Spo0J and SMC are required for normal chromosome segregation in Staphylococcus aureus
Graphical Abstract
Chromosome segregation is an essential but poorly understood process in spherical bacteria such as Staphylococcus aureus. Here, we show that the staphylococcal ParB homologue, Spo0J, and the structural maintenance of chromosomes (SMC) protein act in concert to achieve efficient chromosome segregation in S. aureus. We demonstrate that Spo0J and SMC form a complex that is dependent on correct localization of Spo0J and propose that additional mechanisms are likely involved in accurate staphylococcal chromosome segregation.
Abstract
Bacterial chromosome segregation is an essential cellular process that is particularly elusive in spherical bacteria such as the opportunistic human pathogen Staphylococcus aureus. In this study, we examined the functional significance of a ParB homologue, Spo0J, in staphylococcal chromosome segregation and investigated the role of the structural maintenance of chromosomes (SMC) bacterial condensin in this process. We show that neither spo0J nor smc is essential in S. aureus; however, their absence causes abnormal chromosome segregation. We demonstrate that formation of complexes containing Spo0J and SMC is required for efficient S. aureus chromosome segregation and that SMC localization is dependent on Spo0J. Furthermore, we found that cell division and cell cycle progression are unaffected by the absence of spo0J or smc. Our results verify the role of Spo0J and SMC in ensuring accurate staphylococcal chromosome segregation and also imply functional redundancy or the involvement of additional mechanisms that might contribute to faithful chromosome inheritance.
1 INTRODUCTION
Staphylococcus aureus is a Gram-positive, spherical bacterium that divides in three consecutive, orthogonal planes (Monteiro et al., 2015; Pinho, Kjos, & Veening, 2013; Tzagoloff & Novick, 1977). As is the case for all bacteria, replicated chromosomes must be efficiently segregated to ensure accurate inheritance of genetic material (Badrinarayanan, Le, & Laub, 2015; Bloom & Joglekar, 2010; Graumann, 2014; Toro & Shapiro, 2010; Wang, Llopis, & Rudner, 2013). How this is achieved in dividing S. aureus cells is still poorly understood.
The prototypical DNA segregation system, parABS, was first identified and studied on prophage and Escherichia coli plasmids (Baxter & Funnell, 2014; Gerdes, Howard, & Szardenings, 2010; Hayes & Barilla, 2006; Oliva, 2016; Salje, 2010; Schumacher, 2012), however, homologues are also present on most bacterial chromosomes (Livny, Yamaichi, & Waldor, 2007). The parABS system consists of a Walker-type ATPase (ParA/Soj), a DNA-binding protein (ParB/Spo0J), and repetitive centromere-like sequences (parS) (Baxter & Funnell, 2014; Gerdes et al., 2010; Salje, 2010). Generally, ParB proteins bind specifically to parS sites that are located in the origin-proximal region to form a nucleoprotein complex (Breier & Grossman, 2007; Lin & Grossman, 1998; Minnen, Attaiech, Thon, Gruber, & Veening, 2011; Murray, Ferreira, & Errington, 2006). ParA proteins bind nonspecifically to nucleoid DNA and interact with parS-bound ParB proteins (Lutkenhaus, 2012; Vecchiarelli, Hwang, & Mizuuchi, 2013; Vecchiarelli, Neuman, & Mizuuchi, 2014). Recently, it was found that ParB binds cytidine triphosphate (CTP) and exhibits parS-dependent CTP hydrolysis to modulate parS binding and recruitment of ParA (Osorio-Valeriano et al., 2019; Soh et al., 2019). Interaction with ParB stimulates the ATPase activity of ParA and releases ParA from the ParB/parS complex, ultimately creating a gradient of ParA that drives DNA segregation toward the cell poles (Hatano & Niki, 2010; Sanchez, Rech, Gasc, & Bouet, 2013; Vecchiarelli et al., 2014).
An S. aureus Spo0J homologue exhibits 47% identity with Bacillus subtilis Spo0J. However, similar to Streptococcus pneumoniae, S. aureus does not encode a ParA/Soj homologue, suggesting that the mechanism of chromosome segregation in S. aureus may deviate from canonical mechanisms described thus far.
Studies in S. pneumoniae and B. subtilis show that the structural maintenance of chromosomes (SMC) protein is recruited to origin-proximal sites through interaction with ParB/Spo0J (Gruber & Errington, 2009; Minnen et al., 2011; Sullivan, Marquis, & Rudner, 2009). SMC (MukB in E. coli) is a condensin that, in complex with ScpA and ScpB, contributes to chromosome compaction and organization (Britton, Lin, & Grossman, 1998; Mascarenhas, Soppa, Strunnikov, & Graumann, 2002; Moriya et al., 1998; Wang, Tang, Riley, & Rudner, 2014). In the absence of both B. subtilis Spo0J and SMC, chromosome segregation defects are amplified compared to the absence of either gene alone, suggesting a role for Spo0J and SMC interaction in chromosome segregation (Britton et al., 1998; Lee & Grossman, 2006; Wang et al., 2014). A previous study has shown that SMC also contributes to staphylococcal chromosome segregation, since a significant proportion of cells lacking SMC were anucleate (Yu, Herbert, Graumann, & Götz, 2010). However, little is known about the functional significance of S. aureus Spo0J and its interactions, if any, with SMC.
Based on the function of ParB/Spo0J proteins, we sought to verify the role of Spo0J in staphylococcal chromosome segregation and its potential role in SMC function. We used super-resolution structured illumination microscopy (SIM) to confirm that Spo0J is indeed involved in S. aureus chromosome segregation, but found that it is not essential for growth and viability. We also show that Spo0J is required for correct localization of SMC and that both proteins act in concert to ensure efficient chromosome segregation, however, a role in cell division was not immediately evident. Our data indicate the involvement of additional interactions that influence S. aureus chromosome segregation and cell division, suggesting redundancy or overlapping roles of proteins involved in these essential processes.
2 MATERIALS AND METHODS
2.1 Bacterial strains and growth
Bacterial strains used in this study are listed in Table 1. Molecular cloning and plasmid propagation were performed using E. coli DC10B cells grown in Luria-Bertani (LB) broth (Sigma-Aldrich). Staphylococcus aureus RN4220 strains were grown in tryptic soy broth (TSB; Becton Dickinson Bacto™), minimal media (SSM9PR: 1 × M9 salts [6.8 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 1 g/L NH4Cl], 2 mM MgSO4, 0.1 mM CaCl2, 1% (w/v) glucose, 1% (w/v) casamino acids, 1.5 µM thiamine, 0.05 mM nicotinamide), or on brain heart infusion (BHI) agar (Becton Dickinson Difco™). Liquid cultures were routinely grown at 37°C with aeration, unless otherwise specified.
| Strain or plasmid | Relevant genotype or description | Reference |
|---|---|---|
| Escherichia coli | ||
| DC10B | E. coli K-12 (DH10B) derivative. Δdcm dam+ ΔhsdRMS endA1 recA1 | Monk et al. (2012) |
| BTH101 | F−, cya-99, araD139, galE15, galK16, rpsL1 (Strr), hsdR2, mcrA1, mcrB1 | Karimova et al. (1998) |
| Staphylococcus aureus | ||
| RN4220 | Restrictionless derivative of NCTC 8325–4 | Kreiswirth et al. (1983) |
| HC061 | RN4220 Δspo0J | This study |
| HC080 | RN4220 Δsmc | This study |
| HC094 | RN4220 Δspo0J Δsmc | This study |
| Plasmids | ||
| pHC052 | pSK1 ori, AmpR, NeoR, Pxyl/tetO-spo0J-mRFPmars | This study |
| pHC061 | pMAD containing S. aureus RN4220 spo0J upstream and downstream regions, AmpR, EryR | This study |
| pHC067 | pSK41 ori, AmpR, EryR, Pspac-smc-gfp | This study |
| pHC080 | pMAD containing S. aureus RN4220 smc upstream and downstream regions, AmpR, EryR | This study |
| pHC102 | pSK41 ori, AmpR, EryR, Pspac-gfp | This study |
| pHC111 | pKNT25 derivative encoding Spo0J-T25, KanR, Plac-spo0J-T25 | This study |
| pHC112 | pKNT25 derivative encoding SMC-T25, KanR, Plac-smc-T25 | This study |
| pHC114 | pUT18 derivative encoding Spo0J-T18, AmpR, Plac-spo0J-T18 | This study |
| pHC115 | pUT18 derivative encoding SMC-T18, AmpR, Plac-smc-T18 | This study |
| pKNT25 | Encodes T25 fragment (residues 1–224) of adenylate cyclase, CyaA, fused in frame downstream of MCS, KanR | Karimova et al. (1998) |
| pKT25-zip | pKT25 derivative encoding the leucine zipper of GCN4, KanR, Plac-T25-zip | Euromedex |
| pUT18 | Encodes T18 fragment (residues 225–399) of adenylate cyclase, CyaA, fused in frame downstream of MCS, AmpR | Karimova et al. (1998) |
| pUT18C-zip | pUT18C derivative encoding the leucine zipper of GCN4, AmpR, Plac- T18-zip | Euromedex |
| pMAD | Allelic replacement vector; pBR322 and pE194ts origins of replication in E. coli and staphylococci, AmpR, EryR | Arnaud, Chastanet, and Débarbouillé (2004) |
| pSK9065 | E. coli–S. aureus shuttle vector carrying low-copy number S. aureus pSK1 origin of replication and anhydrotetracycline-inducible Pxyl/tetO promoter, AmpR, NeoR | Brzoska and Firth (2013) |
| pSK9067 | E. coli–S. aureus shuttle vector carrying low-copy number S. aureus pSK41 origin of replication and IPTG-inducible Pspac promoter and optimized lacOid operator, AmpR, EryR | Brzoska and Firth (2013) |
Note
- AmpR, confers ampicillin resistance; EryR, confers erythromycin resistance; KanR, confers kanamycin resistance; NeoR, confers neomycin resistance; ori, origin of replication.
Plasmids were introduced into chemically competent E. coli DC10B or BTH101 cells using heat shock (Sambrook & Russell, 2001). Plasmids isolated from E. coli DC10B strains were introduced into S. aureus RN4220 cells via electroporation (Löfblom, Kronqvist, Uhlén, Ståhl, & Wernérus, 2007; Monk, Shah, Xu, Tan, & Foster, 2012). Where applicable, antibiotics were used at the following concentrations: 100 µg/ml ampicillin, 50 µg/ml kanamycin, 10 µg/ml erythromycin, and 15 µg/ml neomycin. Details on the construction of strains used in this study are provided in Appendix 1.
2.2 Recombinant DNA techniques
Plasmids used in this study are listed in Table 1. Oligonucleotides were synthesized by Integrated DNA Technologies and are listed in Table A1. DNA modification enzymes were purchased from New England Biolabs.
Plasmids were constructed using standard molecular cloning techniques (Sambrook & Russell, 2001) with T4 DNA ligase or Gibson assembly (Gibson et al., 2009) using Q5 high-fidelity DNA polymerase, T5 exonuclease, and Taq DNA ligase. Plasmid DNA was isolated from E. coli DC10B cells using the QIAprep Spin Miniprep kit (Qiagen). Staphylococcus aureus genomic DNA was isolated using the Purelink Genomic DNA Mini kit (Invitrogen), with the addition of 50 µg/ml lysostaphin (Sigma-Aldrich) for cell lysis at 37°C for 30 min. DNA purification was performed using the NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel). Details on the construction of plasmids used in this study are provided in Appendix 1. Plasmid sequences were verified by DNA sequencing (Fasmac).
2.3 Bacterial growth assays
Overnight cultures of strains to be assayed were diluted 1:50 in fresh media (SSM9PR minimal media or TSB rich media) and grown at 37°C until OD600nm ~ 0.05. Cultures were aliquoted in triplicate into separate wells of a 96-well microplate. Optical density was measured every 20 min at 595 nm using a MultiSkan Go microplate reader (Thermo Fisher Scientific) set at 37°C with moderate shaking. Experiments were performed in triplicate for at least three independent experiments.
2.4 Bacterial viability assays
Overnight cultures were diluted 1:50 in fresh media and grown to mid-exponential phase. Cultures were normalized to OD600nm 1.0 and serially diluted in phosphate-buffered saline (PBS), after which 10 µl was spotted onto SSM9PR minimal media agar or BHI agar plates. Plates were incubated overnight at 37°C. Data were obtained from at least three independent experiments.
2.5 Bacterial two-hybrid assays
Escherichia coli adenylate cyclase, cya, mutant cells (BTH101) were cotransformed with pairs of pKNT25 and pUT18 plasmid derivatives. Five single colonies of each strain were patched onto selective LB agar plates containing 100 µg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal; Sigma-Aldrich) and 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG; Sigma-Aldrich). Plates were incubated at 30°C for 3 days. Blue patches indicate β-galactosidase activity from interaction between T25 and T18 fusions. Data shown are representative of three independent assays.
2.6 Fluorescence microscopy
Overnight cultures of S. aureus cells were diluted 1:50 in fresh TSB with appropriate antibiotics and incubated at 37°C with aeration until mid-exponential phase (OD600nm ~ 0.4–0.6). For fluorescence localization of Spo0J-mRFPmars and SMC-GFP, cultures were grown at 30°C until OD600nm ~ 0.1–0.2. Expression of Spo0J-mRFPmars and SMC-GFP was induced for 2 hr with 2.5 ng/ml anhydrotetracycline and 0.1 mM IPTG, respectively. Where required, DNA was stained with 2.5 µg/ml 4′,6-diamidino-2-phenylindole (DAPI), cell membranes were stained with 5 µg/ml FM4-64 (Invitrogen), and cell walls were stained with an equal mixture of vancomycin and a vancomycin BODIPY FL conjugate (0.4 µg/ml, Invitrogen) for 2 min at room temperature. After washing with PBS, a small aliquot of cells was applied to an agarose pad containing 2% (w/v) agarose in TSB on a microscope slide, and then covered with a glass coverslip for imaging.
Super-resolution SIM imaging was performed using a Zeiss ELYRA PS.1 microscope equipped with a 100 × 1.46 NA alpha plan apochromat oil immersion objective and a pco.edge sCMOS camera. Fluorescence images were acquired sequentially using 200–300 ms exposure times per image, for a total of 15 images per SIM reconstruction. All imaging was performed at room temperature (~23°C). Raw data were reconstructed using the SIM algorithms in ZEN 2011 SP7 software (black edition, Carl Zeiss). Brightfield images were captured using widefield imaging mode. Images had a final pixel size of 25 nm × 25 nm.
Images were processed and analyzed using Fiji (Schindelin et al., 2012). Cell diameters were determined by measuring the distance between FM4-64 fluorescence peaks from line profiles drawn along the short axis of cells. Data were obtained from at least three independent experiments.
2.7 Western blot and immunodetection
Staphylococcus aureus cultures were grown using the growth and induction conditions described for fluorescence microscopy. Following induction for 2 hr, cells were harvested (1,800 g, 4°C, 10 min) and then resuspended 1:200 in lysis buffer (0.1 M NaCl, 0.1 M Tris-HCl, 0.01 M MgCl2, pH 7.5) containing 1 × protease inhibitor cocktail (Roche) and 0.1 mg/ml DNaseI (Roche). Cells were mixed with 212–300 μm acid-washed glass beads (Sigma-Aldrich) and lysed at 50 Hz for 6 min using a TissueLyser (Qiagen) with precooled tube adapter. Lysates were cleared by centrifugation (21,100 g, 4°C, 30 min) and then incubated with reducing sample buffer at 95°C for 5 min. Samples were electrophoresed on 4%–15% Tris-glycine polyacrylamide gradient gels and then transferred to 0.45 µm nitrocellulose membranes in Towbin buffer (25 mM Tris, 192 mM glycine, 20% (v/v) methanol, pH 8.3).
Blots were blocked with 5% (w/v) EasyBlocker (GeneTex) in TBST (25 mM Tris, 0.15 M NaCl, 0.05% Tween-20). Spo0J-mRFPmars was detected with a 1:2,000 dilution of anti-RFP or anti-mCherry antibodies (Abcam), and SMC-GFP and GFP were detected with a 1:3,000 dilution of anti-GFP antibodies (Abcam). Primary antibodies were detected with a 1:3,000 dilution of goat anti-rabbit HRP-conjugated antibodies (Bio-Rad). Antibodies were diluted in blocking buffer and incubated with blots for 1 hr at room temperature. Blots were washed three times with TSBT before and after blocking and antibody incubations. Secondary antibodies were detected using Clarity Western ECL substrate (Bio-Rad). Blots were imaged using a ChemiDoc gel imager (Bio-Rad).
2.8 Immunoprecipitation
Immunoprecipitation experiments were performed on S. aureus cell lysates prepared as described for Western blotting. Cell lysates (~1.5 mg total protein) from S. aureus cells expressing Spo0J-mRFPmars (pHC052) and either GFP (pHC102) or SMC-GFP (pHC067) were incubated with pre-equilibrated GFP-Trap magnetic agarose beads (ChromoTek) for 1 hr at 4°C with gentle inversion. Beads were magnetically separated and washed twice with wash buffer (10 mM Tris-HCl, 150 mM NaCl, 0.5 mM EDTA, pH 7.5) at 4°C, before elution of bound complexes with elution buffer (50 mM Tris-HCl, 10 mM EDTA, 1% SDS, pH 8) at 65°C for 15 min. Eluates were magnetically separated from the beads and analyzed by Western blotting and immunodetection.
2.9 Statistical analyses
Analyses of statistical significance were performed using unpaired Student's t tests. p values <.05 were considered statistically significant and are indicated with asterisks.
3 RESULTS
3.1 spo0J and smc are not essential for Staphylococcus aureus growth and viability
There is much variation in the functional significance of ParB and SMC in different bacteria. For example, parB is essential in Caulobacter crescentus, while its homologue spo0J is not essential in B. subtilis nor S. pneumoniae (Ireton, Gunther, & Grossman, 1994; Minnen et al., 2011; Mohl, Easter, & Gober, 2001). Additionally, smc is not essential in S. pneumoniae and C. crescentus, but deletion causes lethality under conditions promoting fast growth in B. subtilis (Gruber et al., 2014; Jensen & Shapiro, 1999; Minnen et al., 2011). In order to determine whether spo0J and smc are essential in S. aureus, we generated separate spo0J and smc deletions in the S. aureus RN4220 parental strain. Strains were constructed using an integrative pMAD derivative followed by double-crossover homologous recombination to generate markerless deletions at the spo0J or smc loci of the S. aureus RN4220 genome (Appendix 1). Furthermore, because spo0J and smc were shown to interact in B. subtilis and S. pneumoniae, we also constructed a Δspo0J Δsmc double mutant to test whether this interaction is essential in S. aureus.
The Δspo0J (HC061), Δsmc (HC080), and Δspo0J Δsmc (HC094) mutants showed similar growth rates to the RN4220 parental strain, with no difference in viability when grown at 37°C in TSB rich medium or SSM9PR minimal medium (Figure 1a–c). As described below, we showed that the lack of significant difference in growth and viability between the strains was not a result of suppressor mutations or polar effects from the gene deletions (Figure A1).
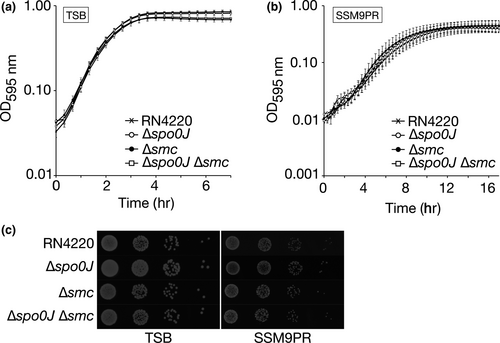
3.2 Spo0J and SMC are involved in Staphylococcus aureus chromosome segregation
The roles of Spo0J and SMC in chromosome segregation are well-studied in B. subtilis and S. pneumoniae. Perturbations to spo0J or smc result in defects in chromosome segregation, with a spo0J smc double mutant having a synthetic lethal phenotype in B. subtilis when grown in rich medium (Britton et al., 1998). Yu et al. (2010) showed that SMC is involved in S. aureus chromosome segregation, but we wondered whether spo0J also plays a role together with SMC. To this end, we performed SIM imaging to examine the DNA content of S. aureus RN4220, Δspo0J, Δsmc, and Δspo0J Δsmc derivatives stained with DAPI.
When grown at 37°C in TSB rich medium, 0.1% of the RN4220 parental strain contained cells that were anucleate, that is, without a nucleoid and, therefore, devoid of DAPI staining (Figure 2a,b). In comparison, 0.6% of Δspo0J and 1.1% of Δsmc cells were anucleate, which are both significantly greater than the frequency observed for the parental strain (p = .0241 and 0.0021, respectively) (Figure 2a,b), suggesting that spo0J and smc are required for normal chromosome segregation in S. aureus. Notably, the Δspo0J Δsmc double mutant showed a significantly higher proportion of anucleate cells compared to the parental strain and the Δspo0J and Δsmc single mutants (3.2% anucleate cells, p = .0017, 0.0032, and 0.0070, respectively) (Figure 2a,b), indicating an increased chromosome segregation defect in the absence of both spo0J and smc.
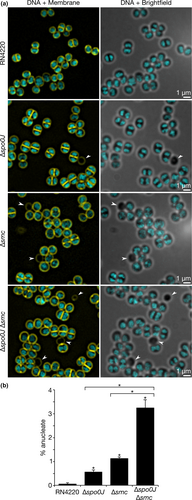
Defects in chromosome segregation are often associated with nucleoid abnormalities such as nucleoid condensation or bisection of unsegregated nucleoids by the division septum (Minnen et al., 2011; Veiga & G Pinho, 2017; Yu et al., 2010). However, in our study, we found no difference in the occurrence of nucleoid abnormalities between the parental and mutant strains and did not observe any instances of septum formation over unsegregated chromosomes.
3.3 Spo0J is required for SMC localization in Staphylococcus aureus
Our finding that Spo0J and SMC are both required together to ensure accurate chromosome segregation led us to speculate that S. aureus Spo0J and SMC might behave similarly to their counterparts in other bacteria. A significant role of ParB/Spo0J in S. pneumoniae, B. subtilis, and C. crescentus is to recruit the condensin, SMC, to origin-proximal sites, where ParB/Spo0J binds (Gruber & Errington, 2009; Minnen et al., 2011; Sullivan et al., 2009; Tran, Laub, & Le, 2017). To determine whether this interaction might also occur in S. aureus, we constructed Spo0J-mRFPmars and SMC-GFP fusion proteins so that their localizations could be visualized by SIM imaging. Both proteins were expressed from compatible, coresident, low-copy number plasmids in S. aureus. Spo0J-mRFPmars expression was induced with 2.5 ng/ml anhydrotetracycline from the Pxyl/tetO promoter, while SMC-GFP expression was induced with 0.1 mM IPTG from the Pspac promoter. Importantly, both fluorescent fusions were functional under the induction conditions tested, since Spo0J-mRFPmars and SMC-GFP could complement the chromosome segregation defects of the Δspo0J and Δsmc mutants, respectively (Figure A1). This further verifies that the growth, viability, and chromosome segregation phenotypes of the Δspo0J and Δsmc strains were the result of gene deletions and not other mutations or polar effects.
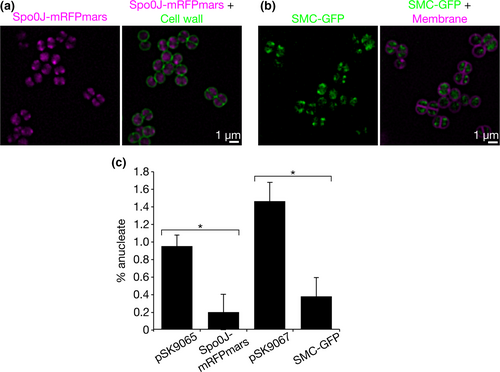
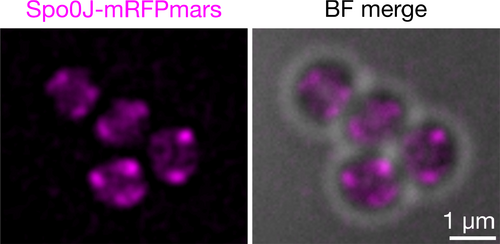
Spo0J-mRFPmars formed fluorescent foci consistent with previously shown localization patterns and indicative of DNA-binding to origin-proximal sites (Pinho & Errington, 2004; Veiga, Jorge, & Pinho, 2011) (Figure 3a and Figure A2). SMC-GFP displayed similar localization patterns to Spo0J-mRFPmars, often with overlapping, or partially-overlapping, foci (Figure 3a). Our results suggest that S. aureus Spo0J and SMC likely localize to similar subcellular positions, consistent with potential complex formation.
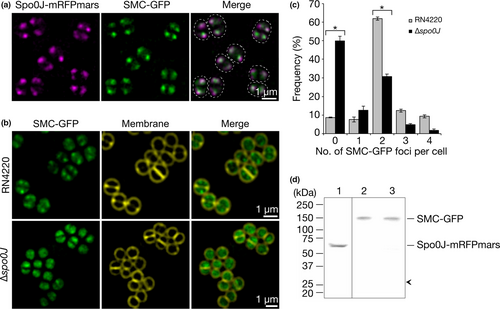
If S. aureus Spo0J recruits SMC to origin-proximal sites as described in B. subtilis, S. pneumoniae, and C. crescentus (Gruber & Errington, 2009; Minnen et al., 2011; Sullivan et al., 2009; Tran et al., 2017), then we predicted that SMC-GFP might be mis-localized in the absence of Spo0J. Therefore, we endeavored to determine whether SMC-GFP localization is dependent on Spo0J. For this purpose, we expressed SMC-GFP in the S. aureus Δspo0J mutant strain and compared its localization to that in the parental RN4220 strain. Subsequent SIM imaging revealed that more than 90% of cells in the parental strain contained at least one fluorescent focus of SMC-GFP, with the majority (62%) containing two SMC-GFP foci per cell (Figure 3b,c). In contrast, SMC-GFP localization appeared more dispersed in the Δspo0J cells, with a significantly higher proportion of cells containing no SMC-GFP foci (50%, p < .0001), and an average of 0.96 foci per cell, compared to 2.1 foci in the parental strain (p < .0001, ntotal = 1,345 and 1,307 cells, respectively, Figure 3b,c). Western blotting using anti-GFP antibodies showed that the loss of SMC-GFP foci in the absence of spo0J was not due to the release of free GFP by proteolysis (Figure 3d). No proteolysis was also detected for Spo0J-mRFPmars (Figure 3d). Taken together, our results suggest that staphylococcal chromosome segregation involves the formation of complexes containing Spo0J and SMC and that Spo0J is required for correct localization of SMC in S. aureus.
3.4 Spo0J and SMC form a complex in Staphylococcus aureus to mediate chromosome segregation
The increased proportion of anucleate cells in the Δspo0J Δsmc mutant and the dependence of SMC localization on Spo0J suggested potential interaction between Spo0J and SMC. Therefore, in order to verify whether Spo0J and SMC form a complex in S. aureus, we performed co-immunoprecipitation experiments on S. aureus RN4220 cells expressing Spo0J-mRFPmars and either GFP or SMC-GFP. Following nuclease digestion of genomic DNA, we used anti-GFP antibodies coupled to magnetic agarose beads to immunoprecipitate GFP or SMC-GFP and their interacting proteins. We were able to detect Spo0J-mRFPmars in the immunoprecipitated complexes isolated from S. aureus cells expressing both Spo0J-mRFPmars and SMC-GFP, but not from cells expressing Spo0J-mRFPmars and GFP (Figure 4a). Thus, Spo0J-mRFPmars was isolated specifically in the presence of SMC-GFP, indicating the formation of complexes containing Spo0J and SMC in S. aureus. Notably, the Spo0J-mRFPmars immunoprecipitated from cell lysates containing SMC-GFP was only weakly detected using anti-RFP antibodies, which may be indicative of weak or transient complex formation.
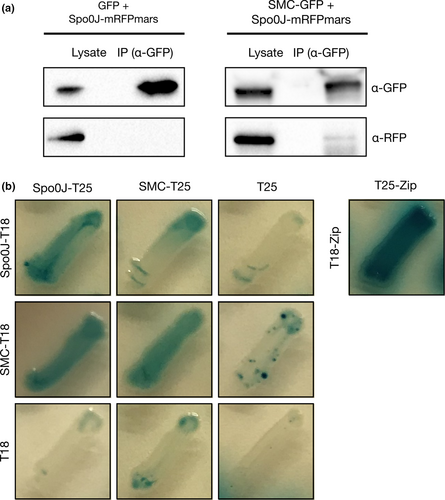
To test whether Spo0J and SMC might interact directly, we conducted bacterial two-hybrid assays using Spo0J and SMC fusions to the Bordetella pertussis adenylate cyclase (CyaA) fragments, T25 and T18 (Karimova, Pidoux, Ullmann, & Ladant, 1998). The assays revealed that in the E. coli cya mutant strain BTH101, β-galactosidase activity was detected from cells expressing the Spo0J-T25 and SMC-T18 fusions (blue patches), however, no activity was detected from cells expressing the reciprocal fusions, SMC-T25 and Spo0J-T18 (white patches) (Figure 4b). We do not exclude the possibility that an interaction between Spo0J and SMC, direct or indirect, may be dependent on Spo0J localization or DNA binding, or on the presence of other S. aureus proteins that were not present in the bacterial two-hybrid assays. The assays also showed self-interaction of Spo0J and SMC (Figure 4b), which is consistent with the fluorescent Spo0J-mRFPmars and SMC-GFP foci observed (Figure 3a).
3.5 Absence of Spo0J and SMC does not interfere with normal Staphylococcus aureus cell division
Spatiotemporal coordination of chromosome segregation and cell division is paramount for successful cell growth. Impairment of parB in C. crescentus blocks FtsZ ring assembly and subsequent cell division (Mohl et al., 2001), while chromosome segregation defects in B. subtilis correlate with elongated cells (Britton et al., 1998; Ireton et al., 1994; Lee & Grossman, 2006). In order to investigate the influence of Spo0J and SMC on S. aureus cell division, we sought to determine whether the chromosome segregation defects observed in the spo0J and smc mutants correspond to abnormalities in staphylococcal cell division.
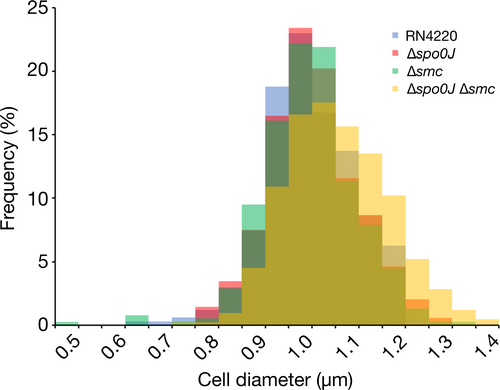
Disruption of staphylococcal cell division, for example, by impeding FtsZ, EzrA, Noc, or DnaK function, results in cell enlargement and aberrant cell division (Bottomley et al., 2017; Jorge, Hoiczyk, Gomes, & Pinho, 2011; Lund et al., 2018; Monteiro et al., 2018; Pereira et al., 2016; Pinho & Errington, 2003; Veiga et al., 2011). Therefore, as an indicator of cell division, we measured the cell diameters of S. aureus cells to determine whether differences in cell size could be attributed to inefficient chromosome segregation. We found no significant difference in cell diameters between the parental and deletion strains (Figure 5a and Figure A3), which suggests that the absence of spo0J or smc, alone or together, does not affect S. aureus cell size.
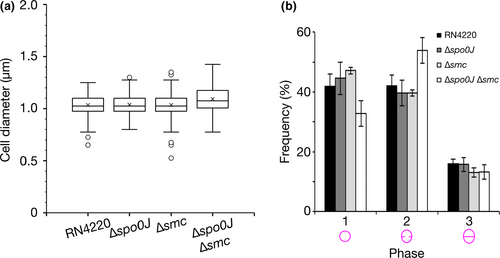
We next investigated whether spo0J and smc play a role in cell cycle progression. We categorized the S. aureus cell cycle into three phases, characterized by cells that had no septum (Phase 1), an incomplete septum (Phase 2) or a complete septum (Phase 3) (Monteiro et al., 2015). In order to provide an indication of the relative duration of each phase of the cell cycle, we stained S. aureus cell membranes with the lipid dye FM4-64 and calculated the proportion of cells in each phase from SIM images. We observed no difference in the distribution of cells in the three cell cycle phases for the parental, Δspo0J, and Δsmc strains (Figure 5b). We did, however, observe a modest, but insignificant, increase in the proportion of cells in Phase 2 for the Δspo0J Δsmc double mutant (54 ± 4.3% compared to 42 ± 3.6% for the parental strain, mean ± SEM, p = .1032) (Figure 5b), potentially indicating slightly prolonged septum closure in these cells. Taken together, these results suggest that spo0J and smc do not significantly affect cell cycle progression and are not essential for normal cell division in S. aureus.
4 DISCUSSION
In this study, we examined the functional significance of Spo0J and SMC in S. aureus, and found that both proteins are required for efficient chromosome segregation, which is consistent with their predicted roles. A single Δspo0J mutant resulted in 0.6% anucleated cells (Figure 2), which is similar to the frequency observed for B. subtilis and S. pneumoniae mutants (Ireton et al., 1994; Minnen et al., 2011). Our observations for the Δsmc mutant are also consistent with the previously shown contribution of SMC to S. aureus chromosome segregation (Yu et al., 2010).
Fluorescence localization of Spo0J-mRFPmars and SMC-GFP in S. aureus showed that the two proteins formed foci that often overlapped or partially overlapped, and were reminiscent of oriC localization (Pinho & Errington, 2004; Veiga et al., 2011) (Figure 3a). Importantly, SMC-GFP localization is dependent on Spo0J, since absence of spo0J resulted in a significant reduction in the number of SMC-GFP foci per cell (Figure 3b–d). This suggests that Spo0J-dependent SMC foci, which likely correspond to complexes containing Spo0J and SMC (Figure 4), are essential for S. aureus chromosome segregation. Note that SMC-GFP foci were not completely absent in the Δspo0J mutant, possibly because SMC itself is capable of binding to chromosomal DNA, albeit less efficiently in the absence of ParB/Spo0J (Gruber & Errington, 2009; Minnen et al., 2011; Sullivan et al., 2009). Although our data did not conclusively indicate a direct interaction between Spo0J and SMC, we nonetheless showed that in S. aureus, formation of complexes containing Spo0J and SMC is dependent on correct localization of Spo0J (Figure 3b–d). It therefore appears that Spo0J-SMC interaction, direct or indirect, is conserved among several bacterial genera, including B. subtilis, S. pneumoniae, C. crescentus, and S. aureus (Gruber & Errington, 2009; Minnen et al., 2011; Sullivan et al., 2009; Tran et al., 2017).
Since S. aureus lacks a ParA/Soj ATPase homologue, it is possible that staphylococcal chromosome segregation occurs passively, instead relying on conformational entropy of replicated chromosomes (Jun & Wright, 2010) or on forces generated from cellular processes such as DNA replication or transcription (Dworkin & Losick, 2002; Kjos & Veening, 2014; Lemon & Grossman, 2001). However, interestingly, we found that absence of both spo0J and smc results in a significantly more severe chromosome segregation defect than absence of either gene alone (Figure 2). This synergistic effect implies that Spo0J and SMC may be partially redundant or offer partial compensation in the absence of the other gene. Furthermore, in light of our finding that accurate chromosome segregation is enhanced by Spo0J in complex with SMC, it is not inconceivable that complexes containing Spo0J and SMC could be involved in additional interactions or processes that are essential for faithful chromosome segregation, such as chromosome organization or compaction. Since a S. pneumoniae ΔparB Δsmc mutant did not show a significant increase in anucleate cells compared to ΔparB or Δsmc single mutants (Minnen et al., 2011), it would be interesting to investigate whether other, perhaps as yet unknown, proteins or interactions might contribute specifically to chromosome segregation in coccoid-shaped staphylococci. Indeed, the observation that chromosome segregation occurs in the absence of both SMC and a ParA homologue in S. aureus suggests that Spo0J facilitates chromosome segregation using a currently unknown mechanism.
A number of bacteria, including Vibrio cholerae, C. crescentus, and sporulating B. subtilis, utilize proteins to anchor chromosomal origins to the cell poles to facilitate chromosome segregation (Ben-Yehuda et al., 2005; Ben-Yehuda, Rudner, & Losick, 2003; Bowman et al., 2008; Ebersbach, Briegel, Jensen, & Jacobs-Wagner, 2008; Yamaichi et al., 2012). Recently, it was shown that S. aureus DivIVA, a membrane-localized divisome protein, interacts with SMC, thereby providing a means of coordinating chromosome segregation with cell division, although the exact mechanism is unclear (Bottomley et al., 2017). Accordingly, one could speculate that SMC-DivIVA interaction might act to anchor oriC-bound complexes containing Spo0J and SMC during staphylococcal chromosome segregation.
It should be noted that our observations were made on segregation of the bulk chromosome, and as such, we do not exclude the possibility that chromosomal loci might be disorganized or mis-segregated in the absence of either spo0J or smc. Taking this into account, we did not observe any bisection of the nucleoid by the division septum in any of the strains analyzed. These events were probably prevented by Noc, which prevents divisome assembly over the staphylococcal nucleoid (Veiga et al., 2011), and FtsK and SpoIIIE, which ensure that DNA is not guillotined by the division septum during staphylococcal cell division (Veiga & G Pinho, 2017; Yu et al., 2010). Indeed, an S. aureus smc spoIIIE double mutant showed aberrations in chromosome organization and content (Yu et al., 2010). Spo0J and SMC are therefore probably involved in the early stages of S. aureus chromosome segregation, immediately following DNA replication, presumably by acting at origin-proximal sites to segregate the origins.
Importantly, although we observed a significant proportion of anucleated cells in the absence of spo0J and smc, the defect in chromosome segregation was not sufficient to severely affect growth, viability, cell division, or cell cycle progression (Figures 1 and 5). However, it would not be surprising that S. aureus utilizes multiple complementary mechanisms, in addition to Spo0J and SMC, to ensure accurate chromosome segregation and coordination with cell division. These additional mechanisms warrant further investigation and may help to better understand how these essential processes are carried out in spherical staphylococci.
ACKNOWLEDGMENTS
We are grateful to Morten Kjos for kindly providing reagents for the bacterial two-hybrid system. Work in the Structural Cellular Biology Unit is supported by OIST core subsidy.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Helena Chan: Conceptualization; Formal analysis; Investigation; Writing-original draft; Writing-review & editing. Bill Söderström: Supervision; Writing-review & editing. Ulf Skoglund: Supervision; Writing-review & editing.
ETHICS STATEMENT
None required.
APPENDIX 1
CONSTRUCTION OF PLASMIDS
pHC052 (Pxyl/tetO-spo0J-mRFPmars). A 0.85 kb fragment containing Staphylococcus aureus RN4220 spo0J was PCR-amplified from S. aureus genomic DNA using primers spo0J_SalI(F) and spo0J_SacI(R), and then restricted with SalI and SacI. The restricted fragment was purified and ligated with SalI and SacI-digested pSK9065 vector DNA using T4 DNA ligase.
pHC061 (Δspo0J). pHC061 was constructed using Gibson assembly (Gibson et al., 2009). Regions of ~2.5 kb upstream and downstream of S. aureus RN4220 spo0J were PCR-amplified from S. aureus genomic DNA using primer pairs spo0J_up(F)/spo0J_up(R) and spo0J_dwn(F)/spo0J_dwn(R), respectively. pMAD vector DNA was PCR-amplified using primers pMAD_dwn(F) and pMAD_up(R). Amplicons were digested with DpnI to remove methylated template DNA, and then the spo0J upstream region, downstream region, and pMAD vector DNA were ligated for 1 hr at 55°C in the presence of 100 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 200 µM of each of the four dNTPs, 10 mM DTT, 5% (w/v) PEG-8000, 1 mM NAD, 3.8 U/ml T5 exonuclease, 23.8 U/ml Q5 DNA polymerase, and 3.8 U/µl Q5 DNA ligase.
pHC067 (Pspac-smc-gfp). A 3.6 kb fragment containing S. aureus RN4220 smc was PCR-amplified from S. aureus genomic DNA using primers smc_RBS_BamHI(F) and smc_SmaI(R), and then restricted with BamHI and SmaI. The restricted fragment was purified and phosphorylated with T4 polynucleotide kinase, then ligated with BamHI and SmaI-digested pSK9067 vector DNA using T4 DNA ligase.
pHC080 (Δsmc). pHC080 was constructed using Gibson assembly (Gibson et al., 2009). Regions of ~2.5 kb upstream and downstream of S. aureus RN4220 smc were PCR-amplified from S. aureus genomic DNA using primer pairs smc_up(F)/smc_up(R) and smc_dwn(F)/smc_dwn(R), respectively. The last 0.2 kb at the 3′ end of smc was left intact to retain the potential promoter region of a putative downstream gene. pMAD vector DNA was PCR-amplified using primers pMAD_dwn(F) and pMAD_up(R). Amplicons were digested with DpnI to remove methylated template DNA, and then the smc upstream region, downstream region, and pMAD vector DNA were ligated for 1 hr at 55°C in the presence of 100 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 200 µM of each of the four dNTPs, 10 mM DTT, 5% (w/v) PEG-8000, 1 mM NAD, 3.8 U/ml T5 exonuclease, 23.8 U/ml Q5 DNA polymerase, and 3.8 U/µl Q5 DNA ligase.
pHC102 (Pspac-gfp). A 0.7 kb DNA fragment containing gfp was amplified from pSK9067 using primers gfp_SalI(F) and gfp_EcoRI(R) and then restricted with SalI and EcoRI. The restricted fragment was purified and then ligated with SalI and EcoRI-digested pLOW vector DNA (Liew et al., 2011) using T4 DNA ligase.
pHC111 (spo0J-T25) and pHC114 (spo0J-T18). A 0.85 kb DNA fragment containing S. aureus spo0J was amplified from S. aureus RN4220 genomic DNA using primers spo0J_BamHI(F) and spo0J_SacI(R) and then restricted with BamHI and SacI. The restricted fragment was purified and then ligated with BamHI and SacI-digested pKNT25 or pUT18 vector DNA using T4 DNA ligase.
pHC112 (smc-T25) and pHC115 (smc-T18). A 3.6 kb DNA fragment containing S. aureus smc was amplified from S. aureus RN4220 genomic DNA using primers smc_BamHI(F) and smc_KpnI(R) and then restricted with BamHI and KpnI. The restricted fragment was purified and then ligated with BamHI and KpnI-digested pKNT25 or pUT18 vector DNA using T4 DNA ligase.
CONSTRUCTION OF Staphylococcus aureus DELETION STRAINS
Electrocompetent S. aureus RN4220 cells were electroporated with the integration plasmid pHC061 or pHC080 to generate spo0J (HC061) and smc (HC080) deletions, respectively. Electroporated cells were incubated at 30°C on BHI agar containing 10 µg/ml of erythromycin and 100 µg/ml of 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) (BHI/Em/X-Gal) to select for transformants. Blue colonies were grown in TSB containing 10 µg/ml of erythromycin at 43°C for ~8 hr, and then streaked on BHI/Em/X-Gal agar. Plates were incubated at 43°C to promote vector integration into the chromosome via homologous recombination. Single blue colonies were selected and continuously subcultured approximately six times at 30°C in TSB without antibiotic selection to promote double cross-over and plasmid loss. The final subculture was serially diluted in PBS, spread on BHI/X-Gal agar plates, and incubated at 30°C. Single colonies were replica-patched onto BHI/X-Gal agar plates with and without erythromycin selection. White patches that were erythromycin-resistant, indicating plasmid excision from the chromosome, were selected and screened for gene deletion. Gene deletions were verified by PCR of isolated genomic DNA.
The S. aureus Δspo0J Δsmc double mutant (HC094) was generated in HC061 (Δspo0J) using the integrative plasmid, pHC080, to generate an additional smc deletion as described above.
| Oligonucleotide | Sequencea |
|---|---|
| gfp_SalI(F) | cgcgtcgacaggaggataattatttatggtttcaaaaggagaagaac |
| gfp_EcoRI(R) | cgcgaattcttatttataaagttcgtccataccg |
| pMAD_dwn(F) | ggatccgatatcgcccgacgcgaggc |
| pMAD_up(R) | ccatggcatgcatcgatagatctgtc |
| smc_BamHI(F) | cgcggatccatggtttatttaaaatcaatag |
| smc_KpnI(R) | cgcggtaccgcttgctcctccttcaacacatc |
| smc_RBS_BamHI(F) | cgcggatccaggaggataattattatggtttatttaaaatcaatag |
| smc_dwn(F) | ctttaaaattgtgataaggagtttaggatggatgaggttgaagctgcactag |
| smc_dwn(R) | tccagcctcgcgtcgggcgatatcggatcccataaactgcttgtctactcac |
| smc_SmaI(R) | gcgcccgggaccagctgatgcagcagaacctccttgctcctccttcaacacatc |
| smc_up(F) | actagacagatctatcgatgcatgccatggcagtagcgttcttagcatcag |
| smc_up(R) | tgcttcatctagtgcagcttcaacctcatccatcctaaactccttatcac |
| spo0J_BamHI(F) | cgcggatccatgagtgaattgtcaaaaag |
| spo0J_SacI(R) | cgcgagctcgctttaccatacctacgatttaattg |
| spo0J_dwn(F) | aactgttataagatattaattagcttacagaggtatggtaaatagttaca |
| spo0J_dwn(R) | tccagcctcgcgtcgggcgatatcggatccgcacctttcacaataccagc |
| spo0J_SacI(R) | gcggagctcggaggcgccgcaggatttaccatacctacgatttaattg |
| spo0J_SalI(F) | cgcgtcgacaggaggataattatttgtgagtgaattgtcaaaaagtg |
| spo0J_up(F) | actagacagatctatcgatgcatgccatgggtttgcccgctatatagtac |
| spo0J_up(R) | atataaaattgtgtaactatttaccatacctgtaagctaattaatatcttataacag |
- a Oligonucleotides are presented 5′–3′, left to right. Restriction sites are underlined. Ribosome binding sites are highlighted in bold. Linker sequences are italicized.
Open Research
DATA AVAILABILITY STATEMENT
Data from this study are available from the corresponding author on reasonable request.