 3 (1.4) | 0 (0.0) | 0.3 |
| Any adverse event leading to death | 4 (1.9) | 12 (6.0) | 0.06 |
| Cerebral hemorrhage | 0 | 4 | |
| Cerebral infarction | 0 | 1 | |
| Heart failure | 1 | 2 | |
| Sudden death | 0 | 1 | |
| Respiratory failure | 0 | 1 | |
| Peritonitis | 1 | 0 | |
| Pulmonary infection | 0 | 1 | |
| Sepsis | 0 | 1 | |
| GI bleeding | 1 | 0 | |
| Unknown | 1 | 1 | |
| Cardiac and cerebral disease | 0.5 | ||
| Heart failure | 12 (5.7) | 4 (2.0) | |
| Coronary artery disease | 2 (0.9) | 2 (1.0) | |
| Cardiac arrhythmias | 3 (1.4) | 2 (1.0) | |
| Cerebral hemorrhage | 2 (0.9) | 5 (2.5) | |
| Cerebral infarction | 2 (0.9) | 3 (1.5) | |
| Gastrointestinal disorders | 0.09 | ||
| Constipation | 2 (0.9) | 2 (1.0) | |
| Gastroenteritis | 3 (1.4) | 1 (0.5) | |
| Diarrhea | 2 (0.9) | 0 (0.0) | |
| Nausea | 2 (0.9) | 2 (1.0) | |
| Vomiting | 3 (1.4) | 2 (1.0) | |
| Abdominal pain | 1 (0.5) | 3 (1.5) | |
| Hernia | 4 (1.9) | 0 (0.0) | |
| Gastrointestinal bleeding | 6 (2.8) | 2 (1.0) | |
| Infections | 0.001 | ||
| Respiratory tract infection | 25 (11.8) | 16 (8.0) | |
| Peritonitis | 26 (12.3) | 0 (0.0) | |
| Urinary tract infection | 4 (1.9) | 1 (0.5) | |
| Catheter related infection | 4 (1.9) | 2 (1.0) | |
| Sepsis | 1 (0.5) | 2 (1.0) | |
| Arteriovenous fistula complication | NA | ||
| Arteriovenous fistula thrombosis | NA | 1 (0.5) | |
| Arteriovenous fistula stenosis | NA | 3 (1.5) | |
| Arteriovenous fistula hematoma | NA | 1 (0.5) | |
| Peritoneal catheter complication | NA | ||
| Peritoneal catheter dysfunction | 2 (0.9) | NA | |
| Hypokalemia | 5 (2.4) | 0 (0.0) | 0.08 |
| Hyperkalemia | 2 (0.9) | 3 (1.5) | 0.9 |
| Secondary hyperparathyroidism | 1 (0.5) | 0 (0.0) | 0.9 |
- Abbreviations: GI, gastrointestinal; NA, not applicable.
4 DISCUSSION
This RCT included a large sample of patients with ESKD who were randomly assigned to PD or conventional in-center HD and followed up for 5 years. ITT analysis showed that patient survival on PD was non-inferior to that on conventional in-center HD. The findings of this large-scale study strongly suggest a need for shared decision-making by physicians and patients regarding the selection of dialysis modality.
The impact of dialysis modality on patient survival has been controversial. Although PD has been associated with high mortality because of infectious complications, patient survival has improved with advances in technology. Therefore, the impact of PD and HD on patient survival should be reassessed. Ideally, the impact of PD and HD on patient survival should be compared in RCTs; however, such trials are difficult to conduct because most patients have a preferred dialysis modality and refuse randomization. Only one RCT was attempted in recent decades and was terminated prematurely as a result of poor recruitment [14]. Several observational studies based on national or regional registries have compared patient survival between PD and HD but have had inconsistent findings. Studies that included more than 690,000 dialysis patients from the US and Canadian registries found that patient survival was similar between PD and HD [15, 18, 19]. A meta-analysis that included 17 cohort studies and 113,578 propensity score-matched incident dialysis patients from Asia, Europe, and North America reported similar findings [20]. Other studies that included 23,718 US patients and 12,095 Danish patients reported that patients treated with PD had better survival than those treated with HD [16, 21]. However, analyses of data from the Korean Society of Nephrology Registry and the Australia and New Zealand Dialysis and Transplant Registry found that mortality was higher on PD than on HD [17, 22]. Potential explanations for these inconsistencies include patient selection bias, differences in study design, methods used for statistical analysis, confounding variables adjusted for, and the impact of switching dialysis modality.
Patient survival on PD was non-inferior to that on HD in our ITT analysis. We also noted that the survival curves for PD and HD overlapped during follow-up, indicating that patient survival may be similar in patients on PD and those on HD within the first 5 years after initiation of dialysis. A potential explanation for this finding is that loss of residual kidney function (RKF) in the early stage of initiation of dialysis is slower in patients on PD than in those on HD. Although RKF contributes to better survival in both PD and HD, patients with RKF have been found to have better volume and blood pressure control, increased protein-bound and middle molecule uremic toxin clearance, less inflammation, and preserved production of erythropoietin and vitamin D [23-25]. Therefore, survival is better in patients with RKF than in those without RKF [25, 26]. Hemodynamic stability is better on PD than on HD, meaning that loss of RKF is slower in patients on PD. Considering that dialysis is a long journey, switching to HD after a few years of PD may be a treatment option for patients with ESKD. We also noted that more adverse events were reported in the PD group than in the HD group but that there was no significant between-group difference in mortality. A possible reason for this finding is that patients in the PD group were more likely to have heart failure related to fluid overload and more likely to have gastrointestinal disorders and peritonitis, which made patients feel uncomfortable and prompted them to present to hospital for treatment. After effective treatment, the patients recovered. Therefore, the mortality rate was lower in the PD group than in the HD group.
It is estimated that 5.439 million patients worldwide will be receiving KRT by 2030 and that most of them will reside in low-income and middle-income countries or regions [4]. Providing these patients with access to KRT will pose a considerable challenge for the economy and health care systems in these areas. Peritoneal dialysis may be a good option because it requires fewer medical resources than HD, costs less in most countries, and will allow more patients with ESKD to access life-saving KRT. Furthermore, PD as a home-based dialysis, may be a good treatment option during pandemics of infectious diseases such as COVID-19. Home-based treatment not only reduces the frequency of travel between hospital and home but also significantly reduces the risk of infection for patients, family members, and health care workers and allows patients to continue to receive dialysis in the event of an emergency lockdown.
The guidelines recommend shared decision-making between health care practitioners and patients when selecting a dialysis modality [27]. However, physicians may have preferences or fixed opinions concerning which dialysis modality patients are suitable for and advise patients accordingly, leading to modality decisions being made by physicians alone. Our findings provide important information regarding patient survival on PD and HD when physicians and patients discuss the selection of dialysis modality. Patients and physicians should fully discuss the pros and cons of PD and HD, including patients' preferences and outcomes, before selecting a dialysis modality.
The strength of this study is that it is the largest RCT ever to compare all-cause mortality between PD and HD. However, some limitations should be kept in mind. First, the trial was terminated early after a planned interim analysis because the mortality rate (0.03 per patient-year) was lower than that assumed at the time of sample size calculation (0.15 per patient-year). Second, we did not find patient survival on PD to be non-inferior to that on HD in the PP analysis, which may reflect the smaller sample size caused by the exclusion of 65 patients who refused random allocation to treatment. Extended follow-up is needed to compare the long-term effect on patient survival of PD with that of HD. Third, our trial was conducted in China, and the study participants were relatively young and less likely to have diabetes or a history of cardiovascular disease. The characteristics of our study population are different from those in the previous studies, and caution is required when generalizing our findings to other populations. Furthermore, automated PD was not common and icodextrin was not available in our trial, which further limits the extrapolation of our results to patients with automated PD and those using icodextrin. However, these scenarios are comparable with those in the real-world setting in low-income and middle-income countries, and our findings could provide scientific evidence for the selection of dialysis modality in these countries. Fourth, given that ambulatory PD and use of icodextrin are associated with better patient survival, our findings regarding survival may be limited by omission of patients on ambulatory PD or icodextrin in our trial. All patients in our HD group received conventional in-center dialysis and no hemodiafiltration was allowed, which may have modified the effect of HD on mortality.
5 CONCLUSION
The findings of this trial show PD to be non-inferior to conventional in-center HD in terms of survival in patients with ESKD. Our findings provide important evidence of a need for shared decision-making between physicians and patients when selecting a dialysis modality.
AUTHOR CONTRIBUTIONS
Xueqing Yu and Jiaqi Qian were responsible for study conception and design. Xueqing Yu and Jiaqi Qian were the principal investigators. Li Fan, Xiao Yang, Huaying Shen, Menghua Chen, Hao Zhang, Zhaohui Ni, Hongli Lin, Hongtao Yang, Qinkai Chen, Hongyu Chen, Gengru Jiang, Jianqin Wang, Jiuyang Zhao, Zhuxing Sun, Aiping Yin, Aili Jiang, Yun Li, Hui Peng, Nan Chen, Chuanming Hao, Yaozhong Kong, and Rong Rong contributed to execution of the trial, patient enrollment and follow-up, and data collection. Xia Zou, Haotian Luo, and Li Fan were responsible for data cleaning and analysis. Li Fan, Xia Zhou, Jie Li, and Xueqing Yu drafted the manuscript. All authors contributed important intellectual content during drafting or revision of the manuscript and accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
ACKNOWLEDGMENTS
The authors thank Han Tao from Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences), Southern Medical University for English editing of the manuscript. The study was funded by Baxter Healthcare Corporation and designed by the sponsor and investigators. The funder had no role in study execution, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the report for publication. Xueqing Yu has received grant/research support from the National Natural Science Foundation of China, Baxter, Wanbang Biopharmaceuticals, AstraZeneca, and GSK as well as consulting and lecture fees from Baxter, Wanbang Biopharmaceuticals, Fresenius, Fresenius Kabi, and Kyowa Kirin.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Xueqing Yu, Jie Li, Chuanming Hao, Hongli Lin and Xia Zhou are Editorial Board Members/Editors-in-Chief for Medicine Advances but were not involved in the editorial review or decision to publish this article.
ETHICS STATEMENT
The study protocol was approved by the Ethics Committee of Clinical Research and Animal Trial of the First Affiliated Hospital of Sun Yat-sen University (No. [2011]153) and all participating sites, and it was compliant with the Helsinki Declaration of 1975, as revised in 2008.
INFORMED CONSENT
The written informed consent form should be applied by all participants.
Open Research
DATA AVAILABILITY STATEMENT
All individual participant data and the data dictionary collected for this study are available. Individual participant data that underpin the results reported in this article (including text, tables, figures, and appendices) will be shared after de-identification. The study protocol is available in the supplementary material. Sharing data will be available beginning 3 months and ending 5 years after publication of the article. Investigators whose proposed use of the data has been approved by an independent review committee will have access to sharing data. Sharing data can be used for analyses to achieve the aims in the approved proposal. Proposals for sharing data should be directed to [email protected], and the requestor needs to sign a data access agreement. After 5 years, sharing data will be available from the Division of Nephrology, Guangdong Provincial People's Hospital, and stored as a .csv file.




