Evaluating Effects of Antibiotics Across Preclinical Models of the Human Gastrointestinal Microbiota
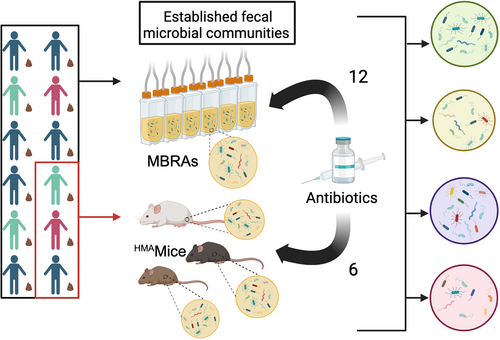
Graphical Abstract
We compared microbiota changes following antibiotic treatment in two preclinical models of the human GI microbiota, minibioreactor arrays (MBRAs) and human microbiota associated mice (HMAmice). MBRAs and HMAmice were colonized with feces from 12 or 3 healthy humans, respectively, before treatment with each of 12 or 6 antibiotics.
ABSTRACT
While antibiotics play important roles in treating infections, disruption of the gastrointestinal microbiota during antibiotic treatment can lead to negative health consequences. However, for many antibiotics, the spectrum of activity has been determined for select isolates rather than for the range of microbes that populate the gastrointestinal tract. Here, we examined the response of communities of gastrointestinal microbes to antibiotics using two different model systems, human fecal minibioreactors and human microbiota-associated mice. Communities established in minibioreactors using 12 different fecal donors were exposed to 12 different classes of antibiotics. Samples from three fecal donors were used to colonize germ-free mice from three different genetic backgrounds; progeny mice were then exposed to 6 of 12 antibiotics tested in minibioreactors. Initial bacterial community diversity was dependent on both the fecal donor and model system. Antibiotics affected a wide range of taxa across the phylogenetic spectrum, with many taxa similarly affected across treatments with different classes of antibiotics. Vancomycin, typically administered to treat Gram-positive bacterial infections, decreased the abundance of diverse taxa, including Gram-negative Bacteroidota species. Effects on some taxa were restricted by model system, indicating the importance of environmental context on antibiotic susceptibility. Altogether, these results indicate the complex interrelationships between microbiota composition and environmental context on antibiotic susceptibility and demonstrate strengths and weaknesses of each preclinical model system for evaluating effects of new antibiotics and other compounds with potential to disrupt the microbiota.
1 Introduction
The microbes that colonize the gastrointestinal tract (GI microbiota) play key roles in many aspects of human physiology, including modulating activities of the digestive (Martinez-Guryn et al. 2018), endocrine (Chimerel et al. 2014), immune (Schluter et al. 2020), and nervous (Buffington et al. 2016) systems. While antibiotics play an essential role in the treatment of infections (Hutchings et al. 2019), antibiotics use can also alter the composition and function of the GI microbiota. Initial, high-throughput sequencing studies demonstrated the strong effect antibiotics can have on multiple taxa abundant in the gut microbiota (Dethlefsen et al. 2008; Dethlefsen and Relman 2011). Since then, there have been studies on factors that affect antibiotic response (Raymond et al. 2016) or recovery (Ng et al. 2019; Palleja et al. 2018; Suez et al. 2018), or examined the functional changes in the microbiome (Zaura et al. 2015). Efforts are complicated by interindividual microbiome diversity, pre-existing conditions and exposures, treatment with more than a single drug, and fluctuations due to altered diet and lifestyle.
Traditional screening platforms for new antibiotics have focused on characterization of antimicrobial activity against a panel of select clinical isolates (Guay 2007; Li et al. 2020), although new innovations continue to emerge that facilitate more robust screening approaches against larger panels of strains and microbial communities (Guzman-Rodriguez et al. 2018; Terekhov et al. 2018; Watterson et al. 2020). Studies conducted in mice (Ajami et al. 2018; Gu et al. 2020) and humans (Rashid et al. 2015; Raymond et al. 2016) typically have other objectives and rarely test multiple classes of antibiotics that would allow broader observations about the taxa affected across treatments. Tools that facilitate testing of new antibiotics against a larger panel of strains or microbial communities play an important role in rapidly identifying off-target effects on the commensal microbiota.
Recently, we reported on studies using fecal minibioreactor arrays (MBRAs), a continuous-flow culture model of the nutritional conditions of the lumen of the distal colon, to characterize mechanisms of colonization resistance to the GI pathogen, Clostridioides difficile, across microbiotas from different healthy individuals (Huang et al. 2025). We demonstrated that changes in conversion of primary to secondary bile salts by the microbiota following antibiotic treatment did not alter colonization resistance. Rather, competition for proline, an amino acid that is a preferred electron acceptor for C. difficile Stickland fermentation, mediated susceptibility to C. difficile colonization (Huang et al. 2025). As part of these studies, we analyzed microbiota composition in fecal communities from 12 healthy individuals in response to treatment with six different clinical antibiotics (Augmentin, azithromycin, cefaclor, ceftriaxone, clindamycin, and fidaxomicin). In this study, we extend upon a subset of data presented in that study to compare susceptibility to antibiotic treatment in the MBRA model to another commonly used model of human GI microbial communities, human microbiota-associated mice (HMAmice). Specifically, we analyzed the effects of treatment with 12 different antibiotics on microbiota composition across 12 fecal microbial communities in the MBRA model. We also characterized the effects of treatment with six antibiotics (Augmentin, azithromycin, cefaclor, ceftriaxone, clindamycin, and fidaxomicin) on microbiota composition in human microbiota-associated mice that were colonized with fecal microbial communities from three of 12 donors studied in the MBRA model. As expected, we observed that antibiotic treatment led to changes in microbiota composition across fecal samples and models. Overall, many Clostridiales species were highly susceptible to the antibiotics tested, whereas the classes of antibiotics that also inhibited Bacteroidales species were more limited. While some taxa responded similarly to antibiotics across the two experimental models, there were several notable differences, which could partially be due to differences in microbiota composition between models, but may also reflect differential responses of taxa to antibiotics between the models. In addition to taxa lost due to direct effects of antibiotics on susceptible microbes, we also observed a decrease in some taxa previously reported to be resistant to antibiotics, suggesting the importance of cross-feeding interactions that would cause additional impacts when disrupted.
2 Materials and Methods
2.1 Fecal Samples
As previously described (Huang et al. 2025), fecal samples were self-collected by 12 healthy individuals who had not been treated with oral antibiotics within the previous 6 months. Samples were collected from children aged 4–17 (n = 3), adults aged 18–65 (n = 7), and older adults aged > 65 (n = 2), with similar numbers of male and female participants. Studies were collected across the age span to capture differences that may be present, but were not specifically powered to evaluate differences in community composition by age. Adult participants provided consent to participate in the study. Children provided assent to participate along with parental consent to participate. Protocols for collection and use of fecal samples were reviewed and approved by Institutional Review Boards at Baylor College of Medicine (protocol number H-38014) and University of Nebraska-Lincoln (protocol number 18585). Details of fecal sample collection and cryopreservation can be found in our previously described study (Huang et al. 2025).
2.2 Bioreactor Experiments
Bioreactor experiments were performed in anaerobic conditions at 37°C using MBRAs as described previously (Auchtung et al. 2015). To prepare 1 L bioreactor medium (BRM3), a solution containing 1 g tryptone, 2 g proteose peptone number 3, 2 g yeast extract, 0.4 g sodium chloride, 1 g bovine bile, 5 mg hemin, 0.01 g magnesium sulfate, 0.01 g calcium chloride, and 2 mL Tween 80 was autoclaved at 121°C for 60 min. A mixture of 0.1 g arabinogalactan, 0.15 g maltose, 0.15 g D-cellobiose, 0.04 g D-glucose, 0.2 g inulin, 2 g sodium bicarbonate, 1 mg vitamin K1, 0.04 g potassium phosphate monobasic, and 0.04 g potassium phosphate dibasic was adjusted to pH 6.8, filter-sterilized, and added to the autoclaved solution. Sources of reagents were previously described (Huang et al. 2025). Media and sterile MBRA were pre-reduced in an anaerobic chamber for > 48 h before use. Reactors were inoculated with fecal slurry to a final concentration of 1% w/v and allowed to equilibrate under anaerobic conditions for 16–18 h. Media flow was initiated after equilibration at 1.875 mL/h media (8 h retention time). As previous studies had demonstrated that microbiota composition stabilized between 6 and 8 days of culture (Auchtung et al. 2015), communities were given 6 days to stabilize before treatment with antibiotics. Antibiotics were dosed every 12 h for 5 days. Research grade antibiotics were obtained from the sources listed in Table A1 at the concentrations indicated in Table 1. Concentrations used for dosing were estimated from previously published measurements in human feces and/or bile (Brogard et al. 1982; Kager et al. 1981; Krook et al. 1981; Neu 1974; Owen and Faragher 1986; Pletz et al. 2004; Sears et al. 2012; Steinbakk et al. 1992; Sullivan et al. 2001), although fidaxomicin dosing was limited by its maximal solubility. Each fecal sample was tested with each antibiotic in duplicate, with the exception of cefaclor, ceftriaxone, and untreated controls, which were tested four times for each fecal sample. Samples were collected for microbial community sequencing before antibiotic treatment (Day 0), after antibiotic treatment (Day 5), and 2 days following the end of antibiotic treatment (Day 7). Day 7 samples were limited to duplicate reactors from cefaclor, ceftriaxone, and untreated communities. One mL samples were collected aseptically through the sampling port, cells were pelleted by centrifugation at ~3000 x g for 5 min, and supernatants were transferred to new 96 well plates or 1.7 mL tubes before storage at −80°C. The heated anaerobic chamber (Coy laboratories) was maintained at 37°C with an atmosphere of 5% H2, 5% CO2, and 90% N2.
| Antibiotic | Class | MBRA concentrationa | HMAmice concentrationb |
|---|---|---|---|
| Augmentin | Penicillins | 25 μg/mL | 23.6 mg/kg |
| Azithromycin | Macrolides: Azalides | 400 μg/mL | 7.1 mg/kg |
| Cefaclor | Cephalosporins: 2nd generation | 67 μg/mL | 20.0 mg/kg |
| Ceftriaxone | Cephalosporins: 3rd generation | 200 μg/mL | 28.6 mg/kg |
| Ciprofloxacin | Fluoroquinolones | 175 μg/mL | ND |
| Clindamycin | Lincosamides | 150 μg/mL | 17.1 mg/kg |
| Doxycycline | Tetracyclines | 14.5 μg/mL | ND |
| Fidaxomicin | Macrolides: Tiacumins | 50 μg/mL | 5.7 mg/kg |
| Imipenem | Carbapenem | 9.5 μg/mL | ND |
| Metronidazole | Nitroimidazoles | 200 μg/mL | ND |
| Sulfamethoxazole | Sulfonamides | 12 μg/mL | ND |
| Vancomycin | Glycopeptides | 150 μg/mL | ND |
- Abbreviation: ND, not done.
- a MBRAs were treated twice daily for 5 days except as noted.
- b HMAmice were treated once daily for 5 days.
2.3 Mouse Experiments
Germ-free Swiss Webster mice were obtained from Baylor College of Medicine; germ-free C3H/HeN and C57BL/6J were obtained from the Nebraska Gnotobiotic Mouse facility. Fecal slurries from three human fecal samples (FS04, FS05, and FS06) were prepared under anaerobic conditions as previously described (Collins et al. 2015) and 100 μL were used to colonize each mouse intended for breeding (both breeding pairs and trios were used) under gnotobiotic conditions. Mice were then housed in individually ventilated cage racks under specific pathogen-free conditions and were fed sterilized chow and water. All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committees of Baylor College of Medicine (AN-6675) and University of Nebraska-Lincoln (IACUC #1668, #1680) and are in accordance with the NIH Office of Laboratory and Animal Welfare (OLAW) guidelines.
All experiments were performed with 6–10 week-old mice, primarily from the F1 generation as indicated in Supporting Information S1: Table S1. Exceptions to testing in the F1 generation included Swiss Webster mice colonized with FS05, which were tested in the F3 generation, and Swiss Webster mice colonized with FS06 and C57Bl/6J mice colonized with FS04 and FS05, which were tested in the F2 generation. Swiss Webster mice colonized with FS05 were originally colonized at Baylor College of Medicine and imported to the University of Nebraska-Lincoln for these studies. Testing of other mice in the F2 generation was a result of limited fecundity of the original breeding pairs and trios (up to 5 per mouse background/fecal slurry). Previous studies had demonstrated that the microbiota composition stabilized following colonization of F1 generation with minimal compositional drift (Collins et al. 2015).
To analyze effects of antibiotics on microbiota composition, mice were administered 100 μL of research grade antibiotics (or sterile PBS) via orogastric gavage once daily for 5 days. To minimize the number of mice used in studies, both male and female mice were used, with animals housed 2-4/cage by sex. Concentrations of antibiotics used in mice were scaled based on doses reported for humans (factors used for scaling: 70 kg human, 25 g mouse, with doses adjusted by mouse body weight as indicated in Table 1). Table A2 provides the number of mice and their sex per treatment. Fecal samples were collected at the end of antibiotic treatment and used for microbial community sequencing analysis as described below. The concentration of antibiotics in fecal samples was not measured.
2.4 Growth of Individual Strains and Testing of Vancomycin Susceptibility
Strains used in this study were originally isolated from 1 of the 12 fecal samples described above or were obtained from ATCC. For strains isolated from fecal samples, diluted fecal material was spread on nonselective agar (M2GSC (Miyazaki et al. 1997) or YCFA (Browne et al. 2016; Table A3) and incubated under anaerobic conditions for up to 72 h. Colonies were passaged on culture media for one to two generations before cryopreservation of a stock in media supplemented with 7.5% DMSO or 15% glycerol. Partial taxonomic identity of strains was determined through sequencing of the V4 region of the 16S rRNA gene as described below. Strains isolated were tested for their ability to grow in medium with or without 150 μg/mL vancomycin. Colonies grown on YCFA agar were inoculated into BRM3 and YCFA broth in a 96-well polystyrene plate. Overnight cultures were inoculated 1:10 in fresh broth, grown 2 h, then diluted 1:30 into wells of fresh broth +/− vancomycin. Growth at 37°C in the anaerobic chamber was monitored continuously by recording optical density at 600 nm in a Tecan Sunrise plate reader, with pre-read mixing on the low setting. Values are the average of two replicates grown in internal wells (to limit evaporation). Controls were uninoculated media and vancomycin-resistant strains Enterococcus faecalis and Lactobacillus reuteri (grown in MRS instead of YCFA).
Akkermansia muciniphila human-isolated strain BAA-835 and mouse-isolated strain YL44 were grown in YCFA, BRM3, or mixtures of components of BRM3 and YCFA (Table A4). Freezer stocks of the strains were struck on M2GSC agar and grown 2–3 days. Colonies were suspended in PBS and used to inoculate different broth media. Four replicates of each culture condition were grown anaerobically in a 96-well polystyrene plate at 37°C. Aliquots of inoculum and 24 h cultures were serially diluted and plated on M2GSC plates, which were incubated anaerobically at 37°C for 3 days before enumeration.
2.5 Microbial Community Analysis Through 16S rRNA Gene Sequencing
Bacteria were targeted for analysis because although protists, fungi, and archaea are also susceptible to some classes of antibiotics, typically only bacteria are abundant in the human GI tract. Frozen cell pellets (MBRA) or fecal samples (HMAmice) were initially disrupted by 0.1 mm bead-beating, followed by BioSprint 96 One-For-All Vet kit processing as described (Huang et al. 2025). DNA was amplified in duplicate with Phusion polymerase using Illumina barcoded primers 515F and 806R as described (Collins et al. 2015), then sequenced on a MiSeq using 2 × 250 kits according to manufacturer's protocol by the investigators in the Nebraska Food for Health Center at the University of Nebraska-Lincoln. 16S rRNA gene data were deposited in NCBI's Sequence Read Archive under BioProject ID PRJNA729569. Fastqs were processed by mothur 1.41.3, removing chimeras identified by uchime, mapping sequences against Silva release 132, and clustering OTUs at 99% identity using the OptiClust algorithm (Quast et al. 2013; Schloss et al. 2009). Because of the size of the study, two sequence processing runs were performed. The first run processed all sequences obtained from MBRAs. The second run processed all samples obtained from HMAmice along with matched MBRA samples from the same fecal donors (FS04, FS05, and FS06) and replicate samples of the fecal inocula. For FS05, we sequenced one aliquot collected at the time of colonization of Swiss Webster mice and three replicates collected at the time of MBRA studies and colonization of HMA mice of other genetic backgrounds. For both studies, mothur version 1.48.1 was used to rarefy samples to 6944 reads before downstream analysis. OTU tables for each study are available in Supporting Information S1 (Mice, Table S1; MBRA, Table S2).
2.6 Analysis of Small Organic Acid Levels by HPLC
We measured short and branched chain fatty acids from Day 5 samples collected from MBRA communities described above. A single sample was analyzed for each fecal donor/treatment combination, with the exception of untreated, cefaclor, and ceftriaxone, for which two replicates for each fecal donor/treatment were tested. Frozen supernatants were thawed and filtered through 0.22 μM filters, before injection of 100 μL into an Agilent 1260 Infinity LC system fitted with an HPX-87H column (BioRad) with a flow rate of 0.6 mL/min in 10 mM H2SO4 running buffer. The column was maintained at 55°C during the run. Peaks were detected by measuring absorption at 215 nm. The area under the curve was determined for every distinct peak present in ≥ 20% samples. Comparisons were made to standard curves of formate, acetate, propionate, butyrate, valerate, lactate, isovalerate, and isobutyrate resuspended in BRM3. Only acetate, butyrate, and isovalerate were detected in ≥ 20% of samples.
2.7 Statistical Testing
A combination of statistical packages was used for analysis including mothur v1.48.1, R v4.2.3, and GraphPad Prism v10.2.3. R packages used included ANCOMBC v1.6.4, DESeq2 v1.36.0, dplyr v1.1.4, ggplot2 v5.1, microbiome v1.18.0, microViz v0.12.0, OTUtable v1.1.2, pairwise Adonis v0.4.1, phyloseq v1.40.0, rstatix v0.7.2, stats v4.2.3 tidyverse v2.0.0, and vegan v2.6-4. Microsoft Excel was also used for data management and calculation of averages as indicated. All code and files for analysis are at Zenodo: https://zenodo.org/records/14728946; doi:10.5281/zenodo.14728946.
For MBRA data, relative abundance of taxa at the OTU and phylum level was plotted using ATIMA, a Shiny-based interface for R packages for microbiome analysis available at atima.research.bcm.edu. The number of observed OTUs was determined using the estimate_richness command of phyloseq, means of replicate data were determined in Excel, data was plotted in GraphPad Prism, and statistical significance of differences before and after treatment was determined by paired one-way ANOVA with Geisser-Greenhouse correction of sphericity of data and Holm-Sidak correction for multiple comparisons. Jaccard and Bray-Curtis dissimilarities were determined using mothur v1.48.1, with a custom Excel template used to extract relevant data, which was plotted in GraphPad Prism. Statistical significance of differences from untreated communities was determined with paired one-way ANOVA with Geisser-Greenhouse correction of sphericity of data and Holm-Sidak correction for multiple comparisons. Differences in relative abundance of taxa in antibiotic-treated communities compared to untreated communities were identified using DESeq2 and ANCOM-BC2 at the genus and OTU levels. For genus-level analysis with DESeq2, OTU abundances were combined at the genus-level using the tax_glom function of phyloseq. Pairwise comparisons between each antibiotic-treated and untreated community were performed with DESeq2 with default parameters. For ANCOM-BC2, analysis was performed with the default parameters recommended in its tutorial (ANCOM-BC2 Tutorial 2025). Log2 ratios of OTU abundance in antibiotic-treated and untreated communities were determined in Excel. Specifically, average abundances of each OTU across replicate samples for each fecal donor/treatment condition were determined. Log2 ratios between antibiotic-treated and untreated communities were then determined for each fecal sample, and the mean of these log2 ratios across all fecal samples was determined. Ratios were matched with the list of genera (Supporting Information S3: Table S3) and OTUs (Supporting Information S3: Table S4) identified as significantly different following treatment with one or more antibiotics across analysis tools. Finally, the mean of log2 ratios across all fecal samples of genera determined to be differentially abundant were plotted as a heatmap using ggplot2. For tentative species-level assignments of 16S rRNA gene sequences, we used a combination of the Unassigner tool in BioConda and BLAST. As recently described (Tanes et al. 2024), the Unassigner tool aligns sequences to the Living Tree project reference database and determines the potential number of mismatches to the full length 16S rRNA gene sequence and the probability of accurate taxonomic assignment. We report taxonomy assigned with < 4 mismatches and a p-value < 0.1. In the case of more than one match, we report the top hit. In the case that high confidence taxonomic assignments could not be provided through Unassigner, BLAST (Altschul et al. 1990) against nr/nt database with default parameters excluding uncultured samples was used for tentative taxonomic assignment (100% minimum query coverage; 100% sequence identity). Representative sequences for each OTU are provided in Table A5 to facilitate reanalysis as taxonomy continues to be refined, although we also acknowledge the limitations of partial 16S rRNA gene sequences for high confidence taxonomic assignments (Tanes et al. 2024).
Similar procedures were used for analysis of HMAmice data and comparisons between HMAmice, fecal inocula, and bioreactor data. Compositional differences in untreated HMAmice and fecal inocula were visualized through a combination of a heatmap of the 100 most abundant OTUs, which was generated using the plot_heatmap function of phyloseq, PCoA plots of Bray-Curtis dissimilarity calculated with vegan and plotted with ggplot2, and plotting of phylum-level abundances, which were generated from OTU data using the tax_glom function in phyloseq, then plotted in GraphPad Prism. Statistical significance between treatment groups in the PCoA plot was determined with pairwise Adonis in R. Statistical significance of differences in phyla abundance was determined with one-way ANOVA with Brown-Forsythe and Welch correction for unequal variance and Dunnett's correction for multiple testing in GraphPad Prism. The number of observed OTUs and Bray-Curtis and Jaccard dissimilarities were determined using mothur v.1.48.1, with a custom Microsoft Excel template used to extract relevant data, and plotted in GraphPad Prism, with statistical tests performed as described above. Species with differential abundance between treated and untreated communities on Day 5 were determined through a combination of ANCOM-BC2 and DESeq2 at the genus and OTU levels as described above. The average log2 fold changes from untreated communities were calculated (Supporting Information S3: Table S5, differentially abundant genera; Supporting Information S3: Table S6, differentially abundant OTUs) and the data were visualized through a heatmap as described above. Comparisons between MBRA and HMAmice models were performed at the OTU level (observed OTUs and Bray-Curtis dissimilarity) and the genus-level (differentially abundant taxa; Supporting Information S3: Table S7). For differentially abundant taxa shared between models, taxa were matched at the genus level and the relative abundance of taxa in antibiotic-treated samples compared to untreated controls was plotted for both models when at least one model had statistically significant values; the heatmap was plotted in GraphPad Prism.
3 Results
3.1 Magnitude of Microbiota Disruption Varies by Fecal Donor and Antibiotic Class in Fecal MBRAs
Independent minibioreactor array (MBRA) communities were established from the stool of 12 healthy human donors (FS01–FS12). After allowing 1 week for communities to stabilize (Auchtung et al. 2015), analysis of microbial communities through sequencing the V4 region of the 16S rRNA gene revealed that communities sampled before antibiotic treatment (Day 0) varied in diversity, with a median of between 108 and 178 observed operational taxonomic units (OTUs; clustered at 99% nucleotide identity) per donor and a mean relative abundance of 45.0% Bacteroidota, 33.5% Bacillota, 18.3% Pseudomonadota, 1.8% Verrucomicrobiota, 0.8% Fusobacteriota, 0.3% Synergistota, 0.1% Cyanobacteriota, and 0.08% Actinomycetota (Figure A1). These communities represent a subset of the starting inoculum (~50% reduction in taxonomic diversity)(Huang et al. 2025), similar to what has been reported for other in vitro and animal models of the GI microbiome (Aluthge et al. 2020; Collins et al. 2015; Rajilić-Stojanović et al. 2010; Van Den Abbeele et al. 2013, 2010). We examined the response of these communities to 5 days of twice-daily treatment with 12 different clinically relevant antibiotics or an equivalent dose of water for untreated controls. Each donor/treatment combination was tested twice, with the exception of cefaclor, ceftriaxone, and untreated samples, which were tested four times. Changes in microbial community composition were assessed by 16S rRNA gene sequencing at the end of antibiotics (Day 5).
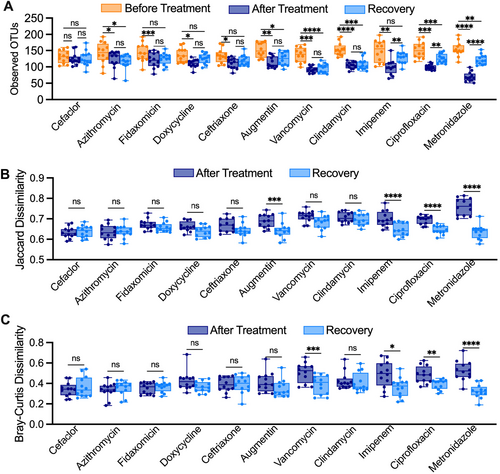
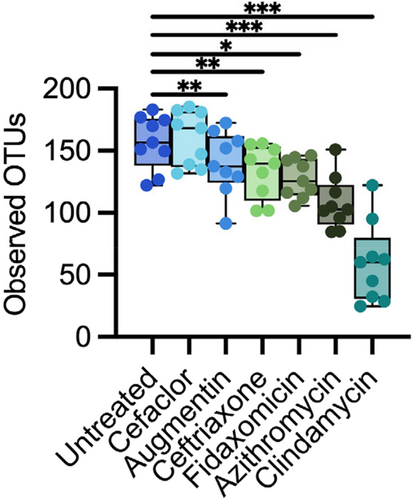
Overall, we observed a range of responses to different antibiotics across all fecal samples (Figure 1). Treatment with all antibiotics except sulfamethoxazole led to significant declines in observed OTUs between samples collected before and after antibiotics (Figure 1A). Levels of observed OTUs were not significantly different following treatment with water in untreated controls. Observations for Jaccard dissimilarity, which assesses compositional similarity based upon the presence of shared OTUs, were similar to those for observed OTUs, with all antibiotics with the exception of sulfamethoxazole and azithromycin showing significantly larger changes in Jaccard dissimilarity compared to untreated communities (Figure 1B). Fewer antibiotics showed significant changes in Bray-Curtis dissimilarity, which assesses changes in community structure based upon abundances of shared OTUs (Figure 1C). The extent to which antibiotic treatment led to loss of OTU richness also varied across fecal donors (Figure 1D), with all fecal communities except for fecal donor #6 (FS06) exhibiting statistically significant decreases in OTU richness. However, decreases in median richness in response to all antibiotics varied from 15% (7%–27% interquartile range [IQR]) for FS06% to 32% (21%–43% IQR) for FS02, pointing to donor-specific responses to antibiotic treatment.

We also examined the persistence of microbiota disruption after antibiotic treatment by assessing community composition 2 days following the end of antibiotic treatment in two replicates per treatment/donor (Figure A2; Recovery). While OTU richness remained significantly lower than before treatment for most antibiotics tested, a subset of antibiotics showed partial recovery of OTU richness (Figure A2A; imipenem, ciprofloxacin, metronidazole), microbiota composition (Figure A2B; Augmentin, imipenem, ciprofloxacin, metronidazole) and/or microbiota structure (Figure A2C; vancomycin, imipenem, ciprofloxacin, metronidazole) 2 days following cessation of antibiotics.
3.2 Conserved Effects of Antibiotic Treatments on Taxonomic Diversity in Fecal MBRAs
We next assessed how antibiotic treatment affected levels of specific taxa across fecal communities cultured in MBRAs using a combination of ANCOM-BC2 (Lin and Peddada 2024) and DESeq2 (Love et al. 2014). We performed analyses primarily at the genus level, as the number of genera shared in ≥ 50% of fecal sample communities after treatment (n = 26 genera) was higher than the number of OTUs shared in ≥ 50% of fecal sample communities after treatment (n = 23 OTUs). We observed a total of 49 genera were differentially abundant following treatment with one or more antibiotics in one or more tests (Figure 2). As had been reported previously (Nearing et al. 2022), ANCOM-BC2 identified a smaller number of genera with significant differences from untreated communities across antibiotics (n = 14) compared to DESeq2 (n = 46), although 11 genera were identified as having significant differences from untreated communities following treatment with one or more antibiotics in both tests (Figure 2, black circles; Supporting Information S3: Table S3).
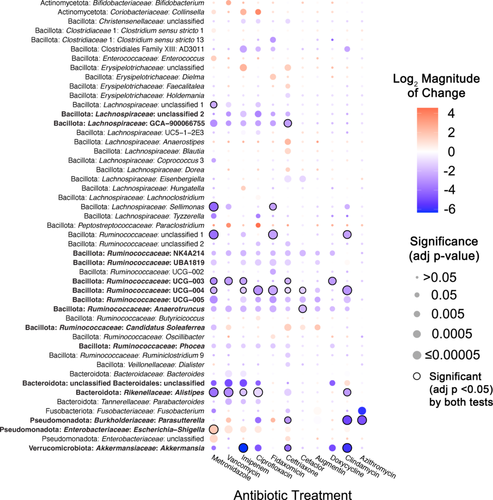
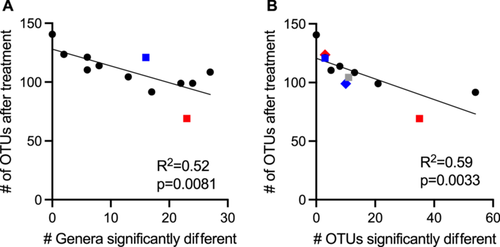
There was a wide range in the effects of different antibiotics on these 49 differentially abundant genera, with each antibiotic demonstrating a unique profile of affected taxa (Figure 2). The largest number of differentially abundant genera was observed in communities treated with ceftriaxone (27 genera), whereas there were no genera whose abundance differed significantly from untreated controls in sulfamethoxazole-treated communities. Overall, there was a strong correlation (R2 = 0.52, p = 0.0081) between lower levels of observed OTUs following antibiotic treatment and the number of genera that were differentially abundant in communities treated with the same antibiotic (Figure A3). Notable exceptions included fidaxomicin and metronidazole-treated communities, which had higher and lower levels of genera that were significantly different from untreated communities than would be predicted by the changes in observed OTUs.
The majority of affected genera were members of Bacillota phylum (38 genera), which is consistent with the prevalence of these families in fecal samples and bioreactor microbial communities, as well as the diversity of genera within these families (Figure A1; Supporting Information S3: Table S2). Four genera within the Bacteroidota showed significant differences in one or more antibiotic-treated communities compared to untreated communities. Identification of a single Bacteroides genus in the Bacteroidaceae family as differentially abundant reflects the more limited diversity of Bacteroidaceae genera in these communities, as similar numbers of OTUs classified as Bacteroidaceae (18 OTUs) and Ruminococcaceae (17 OTUs) were differentially abundant compared to untreated communities (Supporting Information S3: Table S4). Notable changes for members of other phyla include significantly lower levels of Akkermansia (Verrucomicrobiota) and higher levels of Escherichia-Shigella (Pseudomonadota) in communities treated with six different antibiotics. There were also eight abundant genera (total relative abundance across all samples > 0.5%) that were not significantly different between untreated communities and communities treated with antibiotics (Table A6).
In total, 27 genera were significantly lower in one or more antibiotic-treated communities, 17 genera were significantly higher in communities treated with one or more antibiotics, and 5 genera were higher or lower than untreated communities depending upon the antibiotic used for treatment. Some taxa were affected similarly across many classes of antibiotics. Fifteen genera were significantly different in communities treated with five or more antibiotics compared to untreated (Figure 2, taxa in bold text), indicating that they were highly susceptible to disruption following antibiotic treatment.
3.3 Vancomycin Inhibits Growth of Bacteroidota Isolates in Pure Culture
Treatment with vancomycin led to a decrease in nine genera of Bacillota as well as four genera of Bacteroidota. At the OTU level, 13 OTUs classified as Bacillota and 18 OTUs classified as Bacteroidota (including 14 Bacteroides species) were significantly lower in vancomycin-treated communities compared to untreated communities (Supporting Information S3: Table S4). The limited number of studies documenting the sensitivity of gastrointestinal Bacteroidota isolates to vancomycin (Citron et al. 2012; Ednie et al. 2003; Yehya et al. 2013) were done under different conditions and concentrations of vancomycin. The 150 μg/mL used in these studies is higher than typical fecal concentrations when vancomycin is administered intravenously (Currie and Lemos-Filho 2004) but lower than when taken orally (Gonzales et al. 2010; Thabit and Nicolau 2015). To further examine the sensitivity of Bacteroidota species to vancomycin, we tested sensitivity of multiple type strains and fecal isolates to 150 μg/mL vancomycin when grown in bioreactor media (BRM3) and a broad range growth media for gut microbial isolates (YCFA, Browne et al. 2016). This study included eight Bacteroides, two Phocaeicola, two Parabacteroides, and one Alistipes (Figure A4). One strain, Bacteroides cellulolyticus, grew poorly in BRM3. None of the Bacteroidota strains were able to grow in the presence of 150 μg/mL vancomycin in BRM3 or YCFA media. Control strains of Gram-positive Enterococcus faecalis and Limosilactobacillus reuteri and Gram-negative Escherichia coli were also unable to grow at this level of vancomycin when in BRM3, but were resistant in YCFA (or MRS for L. reuteri).
3.4 Magnitude of Microbiota Disruption Varies by Fecal Donor and Mouse Background in HMAMice
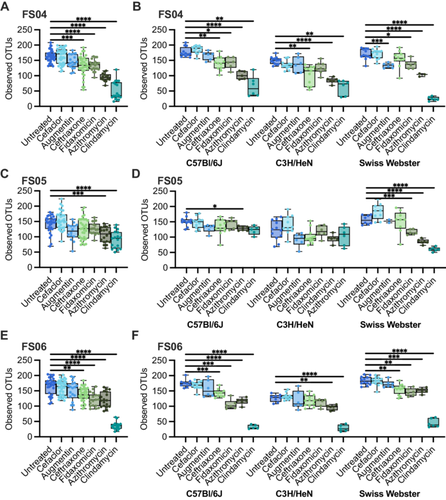
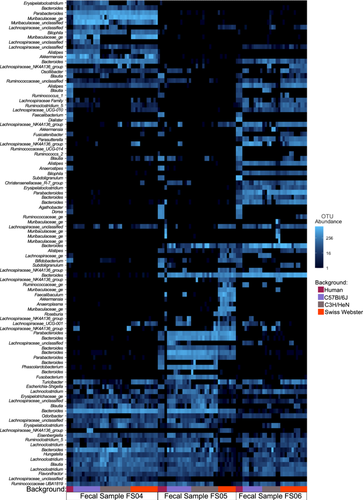
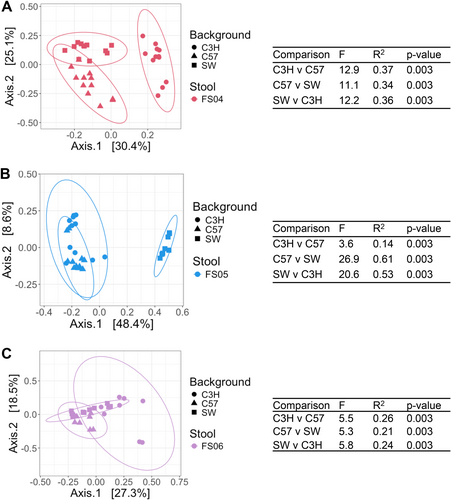
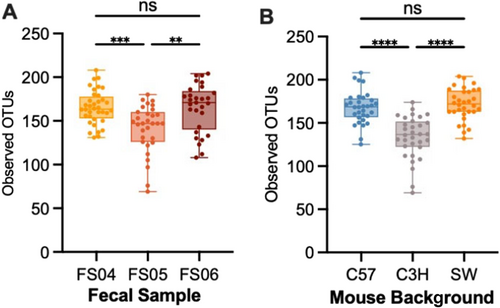
To determine whether the effects of antibiotic treatment on microbial diversity were similar in another commonly used model of the human GI microbiome, we colonized germ-free mice from three genetic backgrounds (C57Bl/6J, C3H/HeN, and Swiss Webster) with fecal samples 4, 5, and 6. Previously, we had used C57Bl/6J mice stably colonized with fecal material pooled from 12 healthy humans to investigate susceptibility to C. difficile infection (Collins et al. 2015; Auchtung et al. 2020). In these previous studies, mice were initially colonized with fecal material under germ-free conditions, then bred under specific pathogen-free conditions. The composition of the fecal microbiota in originally colonized germ-free mice (P1 generation) was most similar to the fecal inoculum, with a shift in microbiota composition observed in the F1 generation that was stable in subsequent generations (F2 and greater). We used the same approach here to colonize mice with fecal material under germ-free conditions and test the effects of antibiotic treatment in progeny mice. Progeny mice were treated once daily by orogastric gavage for 5 days with 1 of 6 different antibiotics (Augmentin, azithromycin, fidaxomicin, cefaclor, ceftriaxone, or clindamycin) or PBS for untreated controls (n = 4–12 mice/treatment; Table A2). Antibiotic concentrations are listed in Table 1. Fecal samples were collected from mice at the end of treatment and microbiota community composition was analyzed through sequencing of the V4 region of the 16S rRNA gene. Analysis of untreated mice demonstrated that both mouse strain and fecal donor influenced taxonomic composition and overall diversity. Differences in taxonomic composition were primarily driven by the fecal donor used for colonization (Figures 3A and A5). However, there were significant differences in microbiota richness (Figure 3B) and microbial community structure (Figure A6) within mice of different genetic backgrounds colonized by samples from a single fecal donor. Overall, mouse communities had a mean relative abundance of 58.0% Bacteroidota, 36.0% Bacillota, 2.9% Verrucomicrobiota, 1.9% Pseudomonadota, 0.8% Fusobacteriota, 0.2% Actinomycetota, 0.2% Mycoplasmatota, and 0.03% Cyanobacteriota (Figure 3C–E). Mice colonized with FS05 had significantly lower numbers of total OTUs, with a mean of 141.5 OTUs/sample, compared to mice colonized with FS04 (mean of 165.3 OTUs) or FS06 (mean of 165.1 OTUs; Figure A7A). C3H/HeN mice also exhibited significantly lower levels of observed OTUs, with a mean of 133.4 OTUs/sample, compared to 167.5 for C57Bl/6J and 171.7 for Swiss Webster (Figure A7B).
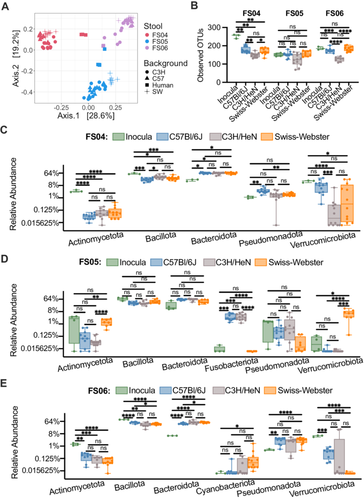
To determine the effects of antibiotic treatment on bacterial richness, we compared differences between antibiotic-treated and control (PBS-treated) mice. Across all mice tested, we observed that treatment with all antibiotics with the exception of cefaclor led to significantly lower levels of observed OTUs compared to untreated (Figure A8). The extent to which treatment with other antibiotics impacted bacterial richness varied by fecal community and mouse background (Figure 4). Ceftriaxone, fidaxomicin, azithromycin, and clindamycin treatment all led to significantly lower levels of OTUs overall in mice colonized with FS04 (Figure 4A) and FS06 (Figure 4E), whereas levels of observed OTUs were not significantly lower than in untreated mice following treatment with ceftriaxone and fidaxomicin in mice colonized with FS05 (Figure 4C). Comparing across mice of different genetic backgrounds colonized with the same fecal sample (Figure 4B,D,F), it is clear that both mouse genetic background and fecal community affect the response to antibiotic treatment. Clindamycin resulted in the most significant differences in richness compared to untreated mice across all mice and fecal communities tested.
3.5 Conserved Effects of Antibiotic Treatments on Taxonomic Diversity in HMAMice
We examined how antibiotics altered taxonomic representation in HMAmice using the approach described above for MBRA communities. Overall, a total of 78 genera were identified as differing significantly between one or more antibiotic-treated groups and the untreated group. Again, DSeq2 testing identified a larger number of genera as significantly different between antibiotic-treated and untreated communities (77 genera) compared to ANCOM-BC2 (51 genera), although there was higher consensus between the two tests (49 genera; Figure 5, circled comparisons; Supporting Information S3: Table S5).
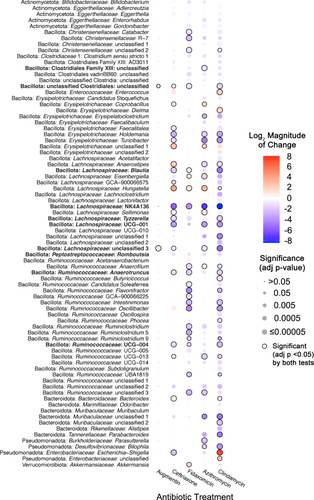
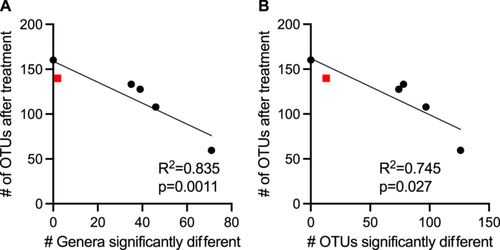
Each antibiotic demonstrated a unique profile of affected taxa (Figure 5), with the largest number of differentially abundant genera observed in mice treated with clindamycin (71 genera), whereas there were no genera whose abundance differed significantly from untreated mice in cefaclor-treated mice. There was a stronger correlation (R2 = 0.835, p = 0.0011) between lower levels of observed OTUs following antibiotic treatment and a higher number of genera that were differentially abundant in communities treated with the same antibiotic (Figure A9), with Augmentin-treated mice the only notable exception, with lower levels of genera that were significantly different from untreated communities than would be predicted by the level of observed OTUs following treatment.
Genera that were differentially abundant in antibiotic-treated mouse communities compared to untreated controls belong to five different phyla (Actinomycetota, Bacillota, Bacteroidota, Pseudomonadota, and Verrucomicrobiota). Again, the majority of affected genera were members of the Bacillota phylum (61 genera), consistent with the prevalence of these families in HMAmice (Figure 3; Supporting Information S3: Table S1). Seven genera within the Bacteroidota showed significant differences compared to untreated communities; as with MBRA communities, higher numbers of Bacteroides OTUs were observed as differentially abundant compared to genera (Supporting Information S3: Table S6). Notable changes for members of other phyla were significantly higher levels of Akkermansia (Verrucomicrobiota) in communities treated with fidaxomicin and azithromycin, lower levels of four Actinomycetota genera in two or more antibiotic-treated communities, and alterations in the levels of four Pseudomonadota genera. There were also two abundant genera (Phascolarctobacterium and Fusobacteria) that were not significantly different between untreated communities and communities treated with antibiotics.
In total, 60 genera were significantly lower in one or more antibiotic-treated communities; 10 of these genera were significantly lower in mice treated with four different classes of antibiotics compared to untreated mice (Figure 5, taxa in bold font). In contrast, six genera were significantly higher in one or more antibiotic-treated communities, and 12 genera were significantly higher or lower than untreated communities, depending upon the antibiotic used for treatment. For example, Escherichia-Shigella was significantly higher in clindamycin-treated mice, but significantly lower in azithromycin and ceftriaxone-treated mice, providing an example of context-dependent effects of antibiotics on specific taxa.
3.6 Comparison of Effects of Antibiotic Treatment Between MBRA and HMAMice
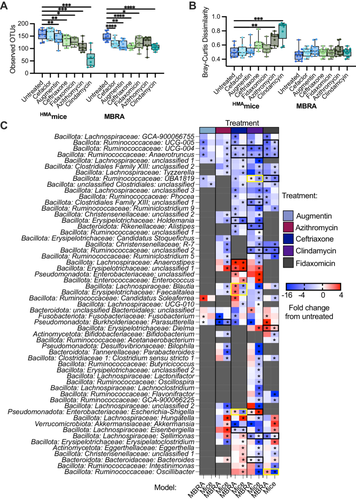
When we compared the subset of antibiotics tested in both models, we observed that most of the antibiotics tested led to reduction in levels of observed OTUs in antibiotic-treated communities compared to untreated controls across both models (Figure 6A). The two antibiotics that did not follow this trend were cefaclor, which led to significantly lower levels of observed OTUs in MBRAs but not in HMAmice, and azithromycin, which led to significantly lower levels of observed OTUs in HMAmice but not in MBRAs. There were no significant differences in microbiota structure between untreated and antibiotic-treated communities in the MBRA model, whereas clindamycin and azithromycin treatment led to significant changes in community composition compared to untreated controls (Figure 6B). In total, 88 genera were identified as significantly different in MBRA (Figure 2, 49 genera) and/or HMAmice (Figure 5, 79 genera) treated with one or more antibiotics compared to untreated controls. Of these 88 genera, we focused on comparisons of the 57 genera that were present in 10 or more samples of HMAmice and MBRA communities to facilitate robust comparisons. Comparing these shared genera demonstrated 22 significant differences in antibiotic-treated communities compared to controls that were similar across both models (Figure 6C, black boxes), as well as five significant differences in antibiotic-treated communities that differed between models (Figure 6C, yellow boxes). Significant differences that were shared between models included lower levels of multiple Ruminococcaceae and Lachnospiraceae genera in response to treatment with ceftriaxone, clindamycin, and/or fidaxomicin, decreases in Alistipes in response to clindamycin-treatment, decreases in Parasutterella in azithromycin-treated communities, increases in the levels of unclassified Erysipelotrichaceae and Enterobacteriaceae genera in ceftriaxone-treated communities, increases in levels of Escherichia-Shigella in clindamycin-treated communities, and increases in Dielma in fidaxomicin-treated communities. For those genera/antibiotic combinations that were significant in one model (Figure 6C starred) and not the other (Figure 6C unstarred) (n = 166), 68% demonstrated a similar, nonsignificant change in taxa abundance in the other model.
4 Discussion
Here, we examined the effect of multiple antibiotics on the communities of human fecal bacteria established in two different model systems. As expected, we observed changes in bacterial richness and community structure, along with changes in the abundance of specific taxa compared to untreated controls for most antibiotics tested. Overall, we observed that abundances of many genera of Gram-positive anaerobes in the Bacillota phylum (primarily members of the Lachnospiraceae and Ruminococcaceae families) were lower in communities treated with most of the antibiotics tested, although there were some variations in responses between models. As many members of the Lachnospiraceae and Ruminococcaceae families are known to produce the beneficial short chain fatty acid (SCFA), butyrate (Singh et al. 2023), loss of these taxa is likely to result in reductions in butyrate levels during antibiotic treatment. Lachnospiraceae have also been shown to inhibit C. difficile colonization (Reeves et al. 2012), so loss of these taxa may also indicate decreased colonization resistance, which is consistent with our previous observations using these models to study C. difficile colonization resistance (Auchtung et al. 2020; Collins et al. 2015; Huang et al. 2025; Robinson et al. 2014).
We also observed differences in the abundance of multiple genera in the Gram-negative phylum Bacteroidota following treatment with several classes of antibiotics. Bacteroidota species have been previously identified as foundational taxa in GI microbial communities (Trosvik and de Muinck 2015), so loss of these taxa and their interactions with other members of the microbiota are likely to have significant effects on microbiota function. Consistent with previous studies (Looft and Allen 2012), we observed higher levels of potential pathobiont Escherichia-Shigella in communities treated with several classes of antibiotics. Enterococcus, another potential pathobiont demonstrated to increase following antibiotic treatment (Dubin and Pamer 2017), was also significantly higher in metronidazole-treated MBRA communities and ceftriaxone and clindamycin-treated mice. Akkermansia were present at significantly lower levels in MBRA communities treated with ceftriaxone, ciprofloxacin, fidaxomicin, imipenem, and metronidazole. Altogether, these data demonstrate that both models can be used to reproduce the disruptive effects of antibiotics on the microbiota that have been previously reported.
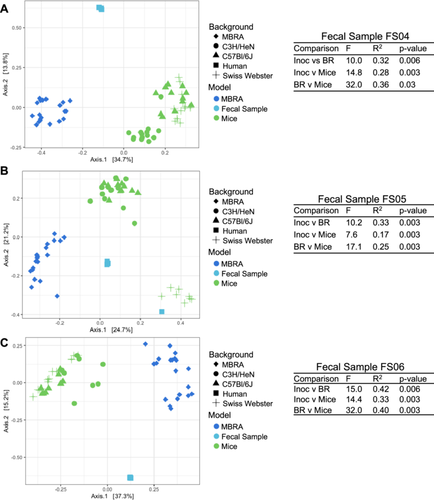
While we observed several taxa that were affected similarly by antibiotic treatment across models, we observed many differences. There are many factors known to play a role in susceptibility to antibiotics, including the composition of phage populations (Modi et al. 2013), bacterial growth rate (Greulich et al. 2015), the presence of mucin (Samad et al. 2019), biofilms (Sharma et al. 2019), immune cells (Ramiro et al. 2016), and pharmacokinetics (Onufrak et al. 2016). While doses of antibiotics were estimated based upon previously published concentrations (as described in Methods), differences in pharmacokinetics in the mouse model are one factor that likely contributed to some of the differences that we observed between HMAmice and MBRA models. Another major difference between the models was the abundance of taxa before treatment. A direct comparison of untreated HMAmice, MBRA communities before antibiotic treatment, and the fecal inocula used to colonize both models (Figure A10) demonstrated significant differences in the structure of each community. For the three fecal donors studied, we observed one fecal donor (FS05) exhibited more similar community structures between HMAmice and fecal inocula, one fecal donor (FS04) exhibited more similar community structures between MBRA communities and the fecal inoculum, and the third fecal donor (FS06) had community structures in MBRA and HMAmice that were equally dissimilar to the starting inoculum. Examining differences in specific taxa between models also illustrated these differences. For example, in mice, Turicibacter was present in 55% of samples, composing ~0.7% of reads overall. It decreased in relative abundance upon azithromycin and clindamycin treatment. In bioreactors, however, it was detected in only 0.38% samples and comprised 0.00025% of reads. Similarly, Dialister, which was present in 33% of MBRA communities and represented ~0.5% of total reads was significantly lower in ceftriaxone-treated communities compared to untreated controls. However, Dialister was found in < 0.4% of mouse samples and represented only 5.7 × 10−5% of reads overall.
4.1 Comparison of Results to Commonly Understood Taxonomic Spectrum of Antibiotics
We compared our results to other studies on the effects of antibiotics on microbiota to better understand how our models compare to previously published data and known antimicrobial spectrums. Overall, we observed several trends that were consistent between our models and previously published studies of effects on the GI microbiota, but we also noted differences. This was not surprising, as even studies with different populations of human subjects report different observations. Below we focus on antibiotics, vancomycin and metronidazole, with a more complete discussion of all antibiotics studied in Appendix 1.
Vancomycin is known to affect Gram-positive cell walls and is most commonly used to target infections of the Bacillota Staphylococcus or Clostridioides difficile (Levine 2006). Gram-negative bacteria such as the Bacteroidota are generally thought to be resistant, and low concentrations of vancomycin are even used in some nominally Bacteroides-selective medias. Although vancomycin has been described as narrow spectrum (Hermans and Wilhelm 1987), previous studies (Isaac et al. 2017; Lewis et al. 2015; Rea et al. 2011) along with the work described here demonstrates its broad effects on the microbiome. Some oral Bacteroides isolates are known to be sensitive to vancomycin (Van Winkelhoff and De Graaff 1983), and there was a decreased relative abundance of Bacteroidota observed in mouse feces (Ajami et al. 2018), C. difficile-infected mice (Lewis et al. 2015; Yamaguchi et al. 2020), and human volunteers (Isaac et al. 2017; Russell et al. 2013) treated with vancomycin. The wide spectrum of vancomycin activity may contribute to its serious side-affects, such as increased susceptibility to developing asthma (Russell et al. 2013), risk of recurrence of C. difficile infection (Cornely et al. 2013), and association with increased prevalence of vancomycin-resistant enterococci (Fridkin et al. 2001).
Although Gram-positive bacteria may be more susceptible to low concentrations of vancomycin; at higher concentrations, Gram-negative bacteria may be equally affected. Vancomycin is a hydrophilic glycopeptide. At low concentrations, hydrophilic molecules primarily travel through the outer membrane via porins; however, vancomycin is too large (Yarlagadda et al. 2016). Other less characterized mechanisms are responsible for cell entry at higher concentrations. In the presence of bile salts, cell membranes will have increased permeability (Begley et al. 2005) and therefore are possibly more susceptible to alternate modes of entry. This observation may explain the higher sensitivity to vancomycin treatment that we observed for control strains grown in BRM3 (which contains bovine bile) compared to YCFA (which lacks bile), although differences in other nutrients between the two media may also contribute to these observations.
Metronidazole does not accumulate to high levels in feces of patients undergoing standard metronidazole treatment (1–24 mg/g stool [Bolton and Culshaw 1986]), so the concentration used for treatment here (200 μg/mL) was higher than would be expected and effects are likely to be of reduced magnitude in patients. The partial recovery in diversity observed after cessation of antibiotic treatment we observed in MBRA communities is consistent with rapid microbiota recovery following cessation of treatment in C57Bl/6 mice (Lewis et al. 2015), although recovery following treatment in dogs (Belchik et al. 2024) and children (Gotfred-Rasmussen et al. 2021) was longer (1–2 months). We also observed similar increases in levels of Bifidobacterium, Enterobacteriaceae (Escherichia-Shigella), and Enterococcus to those reported in previous studies in dogs and rats (Marshall-Jones et al. 2024; Pélissier et al. 2010; Pilla et al. 2020).
4.2 Potential Loss of Taxa Secondarily Affected by Antibiotics
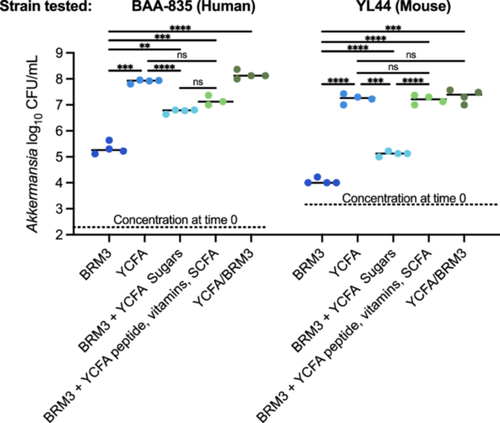
We observed several taxa with lower levels of relative abundance following treatment with many classes of antibiotics. While some of these taxa may be highly susceptible to antibiotics, other taxa may have had lower levels of relative abundance across multiple antibiotic treatments because they were reliant on commensal or synergistic microbial interactions lost during treatment. A. muciniphila was one taxa that was significantly lower in MBRA communities treated with ceftriaxone, ciprofloxacin, clindamycin, doxycycline, imipenem, and metronidazole but was not significantly lower in antibiotic-treated HMAmice. Previous characterization of A. muciniphila indicated that this species is susceptible to imipenem (Dubourg et al. 2017, 2013), ceftriaxone (Dubourg et al. 2017), and doxycycline (Dubourg et al. 2013), but resistant to clindamycin (Cozzolino et al. 2020), metronidazole (Dubourg et al. 2013), and ciprofloxacin (Filardi et al. 2022). Treatment with ciprofloxacin (Rodriguez-Ruiz et al. 2024) and clindamycin (Buffie et al. 2012) also led to increases in Akkermansia in patients and mice, respectively, consistent with resistance to these antibiotics.
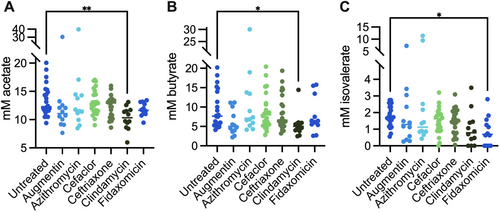
Pure culture isolates of A. muciniphila from human (BAA-835) and mouse (YL44) samples grew poorly in monoculture in bioreactor medium (BRM3), but grew well in the rich medium, YCFA (Figure A11). Addition of the mixture of SCFAs and vitamins found in YCFA to BRM3 allowed for partial (A. muciniphila BAA-835) or full (A. muciniphila YL44) restoration of growth to levels observed in YCFA. This data suggests that BRM3 lacks factor(s) important for A. muciniphila growth that can be provided by YCFA. We propose that these factors are likely also provided by other members of the community when grown in a mixed population. Consistent with a potential role for SCFA supporting growth of A. muciniphila in mixed communities, we observed significantly lower levels of acetate and butyrate in clindamycin-treated communities compared to untreated MBRA communities when we measured levels of these metabolites at the end of antibiotic treatment in a subset of antibiotic-treated communities (Figure A12).
While A. muciniphila itself has been proposed to be a keystone species, as both Anaerostipes caccae (Chia et al. 2018) and Clostridium difficile (Engevik et al. 2020) have been demonstrated to benefit from coculture with A. muciniphila, there is little known about commensal interactions between A. muciniphila and other bacteria. One study demonstrated that the presence of Parabacteroides merdae leads to enhanced growth of A. muciniphila (Olson et al. 2018), but the nature of this interaction was not characterized.
While these data are consistent with the hypothesis that A. muciniphila can be lost in MBRA communities due to loss of commensal interactions with antibiotic-susceptible microbes, the alternative hypothesis that strains present in these communities were susceptible to antibiotics cannot be disproved. Although not statistically significant, Akkermansia levels were also lower in clindamycin-treated HMAmice. While vancomycin treatment led to lower levels of many taxa that were also reduced following treatment with ceftriaxone, ciprofloxacin, clindamycin, imipenem, and metronidazole, but lower levels of Akkermansia were not observed. Previous studies demonstrated resistance of A. muciniphila to vancomycin (Dubourg et al. 2013). Future studies are needed to identify the role of potential synergistic or commensal interactions between Akkermansia and other gut microbes during growth in conditions that may exist in the lumen of the GI tract.
4.3 Limitations of This Study
While several interesting observations emerged from these studies, there are some limitations that may reduce the broader translatability of these observations to human subjects. Both models were limited in their ability to fully recapitulate the complexity of communities found in human fecal samples. While some of the microbes lost from fecal communities were likely nonviable, others, such as Faecalibacterium species (Lopez-Siles et al. 2017), likely contribute important functions to the microbiota. Although this is a common limitation to models of the GI microbiota (Allen-Vercoe 2013; Arrieta et al. 2016), it is important to acknowledge this limitation when drawing parallels with studies in humans.
While minibioreactors allowed more fecal samples to be tested in higher throughput at reduced cost, effects of antibiotics that were influenced by pharmacokinetics, host metabolites, and/or microbiome–immune interactions could not be studied. As pharmacokinetics can alter the concentrations of antibiotics and their metabolites that reach the microbiota, future comparisons between model systems should cross-validate concentrations of antibiotics and metabolites in the ceca of mice with those used in the MBRA model to facilitate more direct comparisons. Although HMAmice provide these more complex interactions, more extensive experimentation was limited by ethical concerns to reduce the number of animals used in research (Hubrecht and Carter 2019) and the higher costs associated with animal experimentation. Additionally, the compositional variation introduced as a result of interactions between mouse genetic background and microbiota composition indicated the potential importance of strain background in understanding the effects of antibiotics on the microbiota when working with HMAmice.
Furthermore, testing a larger number of fecal microbial communities with smaller numbers of replicates also limited the ability to draw statistically significant inferences about microbial communities from a single donor in MBRAs (n = 2/microbiota/treatment combination) and reduced the confidence of these observations within specific mouse populations (n = 4–6 for most mouse/microbiota/treatment combinations). This tradeoff between testing a larger number of fecal samples or testing a smaller number of fecal samples in higher replication is one that is often encountered in microbiota studies. In this case, we decided to test a larger number of fecal samples to identify responses that were conserved across communities rather than within a single community.
Finally, while sequencing of the V4 region of the 16S rRNA gene represents a cost-effective approach to rapidly characterize many microbial communities and compare these communities to previous studies using well-validated analysis pipelines, this approach is limited in its ability to reveal differences in function between communities and to differentiate closely related species that may respond differently as a result of differences in antibiotic susceptibility. Future studies should utilize metabolomics to determine whether antibiotic disruption mediates similar alterations in microbial metabolites and/or metagenomics to investigate potential differences in antibiotic resistance genes among closely related strains that may alter microbiota responses to antibiotics.
5 Conclusions
In summary, this study highlights the importance of testing antibiotics on complex communities of microbes under conditions that mimic the gastrointestinal environment. Testing complex communities facilitates identification of potential context-dependent interactions that increase antibiotic susceptibility among taxa that would not be predicted based on testing with more limited panels of isolated strains. This study also demonstrates the strengths and weaknesses of the two models tested. Both models reproduced some of the observed effects of antibiotics on the microbiota reported in human and other animal studies. However, key differences were also observed. While this is not surprising, as many of the studies cited above also had conflicting observations, it still limits the ability to draw firm conclusions about the effects of each antibiotic on the microbiota of humans. Nevertheless, these data demonstrate that both models can be useful preclinical screening tools during the characterization of new antimicrobial compounds to combat the threats associated with continued spread of antibiotic resistance (Talbot et al. 2006).
Author Contributions
Thomas A. Auchtung: conceptualization, investigation, writing – original draft, formal analysis, data curation, visualization, writing – review and editing, methodology, supervision. Armando I. Lerma: conceptualization, investigation, writing – review and editing, methodology, data curation, formal analysis. Keegan Schuchart: investigation, methodology, data curation, writing – review and editing. Jennifer M. Auchtung: supervision, resources, data curation, writing – original draft, writing – review and editing, funding acquisition, conceptualization, visualization, formal analysis, project administration, methodology. All authors revised, edited, and approved the final manuscript.
Acknowledgments
We thank Hugh McCullough, Austin Johnson, Yining He, and Delphin Mutangana for technical assistance with MBRA experiments and growth of A. muciniphila strains. We thank Amanda Ramer-Tait, Robert Schmaltz, and Rafael Segura-Muñoz of the Nebraska Gnotobiotic Mouse Program and Kylie Farrell, formerly of Baylor College of Medicine, for their assistance in establishing HMAmice models. We thank Devin Rose (University of Nebraska-Lincoln) for use of HPLC for analysis of SCFA. We also acknowledge Kristin Beede and Paula Chacua for providing strains or assisting in their isolation. Sequence analysis was completed utilizing the Holland Computing Center, which receives support from the UNL Office of Research and Economic Development and the Nebraska Research Initiative. This study was supported by CDC contracts 200-2017-96080 and 75D301-18C-02909, as well as Nebraska Tobacco Settlement Biomedical Research Development Funds awarded to Jennifer M. Auchtung.
Ethics Statement
Protocols for collection and use of fecal samples were reviewed and approved by Institutional Review Boards at Baylor College of Medicine (protocol number H-38014) and University of Nebraska-Lincoln (protocol number 18585). Experiments with mice were performed according to protocols approved by the Institutional Animal Care and Use Committees of Baylor College of Medicine (AN-6675) and University of Nebraska-Lincoln (IACUC #1668, #1680) and are in accordance with the NIH Office of Laboratory and Animal Welfare (OLAW) guidelines.
Conflicts of Interest
Jennifer M. Auchtung and Thomas A. Auchtung have a significant financial interest in Synbiotic Health. The remaining authors declare no conflicts of interest.
Appendix 1:

| Augmentin (5:1 amoxicillin sodium salt to potassium clavulanate) | Research Products International |
| Azithromycin dihydrate | Thermo Scientific Chemicals |
| Cefaclor | Thermo Scientific Chemicals |
| Ceftriaxone sodium salt hemiheptahydrate | Thermo Scientific Chemicals |
| Clindamycin phosphate, 97+% | TCI America |
| Fidaxomicin | Apexbio Technology |
| Ciprofloxacin hydrochloride hydrate, 98% | Thermo Scientific Chemicals |
| Doxycycline monohydrate | Thermo Scientific Chemicals |
| Imipenem monohydrate, 98% | Thermo Scientific Chemicals |
| Metronidazole, 99% | Thermo Scientific Chemicals |
| Sulfamethoxazole, 98+% | TCI America |
| Vancomycin hydrochloride, USP grade | VWR |
| C57Bl6/J | C3H/HeN | Swiss Webster | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | FS06 | FS05 | FS04 | FS06 | FS05 | FS04 | FS06 | FS05 | FS04 |
| Augmentin | 6, (33%) | 6, (50%) | 6, (67%) | 6, (50%) | 6, (33%) | 6, (33%) | 6, (50%) | 2, (50%) | 6, (50%) |
| Azithromycin | 6, (50%) | 6, (67%) | 6, (50%) | 6, (67%) | 6, (33%) | 6, (33%) | 6, (67%) | 6, (33%) | 3, (0%) |
| Cefaclor | 12, (42%) | 11, (55%) | 12, (50%) | 12, (67%) | 10, (70%) | 12, (50%) | 12, (50%) | 9, (33%) | 12, (42%) |
| Ceftriaxone | 10, (50%) | 11, (27%) | 11, (36%) | 12, (50%) | 12, (42%) | 12, (58%) | 12, (58%) | 9, (33%) | 10, (50%) |
| Clindamycin | 5, (40%) | 5, (60%) | 6, (33%) | 5, (20%) | 6, (17%) | 5, (80%) | 6, (50%) | 6, (50%) | 6, (33%) |
| Fidaxomicin | 6, (33%) | 6, (67%) | 6, (50%) | 6, (67%) | 6, (83%) | 6, (50%) | 6, (67%) | 6, (50%) | 6, (50%) |
| Untreated | 9, (78%) | 11, (73%) | 12, (50%) | 8, (100%) | 12, (42%) | 12, (33%) | 12, (42%) | 8, (38%) | 12, (33%) |
- a Percent of female mice indicated in parentheses.
| Strain Number | Fecal sample | Media for isolation | Partial 16S rRNA gene sequence | Phylum | Family | Genus species (Best BLAST hit) |
|---|---|---|---|---|---|---|
| JMA0003 | FS06 | M2GSC | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGAGCGTAGGTGGATTGTTAAGTCAGTTGTGAAAGTTTGCGGCTCAACCGTAAAATTGCAGTTGAAACTGGCAGTCTTGAGTACAGTAGAGGTGGGCGGAATTCGTGGTGTAGCGGTGAAATGCTTAGATATCACGAAGAACTCCGATTGCGAAGGCAGCTCACTAGACTGCAACTGACACTGATGCTCGAAAGTGTGGGTATCAAACAGG | Bacteroidota | Bacteroidaceae | Bacteroides caecimuris |
| JMA0010 | FS06 | YCFA | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGAGCGTAGGTGGACTGGTAAGTCAGTTGTGAAAGTTTGCGGCTCAACCGTAAAATTGCAGTTGATACTGTCAGTCTTGAGTACAGTAGAGGTGGGCGGAATTCGTGGTGTAGCGGTGAAATGCTTAGATATCACGAAGAACTCCGATTGCGAAGGCAGCTCACTGGACTGCAACTGACACTGATGCTCGAAAGTGTGGGTATCAAACAGG | Bacteroidota | Bacteroidaceae | Bacteroides fragilis |
| JMA0049 | FS04 | M2GSC | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGTGCGTAGGCGGCCTTTTAAGTCAGCGGTGAAAGTCTGTGGCTCAACCATAGAATTGCCGTTGAAACTGGGGGGCTTGAGTATGTTTGAGGCAGGCGGAATGCGTGGTGTAGCGGTGAAATGCTTAGATATCACGCAGAACCCCGATTGCGAAGGCAGCCTGCCAAGCCATGACTGACGCTGATGCACGAAAGCGTGGGGATCAAACAGG | Bacteroidota | Tannerellaceae | Parabacteroides distasonis |
| JMA0110 | FS12 | YCFA | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGAGCGTAGGCGGACTATTAAGTCAGCTGTGAAAGTTTGCGGCTCAACCGTAAAATTGCAGTTGATACTGGTCGTCTTGAGTGCAGTAGAGGTAGGCGGAATTCGTGGTGTAGCGGTGAAATGCTTAGATATCACGAAGAACTCCGATTGCGAAGGCAGCTTACTGGACTGTAACTGACGCTGATGCTCGAAAGTGTGGGTATCAAACAGG | Bacteroidota | Bacteroidaceae | Bacteroides cellulosilyticus |
| JMA0119 | FS08 | YCFA | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGAGCGTAGGTGGACAGTTAAGTCAGTTGTGAAAGTTTGCGGCTCAACCGTAAAATTGCAGTTGATACTGGCTGTCTTGAGTACAGTAGAGGTGGGCGGAATTCGTGGTGTAGCGGTGAAATGCTTAGATATCACGAAGAACTCCGATTGCGAAGGCAGCTCACTGGACTGCAACTGACACTGATGCTCGAAAGTGTGGGTATCAAACAGG | Bacteroidota | Bacteroidaceae | Bacteroides thetaiotaomicron |
| JMA0127 | FS08 | YCFA | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGAGCGTAGATGGATGTTTAAGTCAGTTGTGAAAGTTTGCGGCTCAACCGTAAAATTGCAGTTGATACTGGATATCTTGAGTGCAGTTGAGGCAGGCGGAATTCGTGGTGTAGCGGTGAAATGCTTAGATATCACGAAGAACTCCGATTGCGAAGGCAGCCTGCTAAGCTGCAACTGACATTGAGGCTCGAAAGTGTGGGTATCAAACAGG | Bacteroidota | Bacteroidaceae | Phocaeicola dorei/vulgatus |
| JMA0128 | FS08 | YCFA | TACGGAGGATTCAAGCGTTATCCGGATTTATTGGGTTTAAAGGGTGCGTAGGCGGTTTGATAAGTTAGAGGTGAAATCCCGGGGCTTAACTCCGGAACTGCCTCTAATACTGTTAGACTAGAGAGTAGTTGCGGTAGGCGGAATGTATGGTGTAGCGGTGAAATGCTTAGAGATCATACAGAACACCGATTGCGAAGGCAGCTTACCAAACTATATCTGACGTTGAGGCACGAAAGCGTGGGGAGCAAACAGG | Bacteroidota | Rikenellaceae | Alistipes onderdonkii/finegoldii |
| JMA0129 | FS08 | YCFA | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGAGCGTAGGCGGACGCTTAAGTCAGTTGTGAAAGTTTGCGGCTCAACCGTAAAATTGCAGTTGATACTGGGTGTCTTGAGTACAGTAGAGGCAGGCGGAATTCGTGGTGTAGCGGTGAAATGCTTAGATATCACGAAGAACTCCGATTGCGAAGGCAGCCTGCTGGACTGTAACTGACGCTGATGCTCGAAAGTGTGGGTATCAAACAGG | Bacteroidota | Bacteroidaceae | Bacteroides uniformis |
| JMA0132 | FS08 | YCFA | TACGGAGGATGCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGTGCGTAGGTGGTAATTTAAGTCAGCGGTGAAAGTTTGTGGCTCAACCATAAAATTGCCGTTGAAACTGGGTTACTTGAGTGTGTTTGAGGTAGGCGGAATGCGTGGTGTAGCGGTGAAATGCATAGATATCACGCAGAACTCCAATTGCGAAGGCAGCTTACTAAACCATAACTGACACTGAAGCACGAAAGCGTGGGTATCAAACAGG | Bacteroidota | Porphyromonadaceae | Parabacteroides johnsonii |
| JMA0135 | FS01 | YCFA | TACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGAGCGTAGGTGGATTGTTAAGTCAGTTGTGAAAGTTTGCGGCTCAACCGTAAAATTGCAGTTGAAACTGGCAGTCTTGAGTACAGTAGAGGTGGGCGGAATTCGTGGTGTAGCGGTGAAATGCTTAGATATCACGAAGAACTCCGATTGCGAAGGCAGCTCACTAGACTGCAACTGACACTGATGCTCGAAAGTGTGGGTATCAAACAGG | Bacteroidota | Bacteroidaceae | Bacteroides ovatus/xylanisolvens |
| YCFA | BRM3 | BRM3 + YCFA Sugars | BRM3 + YCFA peptide, vitamins, and SCFA | BRM3 + YCFA | |
|---|---|---|---|---|---|
| Casitone | 10 g | — | — | 10 g | 10 g |
| Tryptone | — | 1 g | 1 g | 1 g | 1 g |
| Proteose peptone #3 | 2 g | 2 g | 2 g | 2 g | |
| Yeast extract | 2.5 g | 2 g | 2 g | 2.5 g | 2.5 g |
| Cysteine | 1 g | — | — | 1 g | 1 g |
| Sodium chloride | 0.9 g | 0.4 g | 0.4 g | 0.9 g | 0.9 g |
| Magnesium sulfate | 0.09 g | 0.01 g | 0.01 g | 0.09 g | 0.09 g |
| Calcium chloride | 0.09 g | 0.01 g | 0.01 g | 0.09 g | 0.09 g |
| Bovine bile | — | 0.5 g | 0.5 g | 0.5 g | 0.5 g |
| Tween 80 | — | 2 mL | 2 mL | 2 mL | 2 mL |
| Biotin | 10 μg | — | — | 10 μg | 10 μg |
| Cobalamin | 10 μg | — | — | 10 μg | 10 μg |
| p-aminobenzoic acid | 30 μg | — | — | 30 μg | 30 μg |
| Folic acid | 50 μg | — | — | 50 μg | 50 μg |
| Pyridoxamine | 150 μg | — | — | 150 μg | 150 ug |
| Vitamin K1 | 0.5 mg | 0.1 mg | 0.1 mg | 0.5 mg | 0.5 mg |
| Hemin | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg |
| Thiamine | 2 μg | — | — | 2 μg | 2 μg |
| Riboflavin | 2 μg | — | — | 2 μg | 2 μg |
| Sodium acetate | 2.7 g | — | — | 2.7 g | 2.7 g |
| Sodium propionate | 0.86 g | — | — | 0.86 g | 0.86 g |
| Isobutyrate | 1 mM | — | — | 1 mM | 1 mM |
| Isovalerate | 1 mM | — | — | 1 mM | 1 mM |
| Valerate | 1 mM | — | — | 1 mM | 1 mM |
| Sodium bicarbonate | 4 g | 2 g | 2 g | 4 g | 4 g |
| K2HPO4 | 0.45 g | 0.04 g | 0.04 g | 0.45 g | 0.45 g |
| KH2PO4 | 0.45 g | 0.04 g | 0.04 g | 0.45 g | 0.45 g |
| Glucose | 2 g | 0.04 g | 2 g | 0.04 g | 2 g |
| Maltose | 2 g | 0.15 g | 2 g | 0.15 g | 2 g |
| Cellobiose | 2 g | 0.15 g | 2 g | 0.15 g | 2 g |
| Arabinogalactan | — | 0.1 g | 0.1 g | 0.1 g | 0.1 g |
| Inulin | — | 0.2 g | 0.2 g | 0.2 g | 0.2 g |
| pH | 6.8 | 6.8 | 6.8 | 6.8 | 6.8 |
| Bacillotaa | Bacillota | Pseudomonadota | Bacillota | Bacillota | Bacillota | Bacillota | Bacillota | |
|---|---|---|---|---|---|---|---|---|
| Erysipelo-trichaceaeb | Acidamino-coccaceae | Desulfovibrio-naceae | Lachno-spiraceae | Peptostrepto-coccaceae | Erysipelo-trichaceae | Rumino-coccaceae | Lachno-spiraceae | |
| Erysipelato-clostridiumc | Phascolarcto-bacterium | Bilophila | Lachnospira-ceae UCG-004 | Paeni-clostridium | Erysipelotrichaceae UCG-003 | Flavonifractor | Agathobacter | |
| Untreated | 3.38 +/− 7.21% | 2.88 +/− 6.73% | 2.08 +/− 4.13% | 1.38 +/− 2.45% | 0.55 +/− 1.42% | 0.35 +/− 2.24% | 0.60 +/− 1.07% | 0.37 +/− 1.75% |
| Augmentin | 3.89 +/− 9.17% | 2.62 +/− 9.27% | 1.94 +/−7.73% | 0.99 +/− 2.59% | 0.39 +/− 5.01% | 0.76 +/− 4.53% | 0.37 +/− 1.37% | 0.45 +/− 3.19% |
| Azithromycin | 3.41 +/− 7.74% | 2.59 +/− 7.06% | 2.41 +/− 4.25% | 1.35 +/− 1.67% | 0.52 +/− 0.72% | 0.37 +/− 6.32% | 1.07 +/− 1.33% | 0.40 +/− 1.92% |
| Cefaclor | 2.63 +/− 6.50% | 3.33 +/− 7.69% | 2.01 +/− 5.14% | 1.95 +/− 2.99% | 0.73 +/− 2.05% | 1.07 +/− 0.42% | 0.87 +/− 2.08% | 0.80 +/− 2.35% |
| Ceftriaxone | 3.53 +/− 8.14% | 3.22 +/− 7.57% | 1.72 +/− 3.51% | 1.34 +/− 3.22% | 0.77 +/− 1.63% | 1.06 +/− 1.51% | 0.53 +/− 1.86% | 1.21 +/− 2.81% |
| Ciprofloxacin | 3.57 +/− 10.00% | 5.18 +/− 7.50% | 2.44 +/− 5.56% | 1.21 +/− 6.86% | 0.96 +/− 4.83% | 0.98 +/− 3.07% | 0.78 +/− 2.70% | 1.11 +/− 1.40% |
| Clindamycin | 4.10 +/− 9.71% | 3.40 +/− 9.04% | 2.42 +/− 5.19% | 0.98 +/− 4.92% | 1.18 +/− 1.13% | 1.30 +/− 4.16% | 0.79 +/− 2.54% | 0.93 +/− 2.12% |
| Doxycycline | 4.37 +/− 9.92% | 3.81 +/− 7.31% | 3.06 +/− 4.49% | 1.46 +/− 6.27% | 0.72 +/− 1.84% | 0.55 +/− 4.52% | 0.35 +/− 1.34% | 0.91 +/− 1.67% |
| Fidaxomicin | 2.69 +/− 7.97% | 2.70 +/− 5.56% | 4.63 +/− 4.68% | 1.70 +/− 1.95% | 0.61 +/− 1.05% | 0.41 +/− 2.45% | 0.37 +/− 1.41% | 0.57 +/− 2.03% |
| Imipenem | 2.64 +/− 15.22% | 4.69 +/− 13.51% | 2.12 +/− 2.31% | 2.51 +/− 4.02% | 1.61 +/− 3.14% | 0.98 +/− 2.21% | 1.32 +/− 4.65% | 0.60 +/− 1.77% |
| Metronidazole | 3.87 +/− 17.53% | 2.08 +/− 8.90% | 0.45 +/− 6.65% | 1.01 +/− 2.25% | 0.88 +/− 2.33% | 0.32 +/− 3.99% | 0.22 +/− 1.87% | 0.41 +/− 1.63% |
| Sulfamethoxazole | 2.55 +/− 12.36% | 3.27 +/− 3.25% | 2.34 +/− 2.65% | 2.88 +/− 1.80% | 0.36 +/− 0.76% | 0.63 +/− 0.20% | 0.89 +/− 0.36% | 0.61 +/− 0.87% |
| Vancomycin | 3.94 +/− 12.59% | 4.21 +/− 10.92% | 2.51 +/− 10.65% | 2.16 +/− 1.00% | 2.50 +/− 4.59% | 0.70 +/− 0.57% | 0.73 +/− 7.94% | 1.65 +/− 2.10% |
- a Row 1 lists Phylum names.
- b Row 2 lists Family names.
- c Row 3 lists Genus names.
| Comparisons by fecal donor | |||
|---|---|---|---|
| FS05 | FS06 | ||
| FS04 | F = 42.4, p = 0.003 | F = 52.8, p = 0.003 | |
| FS05 | NA | F = 29.4, p = 0.003 | |
| Comparisons by genetic background | |||
| Human | C57Bl/6J | C3H/HeN | |
| C57Bl/6J | F = 8.3, p = 0.006 | NA | F = 3.8, p = 0.024 |
| C3H/HeN | F = 9.3, p = 0.006 | F = 3.8, p= 0.024 | NA |
| Swiss Webster | F = 7.7, p = 0.006 | Not significant (F = 3.2, p = 0.12) | F = 6.7, p = 0.006 |
Appendix 1: Comparisons of effects of antibiotics in GI models to previously published observations.
Augmentin, a broad-spectrum antibiotic that combines amoxicillin and clavulanate, is used to treat Gram-negative and Gram-positive infections (Gerber et al. 2017). A previous study in healthy adults observed decreases in Lachnospiraceae and Coriobacteriaceae and increases in Enterobacteriaceae, Bacteroidaceae, and Porphyromonadaceae, along with a rapid recovery of the microbiota to baseline levels 1 week following treatment (MacPherson et al. 2018). We observed lower levels of a Lachnospiraceae genus in Augmentin-treated HMAmice, but did not observe any of the other changes in microbial taxa reported by MacPherson and colleagues. In the MBRA model, Ruminococcaceae species were significantly lower in Augmentin-treated communities relative to untreated controls, although we observed a similar return to baseline microbial community composition levels (Jaccard diversity) as was reported by MacPherson and colleagues.
Azithromycin is a broad-spectrum antibiotic with extended activity toward Gram-negative pathogens (Retsema et al. 1987). Several studies in human subjects observed loss of Bifidobacterium species following treatment with azithromycin (Chopyk et al. 2023; Sanyang et al. 2024; Wei et al. 2018). Parker and colleagues observed reduced levels of Pseudomonadota (Proteobacteria) and Akkermansia (Parker et al. 2017). Anthony and colleagues observed increases in the Bacillota genera Ruminococcus, Eubacterium, and Anaerostipes (Anthony et al. 2022). In most studies, microbiota disruption was short-term (Anthony et al. 2022; Chopyk et al. 2023; Doan et al. 2019; Parker et al. 2017; Sanyang et al. 2024; Wei et al. 2018), although both Korpela (Korpela et al. 2016) and Anthony (Anthony et al. 2022) observed longer term disruptions. Across the models studied, azithromycin-treatment had much larger impacts on HMAmice than in the MBRA model. Several responses in HMAmice were consistent with observations in human subjects, including loss of Bifidobacterium and decreases in Pseudomonadota. Differences included loss of members of the Bacillota in HMAmice and increases in levels of Akkermansia. The one response conserved across models was lower levels of the Pseudomonadota, Parasutterella in azithromycin-treated HMAmice and MBRA communities. Loss of Parasutterella is consistent with the extended activity of this antibiotic against Gram-negative bacteria. Similar losses of Parasutterella have also been observed in mice treated with azithromycin (Li et al. 2017).
Cefaclor, a second-generation cephalosporin, is a broad-spectrum antibiotic primarily used against Gram-positive aerobes that has limited spectrum against Gram-negative pathogens (Neu and Fu 1978). Very few GI microbiota studies have been performed in humans. In a study reported by Bennett and colleagues, two infants exhibited low levels of E. coli, Bacteroides, lactobacilli, and Bifidobacterium isolates (Bennet et al. 2002). Cefaclor had little (MBRA) or no (HMAmice) significant effects on bacterial richness or taxonomic richness.
Ceftriaxone, a third-generation cephalosporin, has extended spectrum compared to cefaclor, with effects on aerobic Gram-positive and Gram-negative bacteria and limited effects on anaerobic bacteria (Richards et al. 1984). Zhao and colleagues observed decreased Lactobacillus and increased E. coli in BALB/c mice (Zhao et al. 2020). Similarly, Gregoire and colleagues observed decreased Lactobacillus and increased Enterococcus in Swiss Webster mice (Grégoire et al. 2021). Increases in Enterococcus were also observed in C57Bl/6J mice (de Moura e Dias et al. 2023) and Sprague-Dawley rats (Duclot et al. 2024). Akkermansia levels also increased in C57Bl/6J mice (de Moura e Dias et al. 2023). In contrast, Venturini and colleagues observed increased levels of Lactobacillaceae and Enterobacteriaceae as well as decreases in Bacteroidales (Venturini et al. 2021). We observed several conserved responses across both HMAmice and MBRA communities. These included loss of Ruminococcaceae (UCG-04 and Anaerotruncus), Lachnospiraceae (Sellimonas), Erysipelotrichaceae (Holdemania) and increases in an unclassified Erysipelotrichaceae and Anaerostipes (Lachnospiraceae).
Ciprofloxacin is a fluoroquinolone antibiotic with broad-spectrum activity against Gram-negative and Gram-positive bacteria that has been widely used in clinical practice (Bush et al. 2020). Dethlefsen and Relman reported individualized responses to ciprofloxacin treatment and long-lasting microbiota disruption following treatment (Dethlefsen and Relman 2011). Rodriguez-Ruiz and colleagues found that ciprofloxacin treatment led to increased levels of Bacteroides, several genera of Lachnospiraceae (Blautia, Roseburia, Faecalicatena, Dorea), Clostridium (Clostridiaceae), and Eubacterium (Eubacteriaceae), but that levels of Alistipes (Bacteroidota), Faecalibacterium (Bacillota), and Coprococcus (Bacillota) declined in a study of 24 patients (Rodriguez-Ruiz et al. 2024). They also observed increases in Escherichia and Akkermansia in patients undergoing longer term treatment. Faecalibacterium species do not efficiently colonize MBRA communities, so loss of this taxa could not be observed. Consistent with their observations, we observed significant declines in Alistipes and increases in Escherichia-Shigella. We also observed that levels of Coprococcus were lower and levels of Blautia and Dorea were higher in ciprofloxacin-treated communities compared to untreated communities, but these results were not statistically significant. In contrast to their results, we observed significantly lower levels of Akkermansia in ciprofloxacin-treated communities compared to controls.
Clindamycin has long been recognized for its ability to disrupt the microbiome, specifically for its role in increasing susceptibility to Clostridioides difficile infection (Brown et al. 2013; Zhang et al. 2022). Clindamycin's pronounced effects on microbiota are likely due to its broad-spectrum activity against anaerobic Gram-positive and Gram-negative bacteria (Guay 2007). Liu and colleagues studied the effects of clindamycin treatment on the microbiota in Swiss Webster HMAmice colonized with one of three different human fecal samples (Liu et al. 2022) and observed that treatment led to loss of ~33% of rare taxa and increases in the abundance of Enterobacteriaceae, but the microbiota recovered rapidly. In contrast, Buffie et al. observed large, long-lasting decreases in diversity from a single dose of clindamycin in the ileum and cecum of C57Bl/6J mice (Buffie et al. 2012). Many taxa were lost, including members of the Lachnospiraceae, Ruminococcaceae, and Turicibacter (all anaerobic Bacillota), as well as the Barnesiella (Bacteroidota). In addition, they observed increases in Enterobacteriaceae, Mollicutes, Akkermansia, and Blautia. In contrast, Markey and colleagues reported increased Bacteroides and decreased Akkermansia in C57Bl/6 mice (Markey et al. 2021). Across the two models described here, HMAmice exhibited larger changes in richness and taxa abundance, although both models demonstrated loss of Ruminococcaceae, Lachnospiraceae, and Bacteroidaceae genera, as well as increases in Enterobacteriaceae.
Doxycycline, a member of the tetracycline family of antibiotics with higher levels of oral absorption and serum half-life, is another broad-range antibiotic (Holmes and Charles 2009). Sun and colleagues examined effects of doxycycline in CaMK2α-tTA mice (Sun et al. 2024) and observed increased Lactobacillus, Bacteroides, Parabacteroides, and Pseudomonadota and decreased levels of Akkermansia and Lachnospiraceae. In C57Bl/6NCrl mice, Boynton and colleagues also observed increased Bacteroidota and Pseudomonadota and decreased Clostridiales (Boynton et al. 2017). In the MBRA model, we observed very few statistically significant differences compared to controls, with lower levels of Akkermansia, four Ruminococcaceae genera (UCG-003, NK4A214, Phocea, and Anaerotruncus), and an unclassified Bacteroidales genus.
Fidaxomicin is a narrow-spectrum antibiotic used for treatment of C. difficile infection (Goldstein et al. 2012). In 23 patients with mild to moderate C. difficile infection, Tannock and colleagues observed that treatment with fidaxomicin led to increases in Clostridial clusters XIVa (Lachnospiraceae) and IV (Ruminococcaceae) and Bifidobacterium. Nerandzic and colleagues observed increased levels of Bacteroides in C. difficile patients treated with fidaxomicin (Nerandzic et al. 2012). Similarly, Yamaguchi and colleagues observed increased Bacteroidota in C57Bl/6J mice treated with fidaxomicin (Yamaguchi et al. 2020). Across the two models, we observed five Ruminococcaceae and one Lachnospiraceae genera were lower in fidaxomicin-treated models compared to controls and Dielma (Erysipelotrichaceae) was higher compared to controls. Consistent with results described above, higher levels of Bacteroides were observed in HMAmice treated with fidaxomicin compared to controls.
Imipenem is a broad-spectrum antibiotic used to treat severe infections (Rodloff et al. 2006). Characterization of the microbiota by metagenomic sequencing demonstrated few differences in the composition of taxa (Grall et al. 2017). Characterization of the microbiota by culture-based approaches in patients receiving prophylactic imipenem treatment before colorectal surgery demonstrated lower levels of culturable bacteria (Kager et al. 1989), as well as lower levels of Bacteroides, fusobacteria, bifidobacteria, clostridia, and lactobacilli. Similar declines in Bacteroides fragilis group were observed by culture-based approaches in 10 patients, which was accompanied by lower levels of enterococci and Enterobacteriaceae (Nord et al. 1985). However, another study demonstrated increased levels of Enterococcus species in patients being treated with imipenem (Welkon et al. 1986). Similarly to some of the observations described above, we observed lower levels of Bacteroidales and Ruminococcaceae genera, although we also observed higher levels of Collinsella (Actinomycetota), Escherichia-Shigella, and Hungatella (Lachnospiraceae).
Open Research
Data Availability Statement
The raw 16S rRNA gene sequence data that support the findings of this study are openly available in NCBI's Sequence Read Archive (SRA) at https://www.ncbi.nlm.nih.gov/bioproject/, BioProject ID number PRJNA729569. Processed data and code for analysis that support the findings of this study are openly available in Zenodo at doi:10.5281/zenodo.14728946, with reference number 14728946.