Mechanisms of resistance to tyrosine kinase inhibitor-targeted therapy and overcoming strategies
Abstract
Tyrosine kinase inhibitor (TKI)-targeted therapy has revolutionized cancer treatment by selectively blocking specific signaling pathways crucial for tumor growth, offering improved outcomes with fewer side effects compared with conventional chemotherapy. However, despite their initial effectiveness, resistance to TKIs remains a significant challenge in clinical practice. Understanding the mechanisms underlying TKI resistance is paramount for improving patient outcomes and developing more effective treatment strategies. In this review, we explored various mechanisms contributing to TKI resistance, including on-target mechanisms and off-target mechanisms, as well as changes in the tumor histology and tumor microenvironment (intrinsic mechanisms). Additionally, we summarized current therapeutic approaches aiming at circumventing TKI resistance, including the development of next-generation TKIs and combination therapies. We also discussed emerging strategies such as the use of dual-targeted antibodies and PROteolysis Targeting Chimeras. Furthermore, we explored future directions in TKI-targeted therapy, including the methods for detecting and monitoring drug resistance during treatment, identification of novel targets, exploration of dual-acting kinase inhibitors, application of nanotechnologies in targeted therapy, and so on. Overall, this review provides a comprehensive overview of the challenges and opportunities in TKI-targeted therapy, aiming to advance our understanding of resistance mechanisms and guide the development of more effective therapeutic approaches in cancer treatment.
1 INTRODUCTION
Protein tyrosine kinases (PTKs) are a group of enzymes that catalyze the phosphorylation of specific tyrosine residues within target proteins.1 They play a crucial role in cell signaling pathways, which regulate various cellular processes including cell growth, proliferation, differentiation, and apoptosis.2, 3 There are two main types of PTKs: receptor PTKs and non-receptor PTKs.4 Receptor PTKs are transmembrane proteins activated by ligand bindings, which leads to autophosphorylation and subsequent signaling cascades. Epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), insulin receptor, vascular endothelial growth factor receptor (VEGFR), hepatocyte growth factor receptor (also known as mesenchymal epithelial transition, MET), and anaplastic lymphoma kinase (ALK) are classic receptor PTKs, which have been widely and deeply investigated. Non-receptor PTKs are cytoplasmic or membrane-associated proteins, which include BCR–ABL fusion protein, Src family kinases, Janus kinases (JAKs), focal adhesion kinase, Syk kinase, and so on.
Aberrant activation of PTKs due to gain-of-function mutation, genomic amplification, chromosomal rearrangements, or autocrine activation can lead to dysregulated kinase activities, which ultimately results in uncontrolled cell proliferation and tumorigenesis.5, 6 A study analyzing pathway alterations in over 9000 tumor samples across 33 cancer types reveals that gene alterations in receptor PTKs and components of the RAS pathway (RTK–RAS pathways) are prevalent in cancer.7 The pivotal roles played by PTKs and their widespread abnormal activation in various cancers have made PTKs key targets in cancer therapy. Consequently, tyrosine kinase inhibitors (TKIs) have emerged as prominent anticancer agents in clinical practice, specifically designed to inhibit dysregulated kinases.8, 9 The first TKI, imatinib (a BCR–ABL TKI), was approved by the United States Food and Drug Administration (US FDA) in May 2001 for the treatment of chronic myeloid leukemia (CML). Since then, there has been significant growth in the development of small-molecule targeted drugs in malignancies.8, 10 As of 2021, hundreds of TKIs have been researched, and 76 of them have been approved for clinical use, with most of them for cancer treatment.8 Nowadays, TKIs have revolutionized the therapeutic landscape of malignancies, including CML, gastrointestinal stromal tumors (GIST), melanoma, hepatocellular carcinoma (HCC), and non-small cell lung cancer (NSCLC) (Table 1), ushering in an era of more targeted and effective treatment options.10-14 For example, imatinib has transformed CML from a fatal cancer to a chronic disease, by specifically targeting the BCR–ABL fusion protein that drives the proliferation of leukemic cells.8 The 8-year survival of patients with chronic phase CML has significantly improved from ≤15% before 1983 to 87% after the introduction of imatinib in 2001.15 In addition, EGFR-TKIs, such as gefitinib, erlotinib, and osimertinib have become the first-line treatment for patients with EGFR-mutated NSCLC.12, 16 EGFR-TKIs have demonstrated efficacy in prolonging progression-free survival (PFS) and overall survival (OS) in NSCLC patients with EGFR mutations, sparing patients from low-response and high-toxicity platinum-based chemotherapy.17, 18
| Cancer types | Targets | Alteration prevalence (%) | Treatment | References |
|---|---|---|---|---|
| CML | BCR–ABL gene fusion | 100% of CML patients |
First-generation TKIs: imatinib Second-generation TKIs: dasatinib, nilotinib, bosutinib Third-generation TKIs: ponatinib (targeting T315I mutation) |
26 |
| AML | FLT3 mutations | 30% of all AML cases |
First-generation TKIs: sunitinib, sorafenib, midostaurin, lestaurtinib, ponatinib Second-generation TKIs: quizartinib, gilteritinib, crenolanib |
27 |
| B-cell malignancies | Aberrant BTK activation | – |
First-generation TKIs: ibrutinib Second-generation TKIs: acalabrutinib, zanubrutinib Third-generation TKIs: pirtobrutinib |
28 |
| NSCLC |
EGFR mutations (e.g., ex19del, L858R) |
85–90% of EGFR-mutant NSCLC patients |
First-generation TKIs: gefitinib, erlotinib Second-generation TKIs: afatinib, dacomitinib Third-generation TKIs: osimertinib (targeting T790M mutation) Fourth-generation TKIs: EAI045, JBJ-04-125-02, BLU-945/BLU-701 |
23 |
| ALK fusion |
3–7% of NSCLC patients |
First-generation TKIs: crizotinib Second-generation TKIs: alectinib, ceritinib, brigatinib Third-generation TKIs: Lorlatinib Fourth-generation TKIs: TPX-31, NVL-655 |
23 | |
|
EGFR mutation (ex20ins) |
1.5–2.5% of NSCLC patients | Mobocertinib, amivantamab | 29 | |
| ROS1 fusion | 1–2% | Crizotinib, entrectinib, ceritinib, ensartinib, brigatinib, lorlatinib, repotrectinib, taletrectinib | 30 | |
| RET fusion | 0.7–2% of NSCLC patients | Pralsetinib, selpercatinib | 31, 32 | |
| BRAF V600E | 1.5–3.5% of NSCLC patients | Dabrafenib+trametinib | 33 | |
| KRAS G12C |
20–40% of lung adenocarcinoma patients ∼5% of lung squamous patients |
Sotorasib, adagrasib | 34 | |
| HER2 alterations | 2–3% of NSCLC patients | Trastuzumab deruxtecan | 35 | |
| NTRK 1/2/3 fusion | 0.2% of NSCLC patients |
First-generation TKIs: larotrectinib, entrectinib Second-generation TKIs: selitrectinib, repotrectinib, taletrectinib |
36 | |
| GIST | KIT mutations | ∼75% of GIST patients | Imatinib, wunitinib | 37 |
| PDGFRA | 5–7% of GIST patients | |||
| Melanoma | BRAF mutations |
∼50% of cutaneous melanoma patients ∼20% of acral melanoma ∼6% of mucosal melanoma |
Vemurafenib, dabrafenib, | 38 |
| MTC | RET mutations | 25–40% sporadic MTC | Selpercatinib, pralsetinib | 39, 40 |
| PTC | RET fusion | 2.5–73% of sporadic PTC | Sunitinib, selpercatinib, pralsetinib | 41-44 |
| RAS mutations | 10–20% of PTC | Not available | ||
| NTRK fusions | 2−13% of PTC |
First-generation TKIs: larotrectinib, entrectinib Second-generation TKIs: selitrectinib, repotrectinib, taletrectinib |
||
| BRAF fusion | 1% of sporadic PTC | Vemurafenib, dabrafenib | ||
| CRC | KRAS/NRAS mutations | 35–40% of metastatic CRC patients | Not available | 45 |
| BRAF V600E | 5–10% of metastatic CRC patients | Encorafenib+cetuximab | ||
| BRCA | HER2 overexpression | ∼15% of breast cancer patients | Pertuzmab, trastuzumab, lapatinib, neratinib, pyrotinib, tucatinib, margetuximab | 46 |
| HCC | VEGFR, PDGFRβ, RAF1, BRAF, KIT, etc. | – | Sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab | 47 |
| RCC | VEGFR1/2/3, etc. | – | Sunitnib, pazopanib, sorafenib, everolimus, axitnib, cabozantinib, lenvatinib | 48 |
- This table includes selected cancer types and TKIs and does not aim to represent the entirety of all available TKIs in various cancer types. “–” stands for not applicable.
- Abbreviations: ALK, anaplastic lymphoma receptor tyrosine kinase; AML, acute myeloid leukemia; BCR–ABL, breakpoint cluster region-Abelson murine leukemia; BRAF, B-Raf proto-oncogene; BRCA, breast carcinoma; BTK, Bruton tyrosine kinase; CML, chronic myeloid leukemia; CRC, colorectal carcinoma; EGFR, epidermal growth factor receptor; ex19del, exon 19 deletion; ex20ins, exon 20 insertion; FLT3, FMS-like tyrosine kinase 3; GIST, gastrointestinal stromal tumors; HCC, hepatocellular carcinoma; HER2, human epidermal growth factor receptor 2; KIT, KIT proto-oncogene; KRAS, kirsten rat sarcoma viral oncogene; MTC, medullary thyroid carcinoma; NSCLC, non-small cell lung cancer; NTRK, neurotrophic receptor tyrosine kinase; PDGFR, platelet-derived growth factor receptor; PTC, papillary thyroid carcinoma; RAS, rat sarcoma virus oncogene; RCC, renal cell carcinoma; RET, RET proto-oncogene; RMS, rhabdomyosarcoma; ROS1, ROS proto-oncogene 1; SCLL, stem cell leukemia/lymphoma syndrome; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
Despite the great success of TKIs, resistance can develop over time. Resistance to TKIs has remained an ongoing and fundamental challenge in cancer-targeted therapy. Approximately 10−40% of patients receiving front-line imatinib treatment eventually necessitate an alternative treatment due to intolerance or resistance.19, 20 Resistance to EGFR-TKIs is also inevitable in advanced NSCLC patients. Virtually all patients developed acquired resistance within 1−2 years. The median PFS of patients receiving any generation (first, second, or third) of EGFR-TKIs as first-line treatment in phase III clinical trials ranges from 8 to 13.7 months.21, 22 Similar situations can be observed in advanced ALK-positive NSCLC patients receiving crizotinib treatment. Acquired resistance commonly occurs to patients within 1 year with a median PFS of 7.7–10.9 months.23 Furthermore, the majority of patients treated with TKIs do not achieve complete remission, with most experiencing only partial remission, indicating that targeted therapies cannot completely eliminate tumor cells, as some tumor cells exhibit intrinsic resistance to TKIs. This phenomenon is also observed in patients undergoing neoadjuvant-targeted therapy. The pathological complete response rate to neoadjuvant therapy of EGFR-TKIs was shown to be low (0–12%), and neither was the major pathological response rate high (8–24%).24, 25
Various mechanisms of resistance to TKIs, including both primary and acquired resistance, have been investigated. Primary resistance to TKIs typically arises from concurrent genetic alterations. The mechanisms of acquired resistance are usually complex and diverse, including both on-target and off-target mechanisms. In this review, we summarized the molecular mechanisms of resistance to TKIs, highlighted the overcoming strategies, and discussed the future directions of cancer-targeted therapy in research and clinical practice.
2 MECHANISMS OF RESISTANCE TO TKI-TARGETED THERAPY
Although TKIs have shown significant efficacy, previous experiences with targeted therapies suggest that resistance to TKIs, whether first-generation, second-generation, or third-generation, is inevitable and remains a critical unresolved challenge. Therefore, further research to elucidate the potential mechanisms of resistance to these drugs is necessary. In the following sections, we focus on discussing the known mechanisms of TKI resistance, broadly categorized as acquired resistance and intrinsic resistance.
2.1 Acquired resistance
Acquired resistance to TKIs manifests as resistance that emerges after an initial positive response to TKI treatment. Compared with primary resistance, acquired resistance is more frequently observed in TKI-targeted therapy. Acquired resistance can arise from various factors including secondary mutations in the targeted tyrosine kinase, overexpression or amplification of the target proteins/genes, loss of the original targeted mutations, activation of alternative signaling pathways, and tumor histological transformation (Figure 1).
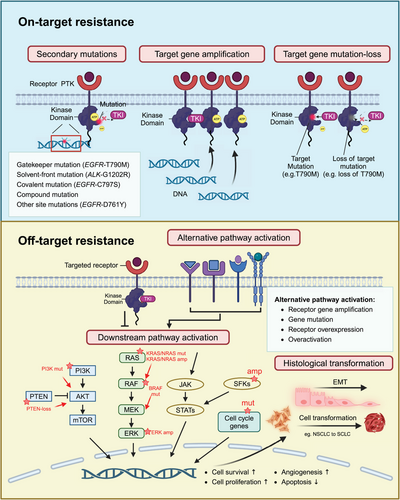
2.1.1 Mutations in the target kinase domain (on-target)
Secondary mutations in the target kinase domain, which are also called on-target mutations, are the most commonly encountered causes of TKI-acquired resistance. According to the location and function of mutated genes in the target kinase domain, secondary on-target mutations can be categorized into five types (Figure 2): (1) gatekeeper mutations, (2) solvent-front mutations, (3) covalent binding site mutations, (4) compound mutations, and (5) other site mutations.
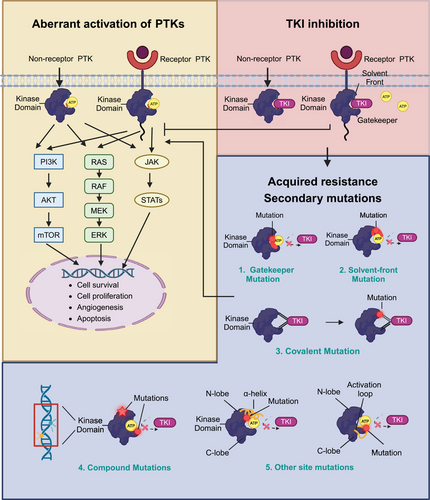
Gatekeeper mutation
Among all types of on-target mutations, gatekeeper mutation is the most frequently identified and extensively investigated TKI-resistance mechanism. Gatekeeper residues situate in the hinge region of tyrosine kinases’ ATP-binding pocket.49 They play a central role in controlling the accessibility of TKIs to the ATP-binding pocket.49 The mutation of gatekeeper residues can influence the interaction between the inhibitors and their targeting kinases, thereby reduce the efficacy of TKIs and lead to drug resistance.50, 51 A gatekeeper point mutation, involving the substitution of threonine with isoleucine at position 315 (T315I) in the BCR–ABL protein, was identified in CML patients resistant to imatinib in 2001.52 Molecularly, the hydroxyl group of threonine 315 can form a hydrogen bond with imatinib upon binding, while the side chain located at position 315 also regulates the steric interaction, governing the inhibitor's binding to hydrophobic regions neighboring the ATP-binding pocket.53, 54 The substitution of threonine with isoleucine leads to the elimination of the hydrogen bond between imatinib and targeted kinase, destabilizing the placement of imatinib.54 Therefore, a point mutation of gatekeeper is sufficient to confer imatinib resistance in patients with CML.
Similarly, a gatekeeper mutation in the kinase domain of EGFR (T790M) was found in 50−60% of NSCLC patients resistant to the first- and second-generation EGFR-TKIs.55-57 The substitution of threonine with methionine in the catalytic cleft of EGFR kinase domain, EGFR T790M, was first identified in a NSCLC patient with disease progression after gefitinib treatment in 2005.57 The methionine substitution introduced a bulkier amino acid side chain to the ATP-kinase-binding pocket, which resulted in a steric hindrance interfering with the binding of gefitinib or erlotinib.57 In addition, the presence of T790M mutation was also proven to enhance the affinity of ATP to the EGFR kinase domain.58 As EGFR-TKIs, such as gefitinib, compete with ATP for binding to the kinase active site, the increased ATP affinity reduces the potency of the inhibitor.58 Later on, other gatekeeper mutations (Table 2), such as ALK-L1196M, ROS1-L2026M, FGFR1-V561F/M, FGFR2-V564F/I, FGFR3-V555E, FGFR4-V550L/E/M, RET-V804L/M, FLT3-F691I/L, KIT -T670I, and PDGFRα-T674I, were identified in patients with IGST, NSCLC, or other cancers after corresponding TKI treatment.59
| PTKs | Mutations | Cancer types | Prior TKIs | Prevalence (%) | References |
|---|---|---|---|---|---|
| BCR–ABL | T315I | CML | Imatinib | 7% | 52, 60, 61 |
| ALL | Dasatinib | 70% | 62 | ||
| EGFR | T790M | NSCLC | First- or second-generation EGFR-TKIs | 50–60% | 23, 57 |
| ALK | L1196M/Q | NSCLC | Crizotinib | 7% | 63 |
| Alectinib | 6% | ||||
| ALCL | Alectinib | Cases reported * | 64 | ||
| ROS1 | L2026M | NSCLC | Crizotinib | 8% | 65 |
| RET | V804L/M | NSCLC | Vandetanib | Cases reported * | 66-68 |
| MTC | Multikinase inhibitors | Cases reported * | |||
| KIT | T670I | GIST | Imatinib | Cases reported * | 69 |
| PDGFRα | T674I | GIST | Imatinib | Cases reported * | 70, 71 |
| BTK | T474I | CLL | Pirtobrutinib | 22% | 72 |
| FGFR1 | V561M | SCLL | AZD4547, E3810 (lucitanib) | Cases reported * | 73, 74 |
| FGFR2 | V564F | CCA | BGJ398 | Cases reported * | 75 |
| FGFR3 | V355M/L | Myeloma | AZ12908010 | Cases reported * | 74, 76 |
| FGFR4 | V550L/M | HCC, RMS | BLU-554 | Cases reported * | 77, 78 |
| NTRK1 | F589L | CCA, CRC | Larotrectinib | Cases reported * | 79, 80 |
| FLT3 | F691I/L | AML | Quizartinib, gilteritinib | Cases reported * | 81 |
- This table includes selected gatekeeper mutations and does not aim to represent the entirety of all reported gatekeeper mutations.
- “Cases reported *” indicates cases with corresponding mutations were reported in case reports, with no available prevalence rate.
- Abbreviations: ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma receptor tyrosine kinase; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCR–ABL, breakpoint cluster region-Abelson; BTK, Bruton tyrosine kinase; CCA, cholangiocarcinoma; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CRC, colorectal carcinoma; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; FLT3, FMS-like tyrosine kinase 3; GIST, gastrointestinal stromal tumors; HCC, hepatocellular carcinoma; KIT, KIT proto-oncogene; MTC, medullary thyroid carcinoma; NSCLC, non-small cell lung cancer; NTRK, neurotrophic receptor tyrosine kinase; PDGFR, platelet-derived growth factor receptor; PTKs, protein tyrosine kinases; RET, RET proto-oncogene; RMS, rhabdomyosarcoma; ROS1, ROS proto-oncogene 1; SCLL, stem cell leukemia/lymphoma syndrome; TKI, tyrosine kinase inhibitor.
Solvent-front mutation
Another predominant on-target mutation mechanism leading to TKI-resistance is solvent-front mutation, which confers acquired resistance to ALK, ROS1, RET, NTK1, NTK2, and NTK3 fusion-targeted therapy.63, 82-86 Solvent-front mutations occur in amino acid residues that are located near the solvent-accessible surface of the ATP-binding pocket.87 For example, solvent-front mutations ALK-G1202R and ALK-S1206Y which sit near the crizotinib-binding site are reported to cause acquired resistance to crizotinib in NSCLC patients.63, 87 Computational modeling suggests that the G1202R mutation results in a bulkier basic residue that would induce steric hindrance, potentially impeding the binding of crizotinib to targeted kinases. And the S1206Y mutation may disrupt the stability of the interaction between the side-chain hydroxyl group of Ser1206 and the carboxylate group of D1203.87 Generally, solvent-front mutations lead to conformational changes in the target kinase domain, diminishing the affinity of crizotinib for the mutant ALK, and thus abrogating the efficacy of crizotinib to inhibit aberrantly activated ALK.87 ROS1-G2032R, involving the substitution of glycine with arginine in the solvent front of ROS1 kinase domain, was found to mediate resistance to crizotinib and lorlatinib in ROS1 fusion-positive NSCLC patients.83, 88 Analogous solvent-front mutations have also been identified in other tyrosine kinases and confer to TKI-resistance, such as RET-G810 solvent mutations after selpercatinib treatment in NSCLC patients,84, 85 EGFR-G706S/R mutation following osimertinib treatment in NSCLC patients,89 TRKA-G595R and TRKA-G667C mutations after entrectinib treatment in colorectal cancer patients.86 The most frequently reported solvent-front mutations are summarized in Table 3.
| PTKs | Mutations | Cancer types | Prior TKIs | Prevalence (%) | References |
|---|---|---|---|---|---|
| EGFR |
G796S/R L792X |
NSCLC | Osimertinib | Cases reported * | 89, 90 |
| ALK | G1202R | NSCLC | Crizotinib | 2% | 63 |
| Alectinib | 21–29% | ||||
| Ceritinib | |||||
| Brigatinib | |||||
| I1171T | NSCLC | Crizotinib | 2% | 63 | |
| Alectinib | 12% | ||||
| RET | G810R/S/C | NSCLC | Selpercatinib | Cases reported * | 85 |
| ROS1 | G2032R | NSCLC | Crizotinib | 38% | 88 |
| Lorlatinib | 32% | ||||
| D2033N | NSCLC | Crizotinib | 2.4% | 88 | |
| L2026M | NSCLC | Crizotinib | Cases reported * | 88 | |
| TRKA | G595R | CRC | Entrectinib | Cases reported * | 79 |
| Larotrectinib | Cases reported * | ||||
| TRKC | G623R | MASC | Entrectinib | Cases reported * | 79 |
| Larotrectinib | Cases reported * | ||||
| BTK | C481S | CLL | Ibrutinib | 50−75% | 91, 92 |
- This table includes selected solvent-front mutations and does not aim to represent the entirety of all reported solvent-front mutations.
- “Cases reported *” indicates cases with corresponding mutations were reported in case reports, with no available prevalence rate.
- Abbreviations: ALK, anaplastic lymphoma receptor tyrosine kinase; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; CRC, colorectal carcinoma; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PTKs, protein tyrosine kinases; RET, RET proto-oncogene; ROS1, ROS proto-oncogene 1; TKIs, tyrosine kinase inhibitors.
Covalent binding site mutation
A less common yet formidable resistance mechanism in targeted therapy is the covalent binding site mutation. As discussed above, the EGFR-T790M mutation can arise in 50−60% of NSCLC patients after receiving treatment with first- and second-generation EGFR-TKIs.55 Osimertinib, a third-generation EGFR-TKI with greater potency and selectivity, was designed specifically to overcome the EGFR-T790M resistance mutation through potent covalent-binding to the ATP-binding pocket.93 In NSCLC patients with T790M acquired resistance mutation treated with first-generation EGFR-TKIs, osimertinib showed a superior overall response rate (ORR) (71 vs. 31%) and longer PFS (10.1 vs. 4.4 months) compared with conventional chemotherapy.94 Following this, osimertinib was compared with standard first- and second-generation EGFR-TKIs in patients with advanced-stage EGFR-positive NSCLC in the pivotal FLAURA trial, revealing a prolonged median PFS (18.9 vs. 10.2 months).95
While osimertinib effectively counteracts the EGFR-T790M resistance mutation, its covalent binding to cysteine 797 (C797) residue in the ATP-binding pocket makes C797 residue vulnerable to mutations.23 Unsurprisingly, EGFR-C797S is reported as the most frequent resistance mutation to osimertinib, which has been identified in 10−33% patients after second-line osimertinib treatment, and 6% patients after first-line osimertinib treatment.96-99 EGFR-C797S mutation occurs at the EGFR C797 codon in exon 20, which is located within the ATP-binding cleft.100, 101 The mutation leads to a substitution of cysteine with serine, resulting in the loss of the covalent bond between osimertinib and EGFR.100-102 Nevertheless, it is noteworthy that the allelic context where C797S arises holds potential implications for treatment. In some circumstances, patients harboring the C797S mutation may remain responsive to quinazoline-based EGFR-TKIs.103-105 When C797S emerges in trans with the T790M mutation, patients are responsive to both first-generation and third-generation EGFR-TKIs to address C797S- and T790M-positive alleles, respectively.103-105 Conversely, when the mutations occur in cis, patients demonstrate resistance to all available EGFR-TKIs, either alone or in combination.103-105 Similar to EGFR-C797S, HER2-C805S has also been reported to cause resistance to HER2-TKI therapy in HER2-mutated preclinical cell models.106 However, the clinical existence and significance of HER2-C805S covalent mutation is yet to be determined.
Compound mutations
Compound mutations refer to the presence of two or more mutations within the same gene or across different genes in the same molecule.107, 108 Compound mutations have the potential to confer cross-resistance to multiple TKIs through steric hindrance mechanisms.107, 109 Compound mutations were first reported in CML patients following sequential therapy with different ABL kinase inhibitors.108 In a study, 20 out of 48 CML patients receiving various ABL-TKI treatments were found to harbor compound mutations.107 However, the genuineness of BCR–ABL compound mutations has been long debated due to their rarity of “low-level mutations,” which can lead to false-positive results in polymerase chain reaction amplification.110, 111 By applying next-generation sequencing (NGS), Deininger et al.111 reported that no single or compound mutation was consistently identified to confer primary or acquired resistance to BAR–ABL inhibitor ponatinib in chronic-phase CML patients. In EGFR-mutant NSCLC, EGFR compound mutations are defined as double or multiple mutations occurring at the EGFR tyrosine kinase domain.112, 113 EGFR compound mutations are usually a combination of an EGFR typical mutation, such as ex19del, L858R, G719X, and atypical mutations.113 It is reported that patients with EGFR compound mutations have poorer clinical outcomes than patients with EGFR-TKI sensitive mutations (ex19del, L858R), but have a more favorable prognosis than patients with resistant mutations (T790M, ex20ins).112, 114 Similarly, compound ALK resistance mutations were identified in ALK-positive patients treated with ALK-TKIs.63 Some studies show that most on-target ALK mutations conferring resistance to third-generation ALK-TKI lorlatinib are ALK compound mutations.23, 115-120
Other site mutations
In addition to the classic secondary mutations discussed above, mutations in other sites of target kinase domains have also been reported to confer TKI resistance. Typically, other site mutations occur in the kinase residues with important functions. They usually are less common. Tyrosine kinase domains exhibit common structural elements, such as the ATP-binding pocket located between an N-terminal lobe containing the αC helix, and a C-terminal lobe containing an activation loop. Mutations in these structural components can cause resistance to inhibitors. For example, EGFR-T854A mutation in the ATP-binding site, EGFR-D761Y, EGFR-L747S mutations near the αC helix, and EGFR-L792F/H (1.2%) in the hinge region have been found to confer resistance to EGFR-TKIs.121-124 D276G mutation, located on the β3-αC loop of BCR–ABL kinase domain, was reported to cause resistance to imatinib by inducing destabilization of the inactive conformation of the kinase.125 Similarly, ATP-binding site mutation (ALK-G1269A) and near αC helix site mutations (ALK-C1156Y, ALK-L1152R, ALK-F1174C, and ALK-1151T insertion) were identified in ALK-rearranged cancers, conferring resistance by causing conformational changes.87, 126-128
2.1.2 Amplification of target genes or loss of target mutations (on-target)
Cancer cells may increase the expression of the target protein, allowing them to maintain signaling despite TKI inhibition. For example, EGFR gene amplification has been reported in many studies as a common resistance mechanism in EGFR-TKI treatment.129 EGFR amplification was identified in 4.1−10% of patients resistant to osimertinib in second-line treatment.129, 130 In addition, wild-type (WT) EGFR amplification, but not the mutant alleles, is proved to be sufficient to confer acquired resistance to third-generation EGFR-TKIs in NSCLC.131 MET gene amplification, overexpression, and constitutive activation are found in cells resistant to MET inhibitors.132 ALK copy number gain, or amplification is reported in NSCLC patients resistant to crizotinib.87, 133
Other than EGFR-C797S secondary mutation, EGFR-T790M mutation-loss represents another major resistance mechanism to osimertinib.129 In a study analyzing paired pre- and posttreatment samples from 49 NSCLC patients resistant to second-line osimertinib treatment, 51% (25 out of 49) of these patients exhibited the loss of T790M.129 Similarly, half of the patients resistant to second-line osimertinib had undetectable plasma EGFR-T790M in the AURA3 trial study.134 Typically, the loss of T790M is always associated with the co-occurrence of acquired off-target resistance mechanisms such as MET amplification, HER2 amplification, KRAS mutation, and cell cycle gene alterations.134 Approximately one-third of patients with EGFR-T790M mutation loss are reported to have at least one acquired resistance mechanism.134
2.1.3 Activation of alternative signaling pathways (off-target)
Gene amplification
Aside from the alterations of targeted genes themselves, cancer cells can bypass the inhibited signaling pathway by activating other tyrosine kinase receptors that promote cell survival and proliferation (see Table 4). For example, MET amplification is the most frequently observed EGFR-independent mechanism of resistance to EGFR-TKIs, representing 5−22% of resistant cases after first/second-generation EGFR-TKI treatment, 7−15% after osimertinib first-line treatment, and 5−50% after osimertinib second-line treatment.135-137 MET encodes the tyrosine kinase receptor for hepatocyte growth factor (HGF). Amplification of MET gene or overactivation of MET receptor by HGF leads to activation of downstream signaling cascades, which are shared among tyrosine kinase family receptors (EGFR, HER2, ALK, and RET), such as phosphatidylinositol 3-kinase (PI3K) /Akt and RAS/RAF/ERK/mitogen-activated protein kinase (MAPK). By amplifying MET gene, cancer cells switch the downstream signaling pathway from the inhibited kinases to the overactivated MET, and then escape from TKI inhibition. MET amplification has been proven to mediate gefitinib resistance by activating Erb-B2 receptor tyrosine kinase 3 (ERBB3)–PI3K/Akt signaling pathway in lung cancer.136 MET amplification is also recognized as a resistance driver to RET-specific inhibitors (selpercatinib), ALK inhibitors (alectinib), and KRAS inhibitors (sotorasib) in NSCLC.84, 138-140 Additionally, the amplification of HER2 is proposed as a resistance mechanism to EGFR TKIs in NSCLC patients, and anti-EGFR monoclonal antibody cetuximab in patients with colorectal cancer.55, 137, 141 EGFR and KIT amplification are reported in patients resistant to ALK inhibitors.87 Proto-oncogeneLYN overexpression is identified to confer resistance to STI571 in CML.142 FGFR (FGFR1, FGFR2, and FGFR3) amplification is reported to be a potential resistance mechanism to osimertinib in NSCLC patients.143
| Alterations | Cancer types | TKIs | Targeted kinases | Prevalence (%) | References |
|---|---|---|---|---|---|
| MET amplification | NSCLC |
Gefitinib erlotinib |
EGFR activated mutations (e.g., ex19del, L858R) |
5–22% | 136, 137 |
| First-line osimertinib | EGFR T790M | 16% | 99 | ||
| Second-line osimertinib | EGFR T790M | 18% | 134 | ||
| Abivertinib | EGFR activated mutations and EGFR T790M | 3.8% | 144 | ||
|
Selpercatinib pralsetinib |
RET fusions | 15% | 84 | ||
| Poziotinib | EGFR ex20ins | 8.7% | 145 | ||
| Crizotinib | ALK fusion | Cases reported * | 146 | ||
| Alectinib | ALK fusion | Cases reported * | 147 | ||
| Adagrasib | KRAS G12C | Cases reported * | 148 | ||
| HER2 amplification | NSCLC |
Gefitinib erlotinib |
EGFR activated mutations (e.g., ex19del, L858R) |
13% | 137 |
| First-line osimertinib | EGFR T790M | 2% | 99 | ||
| Second-line osimertinib | EGFR T790M | 5% | 134 | ||
| Abivertinib | EGFR activated mutations and EGFR T790M | 12.5% | 144 | ||
| FGFR overactivation/amplification | NSCLC | Gefitinib |
EGFR activated mutations (e.g., ex19del, L858R) |
Preclinical only | 149 |
| AZD9291 | T790M | Cases reported * | 150 | ||
|
Selpercatinib Pralsetinib |
RET fusions | Cases reported * | 84 | ||
| AXL overexpression/amplification | NSCLC | Erlotinib | EGFR ex19del | Preclinical only | 151 |
| Osimertinib | EGFR T790M | Preclinical only | |||
| BRCA |
Lapatinib Erlotinib |
HER2 | Preclinical only | 152 | |
| HNC | Erlotinib | EGFR | Preclinical only | 153 | |
| Cetuximab | EGFR | Preclinical only | 154 | ||
| IGF1R activation | NSCLC | EGFR-TKI WZ4002 | EGFR T790M | Preclinical only | 155, 156 |
- This table includes selected cancer types and TKIs and does not aim to represent the entirety of all available TKIs in various cancer types.
- “Cases reported *” indicates cases with corresponding alterations were reported in case reports, with no available prevalence rate.
- Abbreviations: ALK, anaplastic lymphoma receptor tyrosine kinase; AXL, AXL receptor tyrosine kinase; BRCA, breast carcinoma; CRC, colorectal carcinoma; EGFR, epidermal growth factor receptor; ex19del, exon 19 deletion; ex20ins, exon 20 insertion; HER2, human epidermal growth factor receptor 2; HNC, head and neck carcinoma; IGF1R, insulin-like growth factor 1 receptor; KRAS, kirsten rat sarcoma viral oncogene; NSCLC, non-small cell lung cancer; PTC, papillary thyroid carcinoma; RET, RET proto-oncogene; TKIs, tyrosine kinase inhibitors.
Aberrant activation of tyrosine kinase downstream signaling pathways
RAS–RAF–MEK–ERK, PI3K/Akt, and JAK/STAT signaling pathways are crucial in receptor tyrosine kinase (RTK) activation.157 Cancer cells can acquire resistance to TKIs by developing alterations in downstream components of the inhibited kinases and bypassing the need for upstream stimulation. For example, acquired KRAS (G12C) or BRAF (G469A, V599E, or V600E) confer resistance to EGFR, MET, or ALK TKIs in NSCLC.117, 143, 158-161 In these cases, constitutive activation of the RAS–RAF–MEK–MAPK pathways can be directly induced by altered KRAS and BRAF, without the need for upstream stimulation.159, 162 Thus, cancer cells escape from the inhibition of TKIs. Similarly, the mutations in encoding PI3K can lead to constitutive activation of the PI3K/Akt signaling pathway, mediating resistance to MET inhibitors and EGFR-TKIs.55, 163 Additionally, PTEN loss or mutation also contributes to EGFR-TKI resistance by activating Akt.98, 164
2.1.4 Histological transformation (off-target)
Epithelial to mesenchymal transition (EMT), a process by which epithelial cancer cells acquire mesenchymal characteristics (e.g., loss of cell–cell junctions), allows cancer cells to migrate and invade surrounding tissues.165 Cancer cells undergoing EMT become less reliant on the targeted pathway and more resistant to apoptosis induced by TKIs.165 EMT is proven to be a potential resistance mechanism to erlotinib, osimertinib, and lorlatinib in NSCLC in both clinical and preclinical settings.55, 119, 166, 167 The histological transition of EMT typically involves changes in some specific proteins, such as the downregulation of E-cadherin.166 E-cadherin interacts with EGFR, playing an important role in NSCLC progression.168 Reintroduction of E-cadherin into NSCLC cell lines after undergoing EMT can restore cancer cell sensitivity to gefitinib therapy.168 Additionally, activation of Anexelekto (AXL) is shown to play an important role in EMT and TKI resistance. AXL activation was found associated with EMT features and conferring acquired resistance to erlotinib in NSCLC cell lines. Genetically or pharmacologically inhibiting AXL increased cancer cell sensitivity to erlotinib.151 In addition, transforming growth factor-beta (TGF-β) secretion triggered by EGFR inhibition was also found to promote EMT and confer resistance to EGFR-TKI treatment by activating the SAMD pathway in NSCLC.169
Aside from EMT, NSCLC to small cell lung cancer (SCLC) transformation is another major histological transformation mechanism leading to TKI-targeted therapy resistance.170 Approximately 3−14% of patients with resistance to first- or second-generation EGFR-TKIs undergo NSCLC to SCLC transformation.55, 137 When it comes to the third-generation EGFR-TKI, this transformation is even more common.150 One proposed hypothesis for the mechanism of SCLC transformation is tumor heterogeneity.170 It could be possible that patients had tumors with combined histology (both NSCLC and SCLC components) at the time of diagnosis. The SCLC component became dominant after effective TKI treatment. However, gene sequencing of EGFR from both pretreatment and posttreatment biopsy samples revealed that transformed SCLC samples retained their mutant EGFR. This suggests posttreatment SCLC samples are most likely transformed from EGFR-mutant NSCLC.
2.1.5 Tumor microenvironment (off-target)
TKIs have the potential to reshape the tumor microenvironment (TME) by modulating various components, including tumor-infiltrating immune cells, immunomodulatory stromal cells, cytokines, chemokines, and other factors, such as hypoxia-inducible factor-1α (HIF-1α).171-173 Increasing evidence suggests that alterations induced by TKI therapy in the TME may contribute to TKI resistance.174, 175
Tumor-infiltrating immune cells, including T cells, natural killer (NK) cells, and macrophages, play a dual role in tumor progression and response to TKI therapy. While activated T cells and NK cells can recognize and eliminate tumor cells, the immunosuppressive TME can inhibit their antitumor functions. For example, regulatory T cells and myeloid-derived suppressor cells secrete immunosuppressive cytokines (TGF-β and interleukin-6 [IL-6]) and inhibit effector T cell function, contributing to TKI resistance.176 Additionally, tumor-associated macrophages (TAMs) can promote angiogenesis, tumor cell invasion, metastasis, and suppression of T immunity in the TME.177 Various studies have demonstrated that elevated infiltration of TAMs in solid tumors is often associated with unfavorable clinical outcomes.178, 179 Within the TME, TAMs can be categorized into two functionally distinct subtypes, namely classically activated macrophages (M1) and alternatively activated macrophages (M2).180 M1 macrophages typically display an antitumor phenotype, while M2-macrophages are commonly considered as pro-tumor cells.180 During TKI treatment, tumor cells induce TAM M2 polarization. M2-TAMs foster drug resistance through secreting cytokines such as HGF, vascular endothelial growth factor (VEGF), tumor necrosis factor-alpha, and IL-6.177, 181-184
Stromal cells within the TME, such as cancer-associated fibroblasts (CAFs) and tumor-associated endothelial cells, play a critical role in modulating immune responses and promoting tumor survival.185 CAFs secrete growth factors, cytokines, and extracellular matrix proteins that support tumor growth and metastasis.185-187 For instance, stromal cells including fibroblasts could mediate resistance to RAF inhibitors in BRAF-mutant melanoma by secreting HGF and activating MAPK and PI3K signaling pathways in tumor cells.186 CAF-derived SSP1 was found to be a candidate molecule driving resistance to sorafenib/lenvatinib in HCC.175 Furthermore, CAFs can induce EMT in tumor cells, leading to increased invasiveness and resistance to TKI therapy.188
Cytokines, chemokines, and other factors produced within the TME regulate immune cell recruitment, activation, and function, thereby influencing tumor progression and response to therapy. For example, IL-6 and TGF-β promote tumor cell proliferation, survival, and EMT, contributing to TKI resistance.174 Additionally, chemokines such as chemokine (C-C motif) ligand 2 can recruit immunosuppressive myeloid cells to the TME, further exacerbating immune evasion and TKI resistance.176, 189, 190 In solid tumors, the TME is always characterized by hypoxia, leading to the upregulation of HIF-1α in tumor cells.191 HIF-1α is a pivotal regulator that can activate the transcription of genes associated with tumor angiogenesis and cell survival, such as VEGF and its receptor VEGFR.191 As VEGFR and EGFR share common downstream signaling pathways, elevated VEGF and VEGFR expression contribute to the emergence of acquired resistance to EGFR-TKIs in NSCLC.192 Hypoxia was found to induce resistance to gefitinib in EGFR-mutant NSCLC by activating WT EGFR through the upregulation of TGFα.193
2.2 Primary or intrinsic resistance
Primary resistance, defined as the lack of initial responses to TKIs, has been observed in 20−30% of advanced EGFR-mutant NSCLC patients,95 20−40% advanced ALK-positive NSCLC patients,194 10% GIST patients,195 and other cancer patients.196 Primary resistance to TKIs is often associated with coexistence of additional genetic alterations together with TKI-sensitive mutations within the context of tumor heterogeneity.197 Specifically, molecular mechanisms contributing to primary or intrinsic resistance include the presence of TKI-resistant mutations (e.g., existence of de novo EGFR-T790M and EGFR ex20ins are associated with primary resistance to early-generation EGFR-TKIs29, 198), concurrent alterations in other oncogenes (e.g., de novo MET amplifications or KRAS mutation in EGFR-TKI resistant patients and KRAS mutation in imatinib resistance GIST patients195, 199-201), mutations in downstream signaling pathways (e.g., PTEN-loss confers resistance to EGFR-TKIs202), and germline deletions of gene encoding BCL-2-like protein 11 (BIM).203
3 OVERCOMING STRATEGIES FOR RESISTANCE TO TKI-TARGETED THERAPY
“One-drug, one-target, one-disease” has achieved tremendous success in the past 20 years and forms the basis of tumor precision therapy. However, as our research into tumor resistance mechanisms deepens, we have found that aside from tumors developing acquired resistance due to the target itself, most mechanisms are unrelated to the original target of the drug. So far, considerable progress has been made in developing strategies to prolong the duration of treatment benefits to patients. Examples include develop novel TKIs with broader specificity or improved binding affinity. Target-specific mutations that confer resistance to first-line therapies or inhibit multiple signaling pathways simultaneously to prevent bypass mechanisms. Other tumor resistance mechanisms unrelated to the drug target often require combination therapy strategies to overcome. In the following section, new chemical strategies that are based on a different principle to address the emergence of drug resistance are discussed (Figure 3).
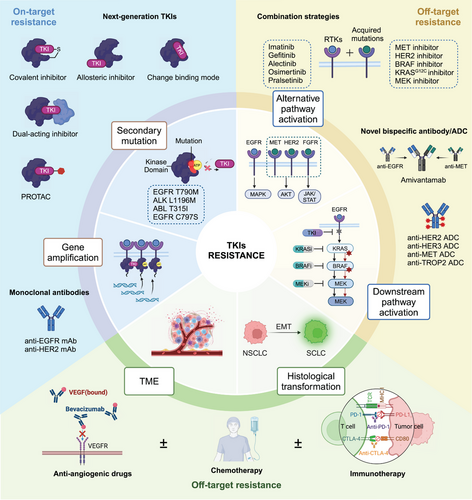
3.1 Development of next-generation TKIs
Acquired drug resistance mutations, including gatekeeper, solvent-front mutations, and aspartic–phenylalanine–glycine (DFG)-loop mutations, most commonly affect the binding of the drug to its target. Developing new TKIs or finding new sites of action is a promising solution for “on-target” resistance. For instance, resistance to first-generation EGFR-TKIs is commonly associated with the EGFR T790M secondary mutation.55, 204 Osimertinib (Tagrisso) is a third-generation TKI designed to target the EGFR T790M to overcome the resistance of first-generation EGFR-TKIs.205 The common strategies to overcome resistance mainly include drug structure optimization, designing covalent inhibitors, and designing allosteric inhibitors.
3.1.1 Drug structure optimization
Drug structure optimization is one of the commonly used strategies to overcome tumor resistance and is also an important means for discovering many best-in-class drugs. By analyzing the cocrystal structure of mutant proteins and small molecules, compound structures can be continuously optimized based on the original small molecule to obtain completely new small molecule compounds with distinct binding modes.206 An important example that demonstrates the clinical impact of this strategy is the ALK inhibitors developed to tackle gatekeeper mutation to crizotinib. ALK fusion-positive NSCLC patients treated with the first-generation ALK inhibitor crizotinib achieve an objective response rate of 60%, with a PFS of 8–10 months, and significantly prolonged OS.207, 208 However, many patients develop resistance to crizotinib approximately 1 year after treatment due to the ALK L1196M “gatekeeper” mutation.209, 127, 133 Cocrystal structures indicate that introducing methionine at the gatekeeper position 1196 likely adversely impacts the conformational changes in the ATP-binding entrance and hinge region, affecting crizotinib binding through steric interference.126 To overcome L1196 gatekeeper mutation, second-generation ALK inhibitors ceritinib and brigatinib with a different binding mode compared with that of crizotinib were designed.126, 210, 211 However, although these agents had improved potency and targeted key resistance mutations, they can only block a subset of resistance-conferring mutant alleles in ALK kinase due to partially overlapped binding modes. G1202R was the major resistance mutation site of the second-generation ALK-TKIs.63, 212, 213 Therefore, the third-generation ALK inhibitor lorlatinib has emerged. Compared with the previous two generations of ALK inhibitors, lorlatinib belongs to the macrocyclic drug class with a certain rigid structure within the molecule.214 It binds more tightly to the active pocket of the ALK protein, thus overcoming resistance caused by mutations such as ALK L1196M and G1202R.63, 215
Therefore, although the above three generations of ALK inhibitors all bind to the active site of ALK protein, they can overcome resistance by utilizing different inhibitor-amino acid residue contact modes. However, not all types of resistance targets are worthy of being developed as new drug targets. Some resistant mutations may become sensitive again to previous generations of drugs targeting the same target. ALK L1198F substitution confers resistance to lorlatinib through steric interference with drug binding. However, L1198F paradoxically enhances binding to first-generation ALK-TKI crizotinib, resensitizing resistant cancers to crizotinib.115, 120, 209 In conclusion, the experience with the development of different ALK inhibitors suggests that designing drugs with different binding modes can help overcome resistance to targeted therapy.
3.1.2 Covalent inhibitors
Another chemical strategy to overcome resistance is to design covalent inhibitors. Covalent bonds are stronger than hydrogen bonds and hydrophobic interactions, and covalent small molecules have a higher affinity with the target protein. This approach depends on the nucleophilic amino acid residues (such as cysteine and lysine) in the active pocket of the target protein to form covalent bonds with electrophilic moiety in inhibitors.216, 217
The most typical example of covalent inhibitors is the design of EGFR inhibitors. Represented by gefitinib and erlotinib, the first-generation EGFR inhibitors are noncovalent ATP-competitive inhibitors of WT EGFR and have shown good efficacy in NSCLC patients with the EGFR L858R/19del mutation.218-220 However, after 8–14 months of treatment with the first-generation EGFR inhibitors, about 50% of patients develop the T790M resistance mutation at the gatekeeper site of the EGFR kinase domain.55, 57, 204, 221, 222 Unlike the analogous L1196M gatekeeper mutation in the ALK kinase domain mentioned above, which introduces a steric impediment for crizotinib binding,126 EGFR T790M only modestly affects gefitinib or erlotinib binding. However, more importantly, it significantly increases its affinity for ATP, similar to that of WT EGFR.58 Competitive noncovalent inhibitors like gefitinib lack sufficient affinity for EGFR T790M to effectively compete with ATP, leading to treatment failure. Cocrystal structure studies have revealed a cysteine residue at position C797 in the ATP-binding site of EGFR. Small molecules that covalently bind to C797 can effectively inhibit the kinase activity of EGFR T790M.205, 206, 223-225 Based on this strategy, the second-generation irreversible covalent inhibitors afatinib and dacomitinib were designed to enhance potency and try to overcome the resistance limitations of EGFR T790M. However, due to the ATP affinity of EGFR T790M being similar to WT EGFR, second-generation covalent inhibitors can cause strong adverse reactions, especially skin rash and diarrhea, and limit the ability to achieve plasma concentrations sufficient to inhibit EGFR T790M.224, 226, 227 This challenge led to embark on innovative strategies to develop third-generation reversible inhibitors osimertinib that were specifically designed to target the EGFR T790M mutant, while maintaining activity against the initial activating mutations and retaining sufficient selectivity over WT EGFR to address the limitations of afatinib and dacomitinib.205 These studies indicate that designing covalent compounds targeting cysteine residues in the active site is feasible and can overcome resistance to prior-generation TKIs.
3.1.3 Allosteric inhibitors
Most approved TKIs, whether in clinical use or in preclinical development, are ATP-competitive inhibitors targeting the ATP-binding pocket of kinases.228 Based on the highly conserved conformation of kinase activation loop starting with DFG, ATP-competitive inhibitors are classified into type I and type II inhibitors. Type I inhibitors (such as gefitinib) bind in the “DFG-in” conformation within the ATP-binding pocket in its active state, while type II inhibitors (such as imatinib) bind to the hinge region and allosteric site of ATP pocket in the inactive state.229-231 However, the conservation of the ATP-binding pocket among different kinases poses a challenge for the development of highly selective kinase inhibitors. Additionally, the efficacy of ATP-competitive or covalent inhibitors can be compromised by active site mutations that can arise upon prolonged drug treatment.96, 232, 233 One strategy is to target kinase allosteric pockets outside the ATP-binding site. Such allosteric modulators bind to sites that are less conserved than ATP-binding sites and only accessible upon conformational changes, providing various advantages such as higher selectivity and extended drug target residence times.228, 234
A good example of the enhanced clinical impact of allosteric inhibitors is provided by ABL1 kinase. The first-in-class BCR–ABL inhibitor imatinib induced resistance in CML through the gatekeeper T315I mutation.235, 236 Asciminib, targeting the allosteric pocket of ABL1 kinase, can effectively block the kinase activity of ABL1 T315I mutation, overcoming resistance to ATP-competitive inhibitors like imatinib.237, 238 Allosteric inhibitors mimic the natural regulatory mechanisms of protein kinases, providing very high specificity. Therefore, targeting the “allosteric pocket” on the target protein instead of the “orthosteric pocket” is one effective strategy to overcome tumor resistance.
3.2 Combination therapies
The development of next-generation inhibitors to overcome drug resistance has become entrenched in current kinase drug discovery. However, as exemplified by osimertinib and lorlatinib, continual development of further next-generation agents that directly target the drug-resistant kinase mutant still acquired resistance.96, 213, 215, 221 Besides, resistance caused by “on-target” mutations only accounts for a minority of cases. The majority of patients develop resistance due to “off-target” pathway activation, while some resistance mechanisms remain unknown. Only a small proportion of patients can continue with monotherapy, while more patients require combination therapy to simultaneously target kinase protein and off-target mutations. Combination therapies have the potential to suppress the emergence of drug resistance by completely and simultaneously suppressing cooperating oncogenic signals or concurrently blocking bypass signaling pathways that may mediate drug resistance. In the following section, we highlight recent advances in rational combination strategies that are based on different principles. Some of these drug combinations are already in clinical practice, whereas others are in earlier stages of clinical development.
3.2.1 Combination therapies with targeted agents
As mentioned above, parallel or downstream activation of oncogenic signaling can reduce the efficacy of primary target inhibition by reactivating the signaling pathway. Such as BRAF or KRAS mutations in EGFR-TKI-treated lung cancer, or MEK mutations in BRAF inhibitor resistant malignant melanoma.143, 160, 239, 240 One approach involves dual inhibition of oncogenic signaling, either upstream or downstream of the driver gene, by inhibiting multiple nodes of a signaling pathway, is a rational and effective strategy to overcome drug resistance. Examples of this strategy are the combination of BRAF and MEK inhibitors to delay resistance in malignant melanoma. Reactivation of the MAPK pathway is the dominant acquired resistance mechanism of BRAF inhibitors.241 The combination of BRAF and MEK inhibitors (e.g., dabrafenib and trametinib) is now a standard regimen for BRAF-mutant melanoma.242, 243 Src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP2) is a central downstream effector of many RTKs activation signal cascades.244 Preclinical studies have shown that inhibiting SHP2 can suppress MAPK pathway activation and inhibit tumor growth dependent on RTK pathway activation and/or carrying oncogenic KRAS mutations.245-248 Therefore, SHP2 inhibitors as part of combination therapy may significantly enhance the therapeutic effects of RTK, RAS, and MAPK pathway inhibitors (e.g., EGFR-TKIs, KRASG12C-TKIs).
Another promising approach is the use of combination therapy that combines inhibitors of the original kinase mutation with inhibitors of a parallel kinase to block the predominant cause of bypass resistance. For example, MET amplification is one of the most frequent mechanisms of acquired resistance to EGFR-TKIs. MET amplification-dependent resistance is caused by persistent activation of signaling pathways downstream of EGFR, such as those mediated by MAPK, signal transduction and activator of transcription, and PI3K–Akt, which bypass EGFR signaling. Several clinical evidence demonstrated that combining an EGFR-TKI with a MET inhibitor may overcome MET-mediated resistance.249, 250 Savolitinib is an oral, potent, and highly selective MET-TKI. In the phase Ib TATTON trial, MET-positive patients with disease progression following osimertinib, treatment with osimertinib plus savolitinib yielded an ORR of 67%, with a median duration of response of 12.4 months.249 Tepotinib, another oral selective MET-TKI, in combination with osimertinib is under investigation in the INSIGH2 trial (NCT03940703). PFS and OS were longer with tepotinib plus gefitinib than with chemotherapy in patients with high MET overexpression or MET amplification.250 Similarly, dual ALK-MET inhibition may also overcome ALK-positive lung cancer with MET-driven resistance.251, 252 Clinical trials of TKI combination therapy based on off-target resistance mechanisms are summarized in Table 5.
| Target | Phase | Clinical trial number | TKIs | Combination | Treatment arms | Trial population | Results | References |
|---|---|---|---|---|---|---|---|---|
| EGFR | II |
NCT03778229 (SAVANNAH) |
Osimertinib | MET inhibitor | Osimertinib+savolitinib | EGFR-mutant, MET-driven NSCLC progressed on osimertinib |
IHC90+ and/or FISH10+: ORR: 49% mDOR: 9.3 m mPFS: 7.1 m |
256 |
| Ib |
NCT02143466 (TATTON) |
Osimertinib | MET inhibitor | Part B: osimertinib+savolitinib | METamp, EGFR-mutated NSCLC, and progression on prior EGFR-TKI |
ORR: 30% (21/69, all PR) DCR: 75% (52/69) mDOR: 7.9months mPFS: 5.4 months |
257 | |
| II |
NCT03944772 (ORCHARD) |
Osimertinib |
MET inhibitor, First-generation EGFR-TKI, anti-EGFR mAbs, CT plus ICIs |
MET alterations: osimertinib+savolitinib EGFR C797X:Osimertinib+gefitinib EGFR alterations:osimertinib+necitumumab ALK rearrangement: osimertinib+alectinib RET rearrangement: osimertinib+selpercatinib Biomarker nonmatched: durvalumab+pemetrexed +carboplatin |
EGFR-mutant NSCLC progressed on osimertinib |
MET alterations: ORR: 41% (7/17, all PR) DCR: 82% (14/17) |
258 | |
| Ib | NCT02374645 | Gefitinib | MET inhibitor | Gefitinib+savolitinib | EGFR-mutant Chinese NSCLC progressed on prior EGFR-TKI |
In EGFR T790M-negative: ORR: 52% (12/23) mDOR: 7.2 m mPFS: 4.2 m |
259 | |
| II |
NCT03940703 (INSIGHT-2) |
Osimertinib | MET inhibitor | Osimertinib+tepotinib | METamp NSCLC and first-line osimertinib resistance |
Interim data: 98 pts with FISH METamp: ORR: 43.9% mDOR: 9.7 m mPFS: 5.4 m 31 pts with NGS METamp: ORR: 51.6% mDOR: 5.6 m mPFS: 4.6 m |
||
| Ib/II |
NCT01982955 (INSIGHT) |
Gefitinib | MET inhibitor | Gefitinib+tepotinib vs. CT | MET overexpression (IHC2+ or 3+) or MET NSCLC progressed on EGFR-TKI |
In EGFR T790M−: ORR: 67 vs. 43% mDOR: 19.9 vs. 2.8 m mPFS: 16.6 vs. 4.2 m mOS: 37.3 vs. 13.1 m |
250 | |
| Ib/II | NCT01610336 | Gefitinib | MET inhibitor | Gefitinib+capmatinib | EGFR-mutated, MET-amplified/overexpressing NSCLC progressed on EGFR-TKI |
ORR: 47% (17/36, all PR) DCR: 75% (27/36) mPFS: 5.5 m |
260 | |
| Ib | NCT05430386 | Almonertinib | MET inhibitor | Almonertinib+HS-10241 | MET-amplified NSCLC progressed after EGFR-TKI | 8 PR and 4 SD among 13 pts | 261 | |
| Ib/II | NCT02335944 | Nazartinib | MET inhibitor | Nazartinib+capmatinib | EGFR-mutant NSCLC (post-EGFR-TKI and treatment naïve) |
MET+: ORR 43.5% MET−: ORR 27.9% TN: ORR 61.7% |
262 | |
| III |
NCT04988295 (MARIPOSA-2) |
Lazertinib | EGFR/MET bispecific antibody |
Lazertinib+amivantamab+CT amivantamab+CT CT |
Osimertinib-relapsed EGFR-mutant NSCLC |
Amivantamab+CT Vs. lazertinib+amivantamab +CT vs. CT: ORR: 64 vs. 63 vs. 36% mPFS: 8.2 vs. 8.3 vs. 4.2 m |
263 | |
| I |
NCT02609776 (CHRYSALIS) |
Lazertinib | EGFR/MET bispecific antibody | Lazertinib+amivantamab | Osimertinib-relapsed EGFR-mutant NSCLC |
ORR: 36% mDOR: 9.6 m CBR ≥ 11 weeks: 64% mPFS: 4.9 m |
264 | |
| I/II |
NCT03784599 (TRAEMOS) |
Osimertinib | Anti-HER2-ADC | Trastuzumab-emtansine+osimertinib | EGFR-mutated NSCLC, progressing on osimertinib and HER2 overexpression |
ORR: 4% (1 of 27) mPFS: 2.8 m |
265 | |
| Ib | NCT04001777 | Osimertinib | Bcl-2 family protein inhibitor | Pelcitoclax(APG1252) +osimertinib | Patients with third-generation EGFR TKI-resistant NSCLC | 3 PR of 20 pts, including 2 pts with osimertinib-resistant NSCLC | 266 | |
| I | NCT03891615 | Osimertinib | PARP inhibitor | Osimertinib+niraparib | NSCLC with EGFR mutation progression on osimertinib | Ongoing | – | |
| I |
NCT03516214 (EATON) |
Nazartinib | MEK inhibitor | Nazartinib+trametinib | Advanced NSCLC harboring EGFR del 19 or p.L858R, first-line or after failure of any EGFR TKI | Ongoing | – | |
| I | NCT01570296 | Gefitinib | PI3K inhibitor | Gefitinib+BKM120 |
Patient Group 1: EGFR TKI Resistant; Patient Group 2: activated PI3K status and overexpress EGFR |
Ongoing | – | |
| Ia | NCT06032936 | Osimertinib | SHP2 inhibitor | Osimertinib+BBP-398 | NSCLC pts with EGFR mutations and with previously third-generation EGFR-TKIs treated or EGFR-TKI naïve | Ongoing | – | |
| ALK | Ib/II | NCT02292550 | Ceritinib | CDK4/6 inhibitor | Ceritinib+ribociclib | Stage IIIB/IV ALK+ NSCLC | ORR was 50% (4/8)in ALKi-naïve pts; 64% (9/14) in pts with prior crizotinib; 0% (0/5;) in pts with prior third-gen ALKi. | 267 |
| I | NCT03087448 | Ceritinib | MEK inhibitor | Ceritinib+trametinib | Patients with refractory NSCLC harboring ALK or ROS1 fusions |
ORR: 22% DCR: 56% mDOR: 7.85 m mPFS: 3 m |
268 | |
| Ib/II | NCT03202940 | Alectinib | MEK inhibitor | Alectinib+cobimetinib | ALK+ NSCLC pts progressed on alectinib | Ongoing | – | |
| I/II | NCT05845671 | – | EGFR/MET bispecific antibody | ALK/ROS1/RET-TKI+amivantamab | NSCLC harboring ALK, ROS1, and RET Gene Fusions progression on at least one prior TKI | Ongoing | – | |
| Ib | NCT04005144 | Ceritinib | Anti-PD-1 mAb | Ceritinib+nivolumab | ALK-TKI naïve and pretreated NSCLC |
ALK-TKI pretreated: ORR: 50% mPFS: 6.4 m |
269 | |
| I | NCT04777084 | Lenvatinib | Bispecific anti-PD-1/PD-L1 antibody | Lenvatinib+IBI318 | Cohort B: advanced NSCLC with EGFR-sensitive mutation/ALK fusion after EGFR-TKI/ALK-TKI resistance. | Ongoing | – | |
| II |
NCT05456256 (HARMONIC) |
– | EGFR, ALK, MET, and ROS1 | LP-300+carboplatin+ pemetrexed | Patients who are never smokers with lung adenocarcinoma and have relapsed after treatment with tyrosine kinase inhibitors | Ongoing | – | |
| KRAS | I/II | NCT03785249 | Adagrasib | SHP2 inhibitor | Adagrasib+TNO155 | Pts with advanced solid tumors harboring a KRAS G12C mutation (excluding NSCLC and CRC) |
ORR: 35.1% mDOR: 5.3 m mPFS: 7.4 m |
270 |
| Ib | NCT04185883 | Sotorasib | SHP2 inhibitor | Sotorasib+RMC4630 | Pts with KRAS G12C-mutated NSCLC, CRC, or other solid tumors |
Pretreated: ORR 27% KRAS G12C naïve: ORR 50% |
271 | |
| I/II | NCT05288205 | JAB21822 | SHP2 inhibitor | JAB21822+JAB3312 | Solid tumors harboring KRAS G12C mutation | Ongoing | – | |
| I/II |
NCT05375994 (RAMP 204) |
Adagrasib | MEK/RAF inhibitor | Adagrasib+VS6766 | Pts with KRAS G12C-mutant NSCLC | Ongoing | – | |
| I/II | NCT04793958 | Adagrasib | Anti-EGFR mAb |
Adagrasib monotherapy Adagrasib+cetuximab |
Pts with metastatic colorectal cancer with mutant KRAS G12C |
Adagrasib monotherapy ORR: 23% mDOR: 4.3 m mPFS: 5.6 m Adagrasib+cetuximab: ORR: 46% mDOR: 7.6 m mPFS: 6.9 m |
272 | |
| I/II |
NCT05002270 NCT05194995 |
JAB21822 | Anti-EGFR mAb | JAB21822+Cetuximab |
Solid tumors CRC |
Ongoing | – | |
| I/Ib | NCT04975256 | Adagrasib | Pan KRAS/SOS1 inhibitor | Adagrasib+BI 1701963 |
Pts with advanced solid tumors haboring KRAS G12C mutation |
Ongoing | – | |
| I/Ib | NCT05178888 (KRYSTAL-16) | Adagrasib | CDK4/6 inhibitor | Adagrasib+pabociclib | Pts with solid tumors harboring a KRAS G12C mutation previously treated with at least 1 standard therapy | |||
| BCR–ABL | II | NCT03610971 | BCR-ABL TKI | JAK1/2 inhibitor | BCR–ABL TKI+ruxolitinib | CML pts relapsed after a prior attempt at TKI discontinuation | Ongoing | – |
| I/II | NCT01914484 | Nilotinib | JAK1/2 inhibitor | Nilotinib+ruxolitinib | Pts with Philadelphia-positive CML or ALL who is resistant to BCR–ABL inhibitor | Ongoing | – |
- Abbreviations: ALK, anaplastic lymphoma receptor tyrosine kinase; ALL, acute lymphoblastic leukemia; Bcl-2, B-cell lymphoma-2; CBR, clinical benefit rate; CDK, cyclin-dependent kinase; CML, chronic myeloid leukemia; CRC, colonrectal cancer; CT, chemotherapy; DCR, disease control rate; EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; ICIs, immune checkpoint inhibitors; IHC, immunohistochemical; JAK, Janus kinase; KRAS, kirsten rat sarcoma viral oncogene; mAb, monoclonal antibody; mDoR, median duration of response; MEK, mitogen-activated protein kinase kinase; MET, mesenchymal–epithelial transition factor; METamp, MET gene amplification; mOS, median overall survival; mPFS, median progression-free survival; NSCLC, non-small cell lung cancer; ORR, overall response rate; PARP, poly-ADP-ribosepolymerase; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; pts, patients; ROS1, c-ros oncogene 1 receptor tyrosine kinase; SHP2, Src homology phosphotyrosyl phosphatase 2; SOS, son of sevenless; TKI, tyrosine kinase inhibitor; TN, treatment naïve.
In addition, combining drugs targeting the same protein kinase but with different binding modes can effectively address drug resistance issues. For example, the combination of the BCR–ABL1 kinase inhibitor dasatinib, which targets the ATP-binding active site, with the allosteric inhibitor asciminib, can significantly overcome resistance to dasatinib and even achieve tumor regression.253 Designing multitarget inhibitors against newly emerging resistant targets also belongs to combination therapy in broad terms. In recent years, cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have made significant breakthroughs in the treatment of advanced or metastatic breast cancer. However, with the widespread clinical use of these drugs, tumor resistance has emerged as a challenge. Resistance to CDK4/6 inhibitors is mainly triggered by the activation of MYC proto-oncogene protein (MYC), G1/S-specific cyclin-E1 (CCNE1), and CDK2.254 Therefore, the combination of CDK4/6 inhibitors with CDK2 inhibitors may overcome resistance to CDK4/6 inhibitors in patients. PF3600 is the first known CDK2/4/6 small molecule inhibitor in clinical development and offers a new approach to overcoming resistance to CDK4/6 inhibitors.255
3.2.2 Antiangiogenic drugs combinations
Combining targeted therapies with antiangiogenic drugs is a new therapeutic approach currently being explored, most notably in the first-line setting, with mixed results to date (Table 6). Antiangiogenic-targeted drugs, represented by bevacizumab and anlotinib, have become indispensable treatment options for a variety of cancers as they can inhibit tumor angiogenesis, promote normalization of tumor blood vessels, and enhance drug delivery.273 Combination therapy with antiangiogenic drugs and immune checkpoint inhibitors (ICIs) or chemotherapy has emerged as a potential strategy to enhance the therapeutic efficacy of cancer.274, 275 In recent years, one of the prevalent research directions is the combination of angiogenic inhibitors with targeted therapies to overcome resistance to TKIs. Preclinical studies have indicated that simultaneous blockade of the VEGF pathway has synergistic antitumor activity and may delay the development of resistance to EGFR-TKIs or ALK-TKIs.276-278 JO25567, NEJ026, CTONG1509, and RELAY studies have shown that the combination of EGFR-TKIs with antiangiogenic inhibitors (e.g., bevacizumab, anlotinib, ramucirumab) may have prolonged PFS in advanced EGFR-mutant NSCLC compared with EGFR-TKIs monotherapy, although these studies have not yielded OS benefit.279-282 The effectiveness of combining antiangiogenic therapy with EGFR-TKIs to enhance efficacy is still controversial. The relevant mechanisms of action are not yet fully understood. Additionally, there is no evidence to suggest that antiangiogenic agents may help EGFR-TKI overcome known resistance mechanisms. In phase I studies of bevacizumab plus osimertinib and ramucirumab plus osimertinib, the summarized resistance mechanisms were not significantly different from those of monotherapy.283, 284 This comparison indirectly suggests that antiangiogenic agents do not help overcome osimertinib monotherapy resistance mechanisms, although we cannot completely rule out the possibility of delaying or reducing the occurrence of osimertinib resistance. Furthermore, the risk of adverse events is higher with combination therapy, with hypertension and proteinuria being particularly noticeable.285 Similarly, the potential benefit from an antiangiogenic agent combined with ALK-TKI in NSCLC patients remains to be determined. In phase I/II small cohort trials, the combination of alectinib and bevacizumab has been demonstrated to be tolerable. However, there was no significant improvement in efficacy.286, 287 To gain further insight and guide future studies, additional translational research and deeper mining of existing studies are necessary.
| Target | Phase | Clinical trial number | TKIs | Combination | Treatment arms | Trail population | Results | References |
|---|---|---|---|---|---|---|---|---|
| EGFR | III |
NCT04028778 (FL-ALTER) |
Gefitinib | MKIs targeting VEGFRs, FGFRs, PDGFRs, c-kit and MET |
Getinib+anlotinib Gefitinib+placebo |
First-line treatment of NSCLC with an EGFR 19del or 21 L858R mutation |
mPFS: 14.75 m vs. 11.20 m ORR: 76.13 vs. 64.52% mDOR: 12.48 vs. 9.46 m |
288 |
| II | ALTER-L004 | Icotinib | MKIs targeting VEGFRs, FGFRs, PDGFRs, c-kit and MET | Icotinib+anlotinib | First-line treatment option for advanced NSCLC carrying EGFR mutation with or without concurrent mutations |
ORR: 68.5% DCR: 98.2% mPFS: 15.1 m mDOR: 13.5 |
289 | |
| Ib/IIa | NCT04770688 (AUTOMAN) | Osimertinib | MKIs targeting VEGFRs, FGFRs, PDGFRs, c-kit and MET | Osimertinib+anlotinib | Treatment-naïve patients of locally advanced or metastatic nonsquamous EGFR-mutated NSCLC |
ORR: 65.2% DCR: 95.7% |
290 | |
| II | ALWAYS | Aumolertinib | MKIs targeting VEGFRs, FGFRs, PDGFRs, c-kit and MET | Aumolertinib+anlotinib | First-line treatment of advanced NSCLC patients with EGFR mutations. |
ORR: 96.15% DCR: 100% CNS ORR: 76.47% CNS DCR: 100% |
291 | |
| II | NCT02803203 | Osimertinib | Anti-VEGF mAb | Osimertinib+bevacizumab | First-line treatment of advanced NSCLC patients with EGFR mutations. |
ORR: 69% PFS-12 months: 0.70 |
292 | |
| II | JO25567 | Erlotinib | Anti-VEGF mAb |
Erlotinib+bevacizumab Erlotinib |
Stage IIIB/IV or recurrent nonsquamous NSCLC with EGFR mutations |
mPFS: 16.0 vs. 9.7 m |
282 | |
| III | NEJ026 | Erlotinib | Anti-VEGF mAb |
Erlotinib+bevacizumab Erlotinib |
Stage IIIB/IV or recurrent nonsquamous NSCLC with EGFR mutations |
mPFS: 16.9 vs. 13.3 m |
281 | |
| III | RELAY | Erlotinib | Anti-VEGFR2 mAb |
Erlotinib+ramucirumab Erlotinib+placebo |
Pts with untreated EGFR-mutated metastatic NSCLC | PFS: 19.4 vs. 12.4 m | 279 | |
| II |
NCT03909334 (RAMOSE) |
Osimertinib | Anti-VEGFR2 mAb |
Osimertinib+ramucirumab Osimertinib |
Treatment-naïve EGFR-mutant NSCLC |
PFS: 24.8 vs. 15.6 m ORR: 76.3 vs. 80.4% DCR: 96.8 vs. 95.7% |
293 | |
| ALK | I/II | NCT02521051 | Ceritinib | Anti-VEGF mAb | Alectinib+bevacizumab | Pts with advanced ALK-rearranged NSCLC |
3(60%) of 5 ALK TKI-pretreated patients had objective responses; mPFS: 9.5 m |
286 |
| I/II | NCT06007937 | Loratinib | Anti-VEGFR2 mAb | Lorlatinib+ramucirumab | Treatment-naïve or progressed of at least one second-generation ALK TKI | Ongoing | – | |
| Ib | NCT04227028 | Brigatinib | Anti-VEGF mAb | Brigatinib+bevacizumab | ALK+ NSCLC pts previously progressed on prior ALK-directed therapy | Ongoing | – | |
| II | ACTRN12622000973718(SHERLOCK) | Sotorasib | Anti-VEGF mAb | Sotorasib+ bevacizumab+carboplatin-pemetrexed | First line treatment of nonsquamous advanced NSCLC with KRAS G12C mutation | Ongoing | – |
- Abbreviations: ALK, anaplastic lymphoma receptor tyrosine kinase; ALL, acute lymphoblastic leukemia; CBR, clinical benefit rate; CML, chronic myeloid leukemia; CRC, colorectal cancer; DCR, disease control rate; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; mAb, monoclonal antibody; mDoR, median duration of response; MET, mesenchymal–epithelial transition factor; METamp, MET gene amplification; MKIs, multiple kinase inhibitors; mOS, median overall survival; mPFS, median progression-free survival; NSCLC, non-small cell lung cancer; ORR, overall response rate; PDGFR, platelet-derived growth factor receptor; pts, patients; TKI, tyrosine kinase inhibitor; TN, treatment naïve; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
3.2.3 Chemotherapy-based combinations
Chemotherapy remains the cornerstone of treatment for advanced-stage cancer. Particularly for patients with resistance to targeted therapy, especially when the resistance mechanism is unclear and targeted therapy is no longer applicable, chemotherapy remains the standard treatment recommended by guidelines. The results of the FLAURA2 study revealed that the median PFS in the osimertinib plus chemotherapy group was significantly longer than that in the osimertinib monotherapy group in first-line treatment (25.5 vs. 16.7 months, hazard ratio 0.62). OS data are currently immature, but they indicate a trend toward benefit.294 The combination of the third-generation BCR–ABL1 inhibitor olverembatinib with the Bcl-2 inhibitor venetoclax has shown high efficacy in relapsed Ph+ acute lymphoblastic leukemia (ALL) patients. A phase II clinical study combining these two drugs with low-dose chemotherapy demonstrated remarkable efficacy and safety.295 The combination of platinum-based chemotherapy with PARP inhibitors has shown promising efficacy in the treatment of ovarian cancer or breast cancer with BRCA1/2 mutations.296, 297 At present, most clinical studies of targeted therapy combined with chemotherapy focus on first-line treatment, some combination regimens have shown certain advantages in certain cancer types. Tumor driver genes typically exhibit strong oncogenicity, leading to a high dependency of tumor cells on activated signaling pathways driven by these genes.298, 299 For example, in NSCLC patients with mutations in driver genes such as EGFR, ALK, and ROS1, after resistance to first- and second-generation TKIs, even upon receiving third- or fourth-generation drugs, the targeting of driver gene signals persists, and some patients still respond effectively.300, 301 Additionally, some patients who develop resistance may still benefit from TKI rechallenge,252, 302 confirming the continued dependency of tumor cells on driver gene signals even after the development of resistance. This so called “driver gene addiction” persists after the development of TKI acquired resistance, leading many clinicians to continue TKI with subsequent chemotherapy.303 Current retrospective data demonstrated that continued first-generation EGFR-TKIs erlotinib and gefitinib with subsequent chemotherapy after progression was tolerated. Improved response rate and PFS were observed, especially in T790M-negative patients.303, 304 However, results from the prospective IMPRESS trial did not demonstrate a clinical benefit of continuing gefitinib with chemotherapy after disease progression. Another two retrospective studies analyzed outcomes of osimertinib pretreated NSCLC patients receiving osimertinib plus chemotherapy as second-line or later therapy. The median duration of treatment was 6.1 and 6.9 months in patients receiving platinum-doublet chemotherapy and osimertinib, respectively.305, 306 Platinum-based chemotherapy plus ALK-TKI also shows modest efficacy in ALK-positive NSCLC after failure of second-generation ALK-TKIs. A retrospective study demonstrated longer PFS in patients who received platinum/pemetrexed in combination with an ALK-TKI compared with those who received platinum/pemetrexed alone (6.8 vs. 3.2 months, respectively). Although the aforementioned studies extended PFS compared with chemotherapy alone, the overall benefit remains limited, and there is a lack of large-sample prospective clinical studies. Further prospective clinical trials are needed to investigate the potential benefit of TKI-chemotherapy combination therapy after resistance.
Combining antiangiogenic inhibitors with chemotherapy is a more widely used combination regimen in clinical practice. Antiangiogenic inhibitors can normalize the surviving tumor vasculature, facilitating the delivery of chemotherapy drugs into the tumor tissue, and thereby enhancing the efficacy of chemotherapy.307, 308 Multiple studies have confirmed the efficacy of combining antiangiogenic drugs with conventional chemotherapy in various fields such as breast cancer, colorectal cancer, and NSCLC.274, 308-310 For NSCLC patients who develop resistance to targeted therapies like EGFR and ALK inhibitors, the current standard treatment recommended by guidelines is chemotherapy combined with bevacizumab.311
3.2.4 Immunotherapy-based combinations
Combining TKIs with ICIs has shown promising results in certain cancers, especially renal cell carcinoma and HCC. Currently, five of the six approved targeted therapy and immunotherapy combinations target angiogenesis. The VEGF–VEGFR pathway plays a key role in almost all immune cell subsets. VEGFRs are expressed on activated and memory T cells.312 VEGF–VEGFR is involved in the activation of downstream signaling pathways of T cells and inhibits the cytotoxic activity of T cells.313, 314 Antiangiogenesis agents, such as levantinib, axitinib, bevacizumab, and cabozantinib, have been reported to improve CD8+ T cell infiltration and activation via tumor vasculature normalization and suppression of inhibitory immune checkpoints including programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4).315, 316 Consequently, multiple studies are now investigating the combination strategy of VEGFR inhibitors with PD-1 or PD-L1 ICIs to improve the efficacy. In 2021, the US FDA approved the combination of multikinase inhibitor lenvatinib plus PD-1 antibody pembrolizumab for first-line treatment of advanced renal cell carcinoma. The efficacy of this combination was investigated in CLEAR (NCT02811861), a multicenter, open-label, randomized phase III trial in patients with advanced RCC in the first-line setting. Patients receiving the combination regimen had a median PFS of 23.9 months compared with 9.2 months for those receiving sunitinib. The objective response rates were 71 and 36%; complete response rates were 16 and 4% on the combination and sunitinib arms, respectively.317 Inspiringly, the combination of pembrolizumab or avelumab (anti-PD-L1 antibody) with another small molecule antiangiogenesis agent axitinib has been approved for the treatment of patients with advanced RCC due to the encouraging results of KEYNOTE 426318 and JAVELIN Renal 101 trials.319
In addition to antiangiogenesis, some kinase inhibitors can potentially stimulate immune responses by preventing the increased expression of PD-L1, enhancing T cell infiltration or inducing immune cell death.320, 321 For example, BRAF inhibition enhances melanoma antigen expression and facilitates T-cell cytotoxicity, leading to a more favorable TME. This provides support for the potential synergy of BRAF-targeted therapy and immunotherapy.321 The combination of BRAF inhibitor vemurafenib and the MEK inhibitor cobinetinib, along with atezolizumab, has now been approved for BRAF-V600-mutant melanoma.322 However, whether used in monotherapy or combination with chemotherapy, ICIs appear to be ineffective in EGFR, ALK, RET, and HER2 driver gene-positive cancers.323-327 This may be attributed to the small number of CD8+ tumor-infiltrating lymphocytes and the low tumor mutational burden of these tumors, characteristics associated with a “cold” immune environment.323, 328-330 IMpower150 trial was the only positive clinical data on the efficacy of immunotherapy for both EGFR-mutated and ALK-rearranged tumors, which combined four drugs including atezolizumab, platin-based chemotherapy, and bevacizumab. Only the combination of atezolizumab and bevacizumab with chemotherapy could provide benefit in EGFR-TKI-mutant patients.331 However, only one patient in the quadruplet therapy group had previously received osimertinib. Thus, it is impossible to draw a conclusive statement regarding the efficacy of the quadruplet therapy in the specific context of EGFR-TKI failure. Moreover, an increased risk of severe toxicities with concomitant or sequential use of ICIs and specific EGFR or ALK-TKIs was demonstrated, particularly grade 3−4 pneumonitis and hepatitis.332-334 Another phase II clinical study of ILLUMINATE explored the efficacy and safety of dual immunotherapy (anti-PD-L1 durvalumab and anti-CTLA4 tremelimumab) combined with chemotherapy in metastatic EGFR-mutation-positive NSCLC that has progressed after EGFR-TKI resistance (NCT03994393). The results of the study found that the dual immunotherapy combined chemotherapy brings moderate antitumor activity, with potential increased benefits in patients with EGFR T790M negative and PD-L1 > 50%. The incidence of grade 3−4 immune-related adverse events was 17%, mainly colitis and hepatitis.335 Overall, the available data do not support the general use of ICIs among patients with driver gene mutations following progression on targeted therapies. US FDA-approved combination therapy of immunotherapy and TKI is summarized in Table 7.
| Indication | TKI | Immunotherapy | Year of approval | Trial name and references |
|---|---|---|---|---|
| First-line advanced RCC | Axitinib | Pembrolizumab | 2019 | KEYNOTE-426318 |
| First-line advanced RCC | Axitinib | Avelumab | 2019 | JAVELIN Renal 101319 |
| First-line advanced RCC | Lenvatinib | Pembrolizumab | 2021 | CLEAR317 |
| First-line advanced RCC | Cabozantinib | Nivolumab | 2021 | CheckMate 9ER336 |
| First-line unresectable HCC | Bevacizumab | Atezolizumab | 2020 | IMbrave 150337 |
| First-line BRAF V600E advanced melanoma | Vemorafenib+cobimetinib | Atezolizumab | 2020 | IMspire 150322 |
| Endometril cancer (not MSI-H or dMMR) | Lenvatinib | Pembrolizumab | 2019 | KEYNOTE-146338 |
- Abbreviations: dMMR, mismatch repair deficient; HCC, hepatocellular carcinoma; MSI-H, microsatellite instability-high; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor.
3.3 Novel dual-targeted bispecific antibodies or antibody–drug conjugates
Antibody–drug conjugates (ADCs) and dual-targeted bispecific antibodies represent innovative therapeutic approaches that are currently in development and hold promise for patients with TKI-resistant cancers. These novel agents are especially intriguing due to their ability to potentially address a wide range of resistance mechanisms. Amivantamab is a bispecific antibody targeting EGFR and MET that received accelerated approval for patients with NSCLCs harboring EGFR exon 20 insertion mutations in May 2021 based on the results of the CHRYSALIS trial.339 The combination of amivantamab with a third-generation EGFR-TKI lazertinib in patients with osimertinib-relapsed, chemotherapy-naïve, EGFR-mutated advanced NSCLC has shown preliminary efficacy, with an ORR of 36% and a median PFS of 4.9 months.264 Inspiringly, amivantamab plus carboplatin–pemetrexed chemotherapy with and without lazertinib also demonstrated antitumor activity in patients with osimertinib refractory EGFR-mutated advanced NSCLC. Objective response rate was significantly higher for amivantamab–chemotherapy and amivantamab–lazertinib–chemotherapy versus chemotherapy (64 and 63 vs. 36%, respectively).263 Of note, an exploratory analysis suggested that EGFR/MET immunohistochemistry (IHC) staining may predict for response to amivantamab plus lazertinib. A retrained signature of MET 3+ stating on 25% tumor cells was identified as predictive of response regardless of molecular resistance mechanism.340, 341
As another example, patritumab deruxtecan is a specifically engineered potential first-in-class HER3-directed ADC. In HERTHENA-Lung01, patritumab deruxtecan was studied in 225 patients with EGFR-mutated locally advanced or metastatic NSCLC following disease progression with an EGFR-TKI and platinum-based chemotherapy, which demonstrated an ORR of 29.8%. The median duration of response was 6.4 months.342 An analysis of 48 paired pretreatment and posttreatment tumor samples showed augmented HER3 expression in EGFR-mutant tumors with acquired resistance to EGFR-TKIs343 and that EGFR inhibition increased membrane expression of HER3 and led to enhanced efficacy of HER3-DXd,344 thus supporting the rational combination of osimertinib and HER3-DXd in EGFR-mutant NSCLC. The activity of patritumab deruxtecan in patients with previously treated EGFR-mutant NSCLCs is currently being investigated further, both as a monotherapy (NCT04619004) and in combination with osimertinib (NCT04676477). BL-B01D1, a bispecific topoisomerase inhibitor-based ADC that targets both EGFR and HER3, is currently being evaluated in a phase I study (BL-B01D1-LUNG101; NCT05194982) for safety and efficacy in individuals with metastatic or unresectable NSCLC. Data from earlier clinical studies of BL-B01D1 were presented in 2023 at the American Society of Clinical Oncology, European Society for Medical Oncology (ESMO) and the San Antonio Breast Cancer Symposium; these data demonstrated impressive antitumor activity in patients with EGFR-TKI resistance, with an ORR of 63.2%.345 Both EGFR and HER3 are highly expressed in most epithelial tumors; thus, BL-B01D1 may have promising antitumor activity in patients with a range of solid tumors.
Trastuzumab deruxtecan (DS-8201, known as dequdacimab), a HER2-targeted ADC, has shown significant clinical efficacy in patients with lung cancer who harbor HER2 gene alterations. The treatment of advanced HER2-mutant NSCLC has historically been guided by the approach for advanced NSCLC without driver gene mutations. HER2-TKIs and anti-HER2 antibodies have shown limited efficacy in this setting,346, 347 while ADC drug have achieved breakthrough progress in HER2-mutant NSCLC. The DESTINY-Lung01 and DESTINY-Lung02 studies have demonstrated the efficacy of DS8201 in HER2-mutant NSCLC who have progressed after standard treatment or targeted HER2-TKI therapy, with ORR of 54.9 and 53.8%, respectively, and a median duration of response of 8.2 months.348, 349 In the DESTINY-PanTumor02 trial, which included 267 patients covering various tumor types such as endometrial cancer, cervical cancer, ovarian cancer, bladder cancer, and bile duct cancer, DS-8201 demonstrated significant efficacy in multiple HER2-positive solid tumors. In IHC3+ HER2-positive patients, the ORR reached an impressive 61.3%, with a PFS of 11.9 months and a median duration of response (DOR) of 22.1 months.350 Based on this study, on April 5, 2024, the US FDA granted accelerated approval to trastuzumab deruxtecan for previously treated and no further treatment options available, unresectable or metastatic HER2-positive (IHC3+) solid tumors. This marks a significant breakthrough for DS8201, allowing for potential treatment across multiple cancer types.
The potential role of alternative ADCs targeting the human trophoblast cell-surface antigen 2 (TROP2) (NCT04152499) and MET351, 352 (NCT03859752) is also being evaluated in patients with lung cancers, potentially including NSCLCs with EGFR mutations or ALK rearrangements. TROP2 is highly expressed in various solid tumors and is associated with worse prognosis.353 It has emerged as a hot target in the field of oncology in recent years, and the development of ADCs targeting this antigen has garnered significant attention. SKB264 (MK-2870) is a novel anti-TROP2 ADC. In a phase II study, 22 cases of EGFR mutation patients were enrolled who experienced progression during or after TKI treatment, 50% of patients had experienced at least one chemotherapy failure. The ORR was 60%, with a median PFS of 11.1 months.354 The ongoing phase III MK-2870-004 study (NCT06074588) will evaluate the efficacy and safety of MK-2870 versus chemotherapy for previously TKI-treated NSCLC with EGFR mutations or other genomic alterations in ALK, ROS1, BRAF V600E, NTRK, MET exon 14 skipping, or RET and those with less common EGFR mutations (exon 20 S768I, exon 21 L861Q, and/or exon 18 G719X). ABBV-399 (also called Telisotuzumab-Vedotin) is currently the only MET-targeting ADC that has entered phase III clinical trials. In January 2022, ABBV-399 was granted breakthrough therapy designation by the US FDA for the treatment of advanced or metastatic MET overexpressing EGFR WT nonsquamous NSCLC patients who have progressed after platinum-based therapy. In the single-arm phase II LUMINOSITY trial (NCT03539536), ABBV-399 demonstrated promising results in the treatment of MET protein overexpressing, EGFR WT, advanced/metastatic non-squamous NSCLC. The ORR was 53.8% in the high MET expression group and 25.0% in the moderate MET expression group, with a median duration of response of 9 and 7.2 months, respectively.355 ABBV-399 is not the only MET ADC drug in development, others like MYTX-011(NCT05652868), SHR-A1403(NCT03856541), TR1801(NCT03859752), and more are also in clinical trial stages. Targeting MET with ADCs provides a new treatment option for patients who develop MET overexpression after developing resistance to previous therapies.
3.4 PROteolysis targeting chimeras
PROteolysis targeting chimeras (PROTAC) is a molecular tool designed for targeted protein degradation. It is a heterobifunctional compound typically composed of three main parts: a binding moiety to target the protein of interest, a binding moiety to recruit the cellular ubiquitin E3 ligase, and a linker.356, 357 The mechanism of PROTAC relies on the dual binding of the target protein and the E3 ligase. When PROTAC binds to both the target protein and the E3 ligase, it forms a ternary complex. The E3 ligase then ubiquitinates the target protein, attaching a chain of ubiquitin molecules to it. The ubiquitinated target protein is recognized and degraded by the cell's ubiquitin-proteasome system, ultimately leading to the degradation of the target protein.358, 359 Traditional small molecule drugs may require higher drug concentrations to effectively inhibit the activity of the target protein, whereas PROTAC molecules can be reused after the degradation of ubiquitinated proteins, resulting in lower effective drug concentrations needed for PROTAC action.360, 361 Additionally, compared with traditional reversible inhibition, targeted degradation provides a longer duration of continuous blockade of the target protein, as the degraded protein needs to be resynthesized.360, 361 These advantages make PROTAC particularly suitable for addressing resistance caused by acquired mutations or amplifications in cancer.
Targeting proteins for proteasomal degradation using the PROTAC approach is now widely pursued in clinical development, mainly targeting proteins in cancer (e.g., BCR–ABL,362, 363 EGFR,361, 364, 365 androgen receptor (AR),366-368 estrogen receptor (ER),369, 370 bromodomain-containing protein 4 (BRD4),371 or Bruton's kinase (BTK)372, 373). Bavdegalutamide (ARV-110) is an orally active, specific AR PROTAC degrader, which is in development for the treatment of men with metastatic castration-resistant prostate cancer (mCRPC) who have progressed on abiraterone and/or enzalutamide.374 Phase I/II study of ARV-110 (NCT0388861) demonstrated clinical activity in heavily pretreated mCRPC, with the greatest PSA50 activity and RECIST responses in patients with AR T878 and/or H875 mutations.368 Another example that demonstrates the clinical impact of this strategy is the BTK degrader developed to tackle on-target resistance to BTK inhibitors. The most common resistance mechanism in patients whose disease progresses on covalent BTK inhibitors is a mutation in the BTK C481 residue, abrogating the covalent binding of irreversible BTK inhibitors such as ibrutinib.91, 72 PROTAC technology itself does not require a strong affinity between the binder and the target protein. In fact, it has been shown that PROTAC molecules with ibrutinib as the binder, although having weaker binding to BTK C481S, can induce degradation of the BTK protein.375 This indicates that protein kinase conferring resistance to anticancer drugs may be targeted using the PROTAC approach.
4 FUTURE DIRECTIONS IN RESEARCH AND CLINICAL PRACTICE
The discovery of actionable driver oncogenes and TKIs launched a paradigm shift in cancer treatment. As TKI-targeted therapy continues to evolve, future directions emphasize several pivotal areas of research and development aimed at overcoming resistance and improving treatment efficacy. Here, we focus on discussing future development strategies in TKI-targeted therapy, including the monitoring of resistance through liquid biopsies, the development of novel kinase inhibitors, and the new nanoscale delivery systems.
4.1 Methods for detecting and monitoring resistance during treatment
Early monitoring of drug resistance mutations can determine the best treatment regimen, particularly with the development of high-quality detection methods in recent years. The current approach to disease management revolves around tumor-specific, stage-based guidelines, primarily relying on pathology and imaging outcomes to optimize patient treatment plans.376 Imaging techniques such as CT scans, MRI, or PET scans are valuable for monitoring disease progression or relapse and assessing treatment response; however, interpreting radiological findings can be challenging, resulting in high rates of false positives and negatives.377 Serial monitoring of tumor markers, such as carcinoembryonic antigen, cancer antigen (CA)-125, CA19-9, PSA, and lactate dehydrogenase, offers a noninvasive means to assess treatment response. However, many of these established biomarkers are deemed unreliable due to potential elevation from noncancer-related conditions, leading to reduced sensitivity and specificity.378-380 NGS allows for the simultaneous analysis of multiple genes and molecular alterations in tumor samples. This provides a comprehensive view of the genomic landscape of cancer, including driver mutations, copy number alterations, and gene fusions.381 Currently, NGS testing is based on tissue biopsy at the time of diagnosis and, when possible, a rebiopsy at the time of progression remains the gold standard, providing direct analysis of genetic mutations or alterations indicative of resistance.382 However, tissue samples are not always feasible, and the sample amount is often insufficient for molecular detection.383 Additionally, tissue samples are not often enough to systematically represent the mutational status of the entire tumor due to tumor heterogeneity and clonal dynamics. In contrast, liquid biopsy offers a minimally invasive alternative, analyzing circulating tumor cells or cell-free DNA to detect real-time genetic changes associated with resistance.384 Dynamic sequencing analysis of circulating tumor DNA (ctDNA) has multiple potential clinical applications, including screening, distinguishing early-stage diseases, detecting molecular residual disease after curative surgery, predicting recurrence, genotyping advanced tumors, early assessment of treatment efficacy, monitoring treatment response, and identifying mechanisms of resistance, serving as an effective alternative to tissue-based genetic testing.385-388 For example, in the monitoring of advanced colorectal cancer, ctDNA enables the dynamic and repeated assessment of changes in multiple key molecules of the tumor, such as EGFR, ERBB2, PIK3CA, and MAP2K1, among others. This facilitates the identification of novel potential therapeutic targets and mechanisms of resistance to targeted therapies, including anti-EGFR, anti-BRAF, and anti-HER2 drugs. Studies have demonstrated a high concordance between ctDNA detection results and tissue biopsy outcomes.386-388 Moreover, ctDNA is advantageous due to its ability to capture molecular heterogeneity within and between tumors, unrestricted by sampling location.389, 390 Dynamic sequencing of ctDNA was also able to decode the evolutionary response of NSCLC patients who received targeted therapy. This approach effectively guides further research directions and clinical treatment practices.391, 392 In metastatic breast cancer, ctDNA demonstrates higher accuracy compared with standard serum markers like CA15-3. Dynamic changes in ctDNA are associated with PFS during chemotherapy, endocrine therapy, and combination targeted therapy.393-396 Additionally, sequential ctDNA analysis can also be employed to assess genomic mechanisms of resistance that arise before clinical progression. In colorectal cancer patients receiving anti-EGFR monoclonal antibody therapy, RAS and EGFR-extracellular domain mutations were detected in ctDNA 10 months before radiographic progression, and these mutations disappeared after discontinuation of anti-EGFR therapy.397, 398 For advanced breast cancer patients receiving aromatase inhibitor (AI) and CDK4/6 inhibitor therapy, monitoring the emergence of estrogen receptor 1 (ESR1) mutations holds potential clinical utility. In the PADA-1 trial, patients with detected ESR1 mutations were randomized to receive fulvestrant with continued CDK4/6 inhibitor therapy, when this group was compared with those continuing AI and CDK4/6 inhibitor therapy, an improvement in PFS was observed. The PFS benefit observed with early fulvestrant use after detection of ESR1 mutations may not be compensated for by later fulvestrant use after disease progression.399 These data suggest that detecting resistance-related mutations in ctDNA earlier rather than later (at radiographic progression) may have more decision-making significance. Although ctDNA holds immense potential as a minimally invasive biomarker for tumor monitoring, its clinical application still faces several limitations and challenges. The optimal timing for dynamic assessment of ctDNA and the most accurate efficacy prediction thresholds require further investigation. To monitor the emergence of resistance mutations before clinical progression, further research is needed to determine the frequency of monitoring. Randomized intervention studies are necessary to assess whether changing treatment based on dynamic ctDNA assessment can improve prognosis or even replace current standard clinical metrics.
4.2 Identification of novel targets
The future of kinase inhibitors partly depends on the identification of novel targets. Current targeted kinase inhibitor therapies focus mostly on known tyrosine kinases, yet there are upward of 500 total kinases in the human genome. During the past 5 years, the number of small molecule kinase inhibitors entering clinical development has increased by over 200%.229 Currently, investigational kinase inhibitors in clinical trials target approximately 110 new kinase targets, accounting for 20% of the human kinome,229 reflecting the active exploration of innovative targets in the kinase field.
Aurora kinase family (Aurora kinase A, Aurora kinase B and Aurora kinase C) has been the most targeted kinase family in oncology, with over 20 drugs entering clinical trials. Aurora kinases are crucial mitotic regulators required for genome stability, and they are often overexpressed in human cancers such as breast cancer, ovarian cancer, gastrointestinal cancer, and this abnormal expression is associated with poor prognosis in cancer patients.400 In recent years, increasing evidence suggests that Aurora inhibitors hold promise for cancer therapy and overcoming resistance to inhibitors targeting CDK4/6 and EGFR.401, 402 Even though Aurora inhibitors hold treatment potential in oncology, so far, no Aurora kinase inhibitor has been approved for clinical use because of the limited clinical efficacy and drug toxicity. Currently, second-generation, isoform-selective Aurora kinase inhibitors are being investigated as monotherapies or in combinations (etc., LY3295668 combined with osimertinib (NCT05017025), MLN8237 and bortezomib in treating multiple myeloma (NCT010345535), VIC-1911 combined with lenvatinib (NCT05718882)) for hematological malignancies and solid tumors.
The CDK family has been a therapeutic target for over 20 years because of its critical roles in controlling cell division, and cancers often show dysregulation of the cell cycle.403 From 2015 to 2017, selective inhibitors targeting CDK4 and CDK6 gained US FDA approval for treating breast cancer, including palbociclib, abemaciclib, and ribociclib. Members of the CDK family continue to be a hotspot for the development of small-molecule kinase inhibitors. Apart from CDK4 and CDK6, there are currently multiple small molecule inhibitors targeting CDK7, CDK8/19, CDK2, and CDK9. CDK2, a critical regulator of the cell cycle, is a promising drug target, especially in cancers with cyclin E1 protein overexpression, including HR+/HER2− breast cancer resistant to CDK4/6 inhibitors.404 Multiple companies, including AstraZeneca, Pfizer, and Blueprint Medicines, are actively developing CDK2 inhibitors. Currently, several CDK2 inhibitors have entered clinical trial stages, being tested both as monotherapy and in combination with CDK4/6 inhibitors.
Hematopoietic progenitor kinase 1 (HPK1) is a family member of mitogen-activated protein kinase kinase kinase kinase, which is a kinases that catalyze the phosphorylation of serine or threonine residues in proteins.405, 406 HPK1 is an intracellular negative regulator of T/B-cell proliferation and signaling. HPK1 kinase knockdown mice demonstrate increased CD8+ T-cell killing function and robust antitumor immune responses even in a cold tumor environment, making HPK1 a high-priority target in immuno-oncology.407, 408 Several lines of preclinical evidence have been collected over the past years that support the development of compounds targeting HPK1, especially in combination with anti-PD-1/PD-L1 ICIs. In 2020, Treadwell Therapeutics' HPK1 inhibitor CFI-402411 became the first to enter phase I clinical trials (NCT04521413). Subsequently, orally highly selective HPK1 inhibitors such as Pfizer's PF-07265028 (NCT05233436), BeiGene's BGB-15025 (NCT04649385), and Zhuhai Yufan Biotechnologies’ PRJ1-3024 (NCT05315167) have entered phase I/II clinical trials. These inhibitors are primarily being investigated as monotherapy or in combination with PD-1/PD-L1 inhibitors for the treatment of advanced solid tumors.
SHP2 is a phosphatase that dephosphorylates substrate proteins. It is a “star target” in the field of cancer therapy. In the RTK pathway, SHP2 effects are upstream of RAS, in mediating tumor proliferation. Virtually all RTKs initiate the RAS signaling pathway by activating SHP2. Thus, inhibitors of SHP2 can block the RTK/RAS/MAPK signaling pathways and inhibit the growth and proliferation of tumor cells driven by RTK or with KRAS, BRAF Class 3 and NF1 loss of function mutations.245-248, 409 SHP2 effects are also downstream of the immune checkpoint regulator, PD-1, in inhibiting the antitumor effect of T cells.410-412 Given the clinical success of anti-PD-1/PD-L1 immunotherapy, SHP2 inhibitors serve as small molecule tumor immunotherapeutic agents and are considered important complements to PD-1/PD-L1 inhibitors, holding great promise in clinical practice. SHP2 also functions downstream of colony stimulating factor 1 receptor, in promoting TAM activity. Thus, targeting SHP2 has the potential for multiple antitumor effects. Currently, there are no drugs targeting the SHP2 target approved for market, but several SHP2 inhibitors have entered clinical trials. These include Novartis’ TNO155 (NCT03114319), Sanofi's RMC-4630 (NCT04916236), Etern BioPharma's ET0038 (NCT05354843) and Jacobio Pharma's JAB-3312 (NCT04720976). The main indications for these inhibitors include combination therapy with KRAS G12C inhibitors, EGFR inhibitors, BRAF inhibitors, MEK inhibitors, ERK inhibitors, and anti-PD-1/PD-L1 antibodies. Among them, the most rapidly advancing is JAB-3312, which has become the world's first SHP2 inhibitor to be approved for phase III pivotal clinical trials in combination with KRAS G12C inhibitors, leading the field of SHP2 inhibitors. Data presented at the 2023 ESMO Congress revealed that in the dose group combining the KRAS G12C inhibitor sotorasib with the SHP2 inhibitor JAB-3312, the objective response rate was 50%, with a disease control rate of 100%.413
At least 70% of the kinome is still unexplored in drug development. New large-scale screening methods such as large-scale quantitative proteomic screens and scRNA-seq may help identify transcript levels of kinases that drive clonal evolution or transcriptional reprogramming, holding promise for the development of novel targeted kinase inhibitor therapies.
4.3 Dual-acting kinase inhibitors
As mentioned above, combination therapy theoretically may have additive or synergistic effects; however, it often leads to unpredictable side effects, such as increased toxicity. As an alternative strategy to combination therapy, dual-target or multitarget drugs are considered to have lower risks of drug–drug interactions and better pharmacokinetics (PK) and safety profiles. Compared with combination therapy, these drugs mitigate issues such as reduced bioavailability, metabolism, and antagonistic effects. The advantage of this approach lies in its ability to simultaneously target multiple points, thereby providing a more comprehensive therapeutic effect against complex disease biology processes. By reducing interactions between drugs, dual-target or multitarget drugs may help reduce the risk of side effects and improve the safety and tolerability of treatment. Currently, many dual-target drugs have been investigated in cancer treatment. For example, alectinib is a dual-target small molecule inhibitor that simultaneously targets ALK and RET. Next-generation RET-TKI TPX-0047 simultaneously targets RET and SRC.414 The dual-target CHK1/FLT3 inhibitor TLX83 can overcome adaptive and acquired resistance to FLT3 inhibitors, offering a new strategy for patients with FLT3-ITD-mutant acute myeloid leukemia (AML).415 The development of EGFR dual-target inhibitors is a promising strategy to decrease the potential drug resistance of EGFR-TKIs induced by aberrant alternative signaling pathways. Preclinical studies have shown that the combination of erlotinib and HDAC inhibitors promotes apoptosis in TKI-resistant NSCLC cells.416 CUDC-101, a multitarget HDAC/EGFR/HER2 inhibitor, has shown effective inhibitory activity against advanced solid tumors in vivo.417 Phase I study (NCT00728793) demonstrated CUDC-101 was generally well tolerated with preliminary evidence of antitumor activity.418 A new MEK inhibitor currently in clinical trials for metastatic melanoma (NCT04720417) simultaneously inhibits RAF feedback activity. So far, various design strategies for dual-target inhibitors targeting different tumor types and pathways have been reported.
As mentioned above, the future development of kinase inhibitors faces challenges beyond the discovery of new targets, particularly in developing compounds with high clinical efficacy and low risk of off-target toxic side effects and resistance. In this regard, developing inhibitors with different binding modes, such as allosteric and covalent inhibitors, as well as developing dual-acting kinase inhibitors, or targeted protein degraders, may play a crucial role in kinase drug development in the next 20 years.
4.4 Nanoparticle-based drug delivery systems
The combination of nanotechnology and targeted therapy has emerged as a promising field in cancer research. Nanoparticle-based drug delivery systems, including polymeric, lipid, carbon nanotubes, dendrimer, and micellar nanoparticles have been extensively designed and investigated in preclinical cancer treatment.419-421 Compared with the conventional delivery routes, nanoparticle-based drug delivery systems possess some unique advantages with improved PKs, increased efficacy, and reduced off-target cytotoxicity. For example, nanoparticle-based drug delivery systems can be utilized to codeliver two different drugs or reagents concurrently. A codelivery system of afatinib and paclitaxel was designed for the treatment of EGFR-TKI-resistant NSCLC.422 The results of this study showed that afatinib and paclitaxel from the system can sustain high concentrations in the lung for up to 96 h, while maintaining lower concentrations in other tissues in Sprague–Dawley rats.422 Besides, as mentioned above, hypoxia in solid tumors can mediate acquired resistance to TKI therapy. Li et al.423 codelivered oxygen-releasing perfluorooctylbromide with erlotinib in liposomal nanoparticles to address hypoxia-induced drug resistance. They found the codelivery system could significantly improve the efficacy of erlotinib when compared with nanoparticles loaded with erlotinib alone.423
However, nearly all US FDA-approved nanomedicines are formulated for intravenous (i.v.) use, while the majority of TKIs are designed for oral. More advanced nanocarriers capable of overcoming the challenges of oral administration will be needed. In addition, most of these studies are limited to preclinical settings. Nano-applications of TKIs are still in their infancy stages of development, and yet to be validated in clinical practice. Despite their immense potential, they still have a considerable journey ahead of them.
4.5 Targeting drug-tolerant persistent cells (DTP cells)
In recent years, DTP cells have gained considerable attention as a promising area in cancer research, particularly regarding their role as nongenetic mechanisms of therapeutic resistance.424 DTP cells are a subpopulation of cancer cells that survive exposure to targeted therapy, despite the treatment being initially effective against the bulk of tumor cells.425 These cells can enter a quiescent or slow-proliferative state, allowing them to evade the cytotoxic effects of the drug.425, 426 While DTP cells may initially respond to treatment, they can eventually lead to disease recurrence or the development of drug resistance.425, 426 Understanding and targeting DTP cells is crucial for improving the long-term efficacy of cancer-targeted therapies.
In cancer, DTP cells were first found and described in the EGFR-mutant NSCLC cell line PC9 by Sharma and colleagues in 2010.427 They observed that these cells exhibited over 100-fold lower drug sensitivity and could revert to their original state upon drug withdrawal.427 An epigenetic regulator, histone demethylase KDM5A, was found to be required for the establishment of DTP cells, and histone deacetylase (HDAC) inhibitors could ablate the generation of DTP cells.427 Subsequently, DTP cells have been identified in various experimental models after targeted therapy, including additional EGFR-mutant NSCLC cell lines, MET-amplified gastric cancer, BRAF-mutant melanoma, prostate cancer, colorectal cancer, and HER2-positive breast cancer.428-433 Two common phenotypic features of DTP cells, including slow proliferation and reversible biological capacity, have been well documented in different studies.434 However, the mechanisms responsible for regulating DTP state are context-specific across various cancer models and therapeutic approaches, which include diverse epigenetic reprogramming, transcriptional regulation, and metabolic remodeling.425, 434
Despite accumulating evidence regarding the existence of DTP cells and the putative mechanisms underlying the regulation of DTP state, universally accepted biomarkers for DTP cells remain elusive. The definition of cancer persistent cell state is yet to be established. Besides, most studies characterized DTP cells by using in vitro cell line experimental models,427, 428 with few studies employing in vivo patient-derived xenograft mouse models.431, 435 Data on patients are still limited. Our clinical understanding of their roles in cancer treatment resistance remains unclear, and therapeutic strategies targeting DTP cells have yet to be explored. Based on the concept of DTP cells, a potential therapeutic strategy of withdrawing targeted therapy and enabling tumor repopulation with drug-sensitive cells has been discussed. However, tumor evolution is unpredictable. What's more, clinical experience over decades has demonstrated attempting to reintroduce the same therapy to a patient is consistently futile.424 Additionally, DTP cells have been proven to be highly heterogeneous and context-specific. With the advancement of single-cell multiomics technologies, including spatial transcriptomics, epigenomics, proteomics, and metabolomics, a more comprehensive understanding of DTP cells may be established and elucidated.
4.6 Investigating resistance mechanisms at single-cell resolution
Most resistance mechanisms discussed above were uncovered through the analysis of bulk tumor specimens. Considering that tumors are highly heterogeneous, bulk-tumor analysis may not completely reveal the entire spectrum of resistance mechanisms.436 Single-cell analyses of matched tumor specimens or cell line models before and after treatment are informative in identifying potential mechanisms of TKI-therapy resistance in various studies.432, 437-440 For example, by performing single-cell RNA sequencing (scRNA-seq) and single-cell ATAC-sequencing analysis on both TKI-resistant cells and clinical specimens, Kashima and colleagues identified CD74 as a novel key factor in regulating tumor drug-tolerant state.437 Although single-cell RNA-seq holds promise in deciphering the transcriptomic profiles of tumor subclones, it lacks coverage of critical mutation information in cells. As we discussed above, most well-reported resistance mechanisms are based on secondary mutations. An innovative genomic technology for single-cell mutational analysis and concurrent transcriptomic sequencing will allow for further understanding of molecular mechanisms underlying treatment resistance, by providing insights into dysregulated pathways in both mutant and nonmutant tumor cells. Alba and colleagues developed such a method for the detection of hotspot mutations, in parallel with unbiased RNA-seq analysis within single cells.441 However, this method has not yet been applied to comprehensively explore resistance mechanisms of cancer treatment.
Multiomics techniques, particularly NGS and scRNA-seq, have revolutionized our understanding of tumor biology by uncovering new biomarkers and molecular regulators linked to oncogenesis, metastasis, and drug resistance.438-440, 442 Nonetheless, these methods have limitations as they do not capture the spatial organization of cells within the TME, thus providing an incomplete picture of tumor biology. There is an increasing demand for image-based methodologies with single-cell resolution to visualize intratumor heterogeneity, cell–cell interactions, and protein expression information within the TME. Emerging technologies that utilize multiplexed fluorescence, RNA, DNA, and isotope labeling now enable the deep investigation of cancer biology within their spatial context.443, 444 By conducting single-cell multiomic assays including nucleus RNA, open-chromatin, spatial transcriptomics, spatial proteomics and exome sequencing, on 86 matched primary-recurrent tumor specimens, Lin and colleagues comprehensively described a single-cell atlas of glioblastoma evolution under treatment.445
While multiomics techniques offer immense potential in cancer research, several challenges hinder their widespread application. One major obstacle is the resistance mechanisms identified by multiomics techniques are usually individual-specific and context-specific, making it difficult to develop universal therapeutic approaches applicable to all patients. Moreover, the cost and time required for multiomic analyses are substantial, limiting their accessibility and scalability. Besides, tumors exhibit high levels of heterogeneity, both within and between tumors. Thus, single-cell resolution is crucial for capturing this heterogeneity, but it also introduces complexity to data analysis, further complicating the interpretation of results. Overcoming these challenges will require advancements in technology, computational methods, and collaborative efforts within the scientific community to fully leverage the potential of single-cell multiomic technologies in cancer research.
5 CONCLUSIONS
TKI resistance poses significant clinical challenges, with multiple resistance mechanisms now elucidated. Recent progress in developing next-generation TKIs, combination therapies, ADCs, and PROTACs has shown promise in overcoming these mechanisms, demonstrating encouraging results in clinical trials. However, many cancers treated with TKIs, such as NSCLC, exhibit high heterogeneity, leading to inevitable drug resistance. There is still an urgent need for novel therapeutic strategies either targeting TKI resistance or employing de novo strategies to extend patients’ lives and improve their life quality of life while living with cancer. The advancement of new technologies, including liquid biopsy, nanotechnologies, image-based single-cell methodologies, and multiomics approaches, holds potential for deeper insights into TKI resistance and tumor biology. These advancements can facilitate the development of innovative cancer treatment strategies.
AUTHOR CONTRIBUTIONS
Xuejin Ou and Ge Gao wrote the manuscript. Inbar A. Habaz conducted a thorough review of relevant literature and provided critical revisions to the manuscript. Yongsheng Wang conceived the idea for the review paper, provided funding support, and edited the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 81872489 to Yongsheng Wang) and China Postdoctoral Science Foundation (2024M752235 to Ge Gao).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known financial or nonfinancial interests that are directly or indirectly related to the work submitted for publication.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




