Complete nucleotide sequence and comparative genomic analysis of microcin B17 plasmid pMccB17
Graphical Abstract
We have determined the complete sequence of plasmid pMccB17 (isolated in 1977), which carries the prototypical microcin operon mcb. The plasmid is 69,190 bp and assigned to the IncFII group of F2:A-:B- replicon type with a conjugation system from the MOBF12 group. While the mcb operon is the major noteworthy feature, pMccB17 is unlike many contemporary IncFII plasmids in that it lacks transposable elements and antibiotic resistance genes.
Abstract
We present a comprehensive sequence and bioinformatic analysis of the prototypical microcin plasmid, pMccb17, which includes a definitive sequence for the microcin operon, mcb. Microcin B17 (MccB17) is a ribosomally synthesized and posttranslationally modified peptide produced by Escherichia coli. It inhibits bacterial DNA gyrase similarly to quinolone antibiotics. The mcb operon, which consists of seven genes encoding biosynthetic and immunity/export functions, was originally located on the low copy number IncFII plasmid pMccB17 in the Escherichia coli strain LP17. It was later transferred to E. coli K-12 through conjugation. In this study, the plasmid was extracted from the E. coli K-12 strain RYC1000 [pMccB17] and sequenced twice using an Illumina short-read method. The first sequencing was conducted with the host bacterial chromosome, and the plasmid DNA was then purified and sequenced separately. After assembly into a single contig, polymerase chain reaction primers were designed to close the single remaining gap via Sanger sequencing. The resulting complete circular DNA sequence is 69,190 bp long and includes 81 predicted genes. These genes were initially identified by Prokka and subsequently manually reannotated using BLAST. The plasmid was assigned to the F2:A-:B- replicon type with a MOBF12 group conjugation system. A comparison with other IncFII plasmids revealed a large proportion of shared genes, particularly in the conjugative plasmid backbone. However, unlike many contemporary IncFII plasmids, pMccB17 lacks transposable elements and antibiotic resistance genes. In addition to the mcb operon, this plasmid carries 25 genes of unknown function.
1 INTRODUCTION
Microcin B17 (MccB17), is a small (3093 Da), ribosomally synthesized and posttranslationally modified peptide (RiPP) that is produced by Escherichia coli (Arnison et al., 2013; Duquesne et al., 2007). This antimicrobial natural product inhibits the class II topoisomerase DNA gyrase in a similar way to quinolone antibiotics while differing slightly in that MccB17 targets gyrase subunit B, whereas quinolones bind gyrase subunit A (Heddle et al., 2001; Pierrat & Maxwell, 2003). Biosynthesis of MccB17 is carried out by the products of 7 genes (mcbA–G) that encode precursor peptide, synthase, immunity, and export functions and are arranged in an operon found on the plasmid pMccB17 (Garrido et al., 1988; Genilloud et al., 1989; Yorgey et al., 1994). The 69 amino acid promicrocin (precursor peptide) McbA is posttranslationally modified by the synthetase complex McbBCD, resulting in the formation of heterocycles (thiazole and oxazole) in the sequence. This modified peptide sequence is exported from the host cytoplasm by an efflux pump encoded by mcbE and mcbF. The last gene, mcbG (the product of which binds to the host DNA gyrase), together with mcbE and mcbF functions to offer immunity to the host cell (Collin et al., 2013). MccB17 is one of the best-studied microcins and is gaining recognition as a promising template for developing new antibacterial agents (Collin & Maxwell, 2019; Ghilarov et al., 2019; Withanage et al., 2013)
Conjugative plasmid pMccB17, previously known as pRYC17, was originally found in E. coli strain LP17 isolated from the intestinal tract of a healthy newborn at Hospital La Paz, Spain, and transferred by conjugation to E. coli K-12 (Baquero et al., 1978). This is a low copy number plasmid (approximately two copies per chromosome) belonging to the IncFII group that includes the archetypes R100 and R1. Plasmid pMccB17 is not known to possess any conventional antibiotic resistance markers and its size was previously estimated as 70 kb (San Millan et al., 1985).
Here we report the complete sequence of pMccB17, with some comparative genomic analysis. This sequence provides an insight into the biology of a prototypical microcin plasmid and a definitive sequence for the mcb microcin operon
2 MATERIALS AND METHODS
2.1 Bacterial strains and plasmids
The E. coli K-12 strains ZK0005 (RYC1000 [pMccB17]) and ZK359 (MC4100 [pPY113]) were provided by Professor Roberto Kolter of Harvard Medical School.
2.2 DNA purification
Bacterial genomic DNA was isolated from strain ZK0005 as part of the initial DNA sequencing process as follows (this was carried out by microbesNG, details below). Cells were lysed in TE buffer containing lysozyme (final concentration 0.1 mg/mL) and RNase A (0.1 mg/mL) with incubation for 25 min at 37°C. Proteinase K (0.1 mg/mL) and sodium dodecyl sulfate (final concentration 0.5% v/v) were then added and this mixture was incubated for 5 min at 65°C. Genomic DNA was purified using an equal volume of solid-phase reversible immobilization beads and resuspended in elution buffer (EB;10 mM Tris-HCl, pH 8.5).
Plasmid DNA (pMccB17) was isolated from strain ZK0005 by alkaline lysis followed by anion-exchange chromatography (Plasmid Midi kit, Qiagen) according to the manufacturer's recommendations for “very low-copy” plasmids. DNA was eluted in EB buffer as above.
Plasmid DNA (pPY113) was isolated by alkaline lysis followed by chromatography using a silica matrix (Monarch Plasmid Miniprep Kit, New England Biolabs. DNA was eluted in DNA elution buffer (10 mM Tris-HCl, 0.1 mM EDTA, pH 8.5).
2.3 DNA sequencing
Plasmid pMccB17 was sequenced twice using an Illumina short-read next-generation method by microbesNG (http://www.microbesng.com). First, the plasmid was sequenced together with the E. coli host bacterial chromosome; subsequently, plasmid DNA was purified as described above and sequenced separately. Genomic DNA libraries were prepared using the Nextera XT Library Prep Kit (Illumina) following the manufacturer's protocol with the following modifications: input DNA was increased twofold and polymerase chain reaction (PCR) elongation time was increased to 45 s. DNA quantification and library preparation were carried out on a Hamilton Microlab STAR automated liquid handling system (Hamilton Bonaduz AG). Libraries were sequenced on an Illumina NovaSeq. 6000 (Illumina) using a 250 bp paired-end protocol. Reads were adapter trimmed using Trimmomatic version 0.30 (Bolger et al., 2014), with a sliding window quality cutoff of Q15. De novo assembly was performed on samples using SPAdes version 3.7 (Bankevich et al., 2012) and contigs were annotated using Prokka version 1.11 (Seemann, 2014).
Plasmid pPY113 was sequenced using a nanopore platform (PromethION with v14 chemistry and R10.4.1. flow cells; Oxford Nanopore Technologies). Sequencing and annotation were performed by Plasmidsaurus.
Sanger DNA sequencing of PCR amplicons, for plasmid genome finishing/gap resolution, was carried out by DBS Genomics (Durham University, UK).
2.4 Plasmid sequence gap closing
PCR Primers were designed to amplify across gaps in the draft sequence, and the corresponding oligonucleotides were synthesized by Integrated DNA Technologies (Appendix Table A1). PCR amplification used ImmoMix (Bioline) or Q5 High Fidelity (New England Biolabs) PCR master mixes, following the manufacturer's recommendations. Thermal cycling was carried out in a T100 thermal cycler (BioRad) and amplified products were electrophoresed in 1% agarose gels at 6 V/cm for 1 h, stained with GelRed, and visualized using a ChemiDoc XRS+ imaging system (BioRad). Products of the expected sizes were excised, and gel-purified using Monarch DNA Gel Extraction Kit (NEB) and then sequenced (Sanger method, as above).
2.5 Annotation of pMccB17
The draft genome sequence was annotated automatically by MicrobesNG using Prokka 1.11 (Seemann, 2014). These annotations were manually checked using BLASTn (Zhang et al., 2000) and BLASTp (Altschul, 1997) retaining the default parameters. Coding sequences (CDSs) without proper annotation were manually assigned one where possible, using the BLAST result as a guide and Artemis to edit the annotation (Berriman, 2003; Rutherford et al., 2000).
2.6 pMccB17 plasmid replicon and conjugation system typing
Replicon typing was carried out using the IncF RST (replicon sequence typing) scheme, as implemented by pubMLST (https://pubmlst.org/plasmid/; Jolley et al., 2018) and pMLST (https://cge.cbs.dtu.dk/services/pMLST/; Carattoli et al., 2014). Initial classification of the relaxase was carried out using MOBscan (https://castillo.dicom.unican.es/mobscan/; Garcillán-Barcia et al., 2020) and manually refined by pairwise comparisons with selected relaxase proteins using EMBOSS Needle (https://www.ebi.ac.uk/Tools/psa/emboss_needle/; Needleman & Wunsch, 1970).
2.7 Phylogenetic tree building
The phylogenetic tree of ParB-fusion (Pbf) protein homologs was built using a maximum-likelihood method as implemented by MEGA X (Kumar et al., 2018). The tree used the LG + G model (Le & Gascuel, 2008) for amino acid substitution.
2.8 Identification of resistance genes, insertion sequence, and virulence factors
Identification of resistance genes was performed by submitting the complete plasmid nucleotide sequence to the ResFinder web server (https://cge.cbs.dtu.dk//services/ResFinder/; Zankari et al., 2012). The nucleotide sequence was also submitted to the IS-Finder web server (https://www.issaga.biotoul.fr/; Varani et al., 2011), and VFDB web server (https://www.mgc.ac.cn/VFs/; Liu et al., 2019) to identify insertion sequences and virulence factors, respectively.
3 RESULTS AND DISCUSSION
3.1 Plasmid pMccB17 genome assembly
Draft genomes were obtained using a short-read, high coverage (Illumina) approach as described in Methods. Plasmid contigs from the draft genome were scaffolded as follows. Two contigs, 1.27 and 1.52, from the first draft sequence (of plasmid and chromosomal DNA), were discovered to have standard plasmid-related features by manually examining the genome sequence. Contig 1.27 (60,265 bp) had 71 genes including the MccB17 operon, conjugative transfer system, replicon system, and some plasmid maintenance genes (stbA, stbB, parE), whereas contig 1.52 (8894 bp) had 12 genes, half of which have plasmid maintenance related functions, that is, ssb, pbf, psiA, psiB, and hok/sok. Contig 2.1, the first contig in the second draft sequence (from purified plasmid DNA), contained all the genes present in contigs 1.27 and 1.52, and when the three contigs were aligned using Artemis Comparison Tool (Carver et al., 2005, 2008), it was evident that contigs 1.27 and 1.52 make up contig 2.1. The ends of contig 2.1 were found to be within a CDS, gene_81, found in contig 1.27. PCR primers were designed to flank gaps in the plasmid sequence and were employed to close these gaps by PCR amplification followed by Sanger sequencing of the amplicons.
Finally, 114 nucleotides missing from contig 2.1 were added manually using Artemis, resulting in a complete circular genome sequence for pMccB17. This sequence was submitted to GenBank (accession number ON989342).
3.2 Plasmid replicon and conjugation system typing
The replicon sequence type was assigned using the IncF RST scheme as implemented by pubMLST and pMLST, indicating a FAB type of F2:A-:B-; so pMccB17 has an FII replicon (allele 2) without additional FIA or FIB replicons (Villa et al., 2010). Initial classification of the relaxase using MOBscan assigned it to the MOBF family. The first 300 N-terminal amino acids of the TraI relaxase/helicase protein (that is, the relaxase domain) were then compared in a pairwise manner with archetypal IncF plasmid relaxases. The pMccB17 relaxase domain was 99.3% identical to that of the IncFII plasmid R100 (GenBank: NC_002134) and 91.3% identical to that of F plasmid (GenBank: NC_002483), placing it in the MOBF121 type (Garcillán-Barcia et al., 2011), or group A according to a recent phylogeny of IncF relaxases (Fernandez-Lopez et al., 2016).
3.3 Plasmid pMccB17 genome analysis overview
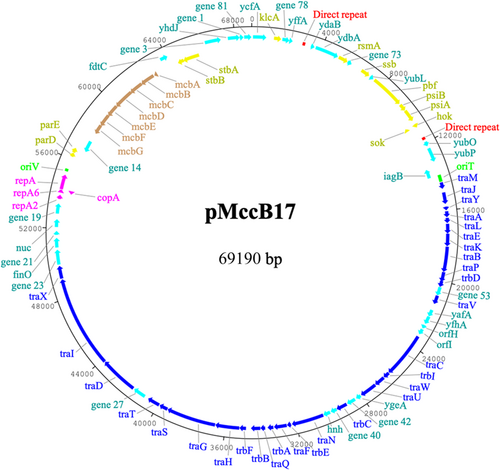
pMccB17 is a circular IncFII plasmid (69,190 bp) with an average GC content of 51% and 83 CDS (Figure 1 and Appendix Table A2). This plasmid encodes MccB17, having the MccB17 operon. The tra region (traMJYALEKBPVCWUNFQHGSTDIX and trbDICEABF) encodes conjugative transfer functions and takes up about half of the plasmid backbone. pMccB17 encodes two types of toxin–antitoxin (TA)/plasmid addiction system, for eliminating plasmid-free segregants. A type I hok/Sok system is located downstream of psiA and a type II system encoding ParDE is encoded downstream of the replication gene repA. The plasmid has no known antibiotic resistance genes, virulence factors, or transposable elements, according to ResFinder, IS-Finder, and VFDB web servers, respectively.
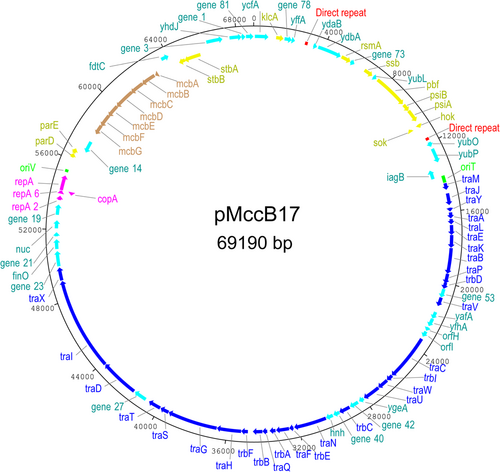
3.4 MccB17 operon
The mcb operon, consisting of genes mcbABCDEFG encoding biosynthetic and immunity functions for MccB17, is located between gene_14 (encoding a hypothetical protein) and fdtC (encoding an acetyltransferase). The nucleotide and amino acids sequences of genes in the mcb operon of pMccB17 (ON989342) were compared with some historical published sequences (Table 1). The M24253 (Genbank) sequence, derived by Sanger sequencing of a cloned DNA fragment of pMccB17, provided the first available sequence of mcbA, mcbB, mcbC, and mcbD (Genilloud et al., 1989). The X07875 (Genbank) sequence, also obtained via Sanger sequencing of a cloned fragment of pMccB17, includes the remaining three genes mcbE, mcbF, and mcbG (Garrido et al., 1988). The ON989342 sequence should in theory be identical to the M24253 and X07875 sequences and the plasmid pMccB17 as sequenced here was provided by one of the publishing authors (Roberto Kolter) of the historical sequences (Garrido et al., 1988; Genilloud et al., 1989). The FM877811 sequence provides a complete mcb operon sequence and was selected because of this. This is not the original mcb operon, rather it is from the whole genome sequence of E. coli strain L1000, isolated from human feces, and appears to be chromosomal rather than plasmid-borne (Zihler et al., 2009). Nucleotide sequences of mcbA, mcbC, mcbE, and mcbG were 100% identical to those in the historical sequences M24253 and X07875. However, mcbB and mcbD each differed by a single base pair from M24253, resulting in a single nonconservative amino acid substitution in each case (the amino acid in our sequence is shown first): S117C for McbB and R171T for McbD. Our McbB and McbD sequences also differed from FM877811 by a single (but different) substitution in each case: E198D and A113T respectively. As sequenced here, mcbF was annotated as 732 bp versus 744 bp in the historical X07875 sequence, reflecting a frameshift towards the 3′ end of the latter gene due to the C at position 688 of the new sequence is missing from X07875. There are also five substitutions in the sequence 5′ of this indel and overall this gene and hence the encoded McbF protein has the greatest difference from historical published sequences for the mcb operon (Table 1).
| Protein | Predicted function | Number of amino acids (% identity to ON989342) | |||
|---|---|---|---|---|---|
| GB:ON989342 | GB:FM877811 | GB:M24253 | GB:X07875 | ||
| McbA | Microcin precursor | 69 | 69 (100) | 69 (100) | − |
| McbB | Microcin B synthase enzyme complex | 295 | 295 (99.7) | 295 (99.7) | − |
| McbC | 272 | 272 (100) | 272 (100) | − | |
| McbD | 396 | 396 (99.7) | 396 (99.7) | − | |
| McbE | Self-immunity and export | 241 | 241 (100) | − | 241 (100) |
| McbF | Self-immunity and export | 243 | 243 (100) | − | 247 (91.5) |
| McbG | Self-immunity | 187 | 187 (99.5) | − | 187 (100) |
We do not believe that the RYC1000 [pMccB17]) strain sequenced here has been passaged extensively since it was derived from the original capture of pMccB17 by conjugation into BM21 (Baquero et al., 1978). Due to the time elapsed, we are unable to confirm how many passages the pMccB17 plasmid has undergone between the original sequencing and our sequencing reported here. It is possible that the differences we observed, compared to the original sequences, are due to mutations accumulated during passage. However, we hypothesized that the differences observed were due to Sanger sequencing errors in the historical sequences i.e. that our pMccB17 sequence is correct. To confirm the mcb operon sequence presented here, we obtained pPY113 another clone of the mcb operon that was independently derived from the parental BM21 [pMccB17] strain, and sequenced it using an orthogonal high-coverage long-read approach (Yorgey et al., 1994). The resulting pPY113 plasmid sequence (Genbank: OR091272) is identical to our sequence of pMccB17 throughout the shared mcb operon sequences. Hence, we believe that it is unlikely that these differences reflect mutations accumulated by our pMccB17 strain and much more likely that they are due to inaccuracies in Sanger sequencing, which is a relatively error-prone process.
3.5 Direct repeats
In the process of aligning contigs 1.27 and 1.52 with contig 2.1, to ascertain the complete plasmid sequence, we discovered a 149 bp direct repeat in contig 2.1 (Appendix Figure A1). This 149 bp sequence is repeated twice (8639 bp apart) in the sequence of pMccB17. These repeats are identical and each has an intragenic location: one is located between yffA and ydaB, whereas the other is located between hok/sok and yubO. These repeats have 12 genes in between them, including ssb, pbf, psiAB, and the hok/sok TA system. This approximately corresponds to the plasmid leading region as originally defined for F plasmid (Loh et al., 1989). A large perfect palindrome [5′-CAAAATTTTTTACC]CAAAACCC[GGTAAAAAATTTTG-3′] is present at the center of this sequence, with some imperfectly palindromic sequences to either side (Appendix Figure A1). This may result in the formation of functional secondary structures, in either single-stranded DNA produced during conjugation or mRNA produced during transcription.
Searches using BLASTn against the nucleotide collection (nr/nt) database at NCBI revealed that the repeated sequence is present in IncF plasmids of Gammaproteobacteria, mainly from the Enterobacterales, In 20 plasmids examined, having query coverage and identity of 100%, copy number ranged from 1 to 3 and all were members of the IncF family. The exact function of these repeated sequences is unknown, but several genes are commonly flanked by these repeats, including ssb, pbf (see below), and psiAB.
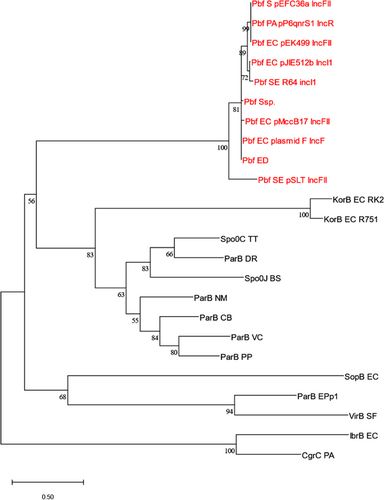
3.6 Pbf protein
Upstream of psiB is a gene encoding 652 amino acids that we have annotated as “pbf” (for ParB fusion) as it features a ParB-like N-terminal domain joined to a C-terminal region that does not include any known conserved domain. A BLASTp search of its amino acids against the nonredundant protein sequences (nr) database at NCBI confirmed that its N-terminal region (the first 250 amino acids) contains a conserved domain, annotated as “ParB/RepB/Spo0J family partition protein.” Thus, Pbf is evolutionarily, if not functionally, related to proteins that (via interactions with an NTPase partner ParA and a centromere-like partition site on the DNA, parS) are involved in the active partitioning of bacterial chromosomes and low copy-number plasmids (McLean & Le, 2023; Appendix Figure A2). The classical plasmid F carries a homologous gene (orf652 which is 94% identical to pbf) and the similarity of the encoded protein to ParB was first described by Manwaring et al. (1999).
4 CONCLUSIONS
Plasmid pMccB17 seems a typical member of the IncFII family, apart from its carriage of the MccB17 biosynthetic gene cluster mcb. This plasmid does not carry any identifiable insertion sequence or other mobile genetic elements, nor does it encode any known antibiotic resistance genes (apart from those conferring immunity to MccB17) or pathogenicity factors. We have reported here a complete and accurate sequence of the mcb operon, which will be useful for future studies and manipulation of the biosynthetic pathway for this prototypical RiPP.
AUTHOR CONTRIBUTIONS
Mayokun Ajeigbe: Conceptualization (equal), writing—original draft (lead), formal analysis (lead), writing—review and editing (equal). Stephen Childs: Conceptualization (equal), writing—review and editing (equal). Timothy Paget: conceptualization (equal), writing—review and editing (equal). Lewis Bingle: Conceptualization (lead), formal analysis (supporting), writing—review and editing (equal).
ACKNOWLEDGMENTS
The authors would like to thank Professor Roberto Kolter and Dr Nicolas Lyons of Harvard Medical School for supplying pMccB17 DNA and for helpful discussion. Genome sequencing was provided by MicrobesNG (http://www.microbesng.uk), supported by the BBSRC (grant number BB/L024209/1). We are grateful to Dr Linda Henry and Mrs Kayleigh Ironside for technical assistance, and to Dr Callum Cooper and Dr Jon Marles-Wright for their useful comments.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
None required.
APPENDIX
| Gaps | 5′ Forward 3′ | 5′ Reverse 3′ | Annealing temperature (°C) and PCR system |
|---|---|---|---|
| Contig 1.27 | CTGATACAGCCACAACCTGC | GTTTGAGAACCTTCGCCGC | 65, Q5 Master Mix |
| Contig 1.52 | CTGATACAGCCACAACCTGC | CTGTTTGAGAACCTTCGCCG | 65, Q5 Master Mix |
| Contig 2.1 | AGAAATACCGGATTCAGTCCG | GAAATCAGGACTGTCCTGGC | 50-60, ImmoMix |
- Abbreviation: PCR, polymerase chain reaction.

| CDS | Position (bp) | Protein function |
|---|---|---|
| ycfA | 49. .819 | Hypothetical protein |
| klcA | 1235. .1660 | Antirestriction protein |
| gene_78 | 1707. .2129 | Hypothetical protein |
| yffA | 2126. .2317 | Hypothetical protein |
| ydaB | 3355. .3585 | Hypothetical protein |
| ydbA | 3637. .4998 | Hypothetical protein |
| rsmA | 5045. .5608 | Ribosomal RNA small subunit methyltransferase A |
| gene_73 | 5608. .5874 | Hypothetical protein |
| ssb | 6517. .7056 | Plasmid-derived single-stranded DNA-binding protein |
| yubL | 7118. .7351 | Hypothetical protein |
| pbf | 7416. .9374 | ParB fusion protein |
| psiB | 9429. .9863 | Plasmid SOS inhibition protein B |
| psiA | 9860. .10579 | Plasmid SOS inhibition protein A |
| sok | Complement (10748. .10813) | Antisense RNA of hok transcripts |
| mok | 10801. .11013 | Modulation of hok protein |
| hok | 10859. .11017 | Postsegregation killing protein |
| yubO | 11944. .12231 | Hypothetical protein |
| yubP | 12350. .13171 | Hypothetical protein |
| iagB | Complement (13466. .13975) | Invasion protein |
| traM | 14391. .14774 | Relaxosome protein |
| traJ | 14968. .15639 | Conjugal transfer transcriptional regulator |
| traY | 15776. .16003 | Relaxosome protein TraY |
| traA | 16036. .16395 | Pilin TraA |
| traL | 16410. .16721 | Peripheral membrane protein TraL |
| traE | 16743. .17309 | Bacterial sex pilus assembly and synthesis proteins TraE |
| traK | 17296. .18024 | Pilus assembly TraK |
| traB | 18024. .19475 | Involved in F pilus assembly |
| traP | 19465. .20031 | Conjugation protein |
| trbD | 19970. .20341 | Conjugal transfer protein TrbD |
| gene_53 | 20334. .20843 | Hypothetical protein |
| traV | 20836. .21348 | Conjugative transfer system lipoprotein |
| yafA | 21697. .22110 | Hypothetical protein |
| yfhA | 22103. .22576 | Hypothetical protein |
| orfH | 22657. .22875 | Hypothetical protein |
| orfI | 22903. .23250 | Hypothetical protein |
| traC | 23376. .26006 | Conjugative transfer ATPase |
| trbI | 26003. .26389 | Type-F conjugative transfer system protein |
| traW | 26386. .27018 | Type-F conjugative transfer system protein |
| traU | 27015. .28007 | Conjugal DNA transfer protein |
| ygeA | 28034. .28342 | Hypothetical protein |
| gene_42 | 28405. .28923 | Hypothetical protein |
| trbC | 28950. .29588 | Type-F conjugative transfer system pilin assembly protein |
| gene_40 | 29585. .29956 | Hypothetical protein |
| hnh | 29981. .30403 | Hnh nuclease |
| traN | 30400. .32250 | Type-F conjugative transfer system mating-pair stabilization protein |
| trbE | 32277. .32534 | Type IV conjugative transfer system protein |
| traF | 32527. .33270 | Type-F conjugative transfer system pilin assembly protein |
| trbA | 33284. .33628 | Conjugal transfer repressor |
| traQ | 33747. .34031 | Type-F conjugative transfer system pilin chaperone |
| trbB | 34018. .34563 | Type-F conjugative transfer system pilin assembly thiol-disulfide isomerase |
| trbF | 34821. .35213 | Plasmid mobilization |
| traH | 35200. .36573 | Type IV conjugative transfer system protein |
| traG | 36570. .39395 | Mating pair stabilization protein |
| traS | 39392. .39901 | Conjugal transfer entry exclusion protein |
| traT | 39915. .40646 | Lipoprotein TraT |
| gene_27 | 40849. .41586 | Hypothetical protein |
| traD | 41637. .43835 | Type IV conjugative transfer system, coupling protein TraD |
| traI | 43835. .49105 | Conjugative transfer relaxase protein |
| traX | 49125. .49871 | Type-F conjugative transfer system pilin acetylase |
| gene_23 | 49930. .50790 | 2,6-dihydropseudooxynicotine hydrolase |
| finO | 50893. .51453 | Fertility inhibition protein |
| gene_21 | 51598. .51840 | Hypothetical protein |
| nuc | 52039. .52500 | Thermonuclease nuc |
| gene_19 | 52794. .53384 | Hypothetical protein |
| repA2 | 53624. .53881 | Replication regulatory protein |
| copA | Complement (54012. .54104) | Leader peptide regulator for RepA expression |
| repA6 | 54116. .54190 | |
| repA | 54183. .55040 | Plasmid replication initiator |
| relB/parD | 56110. .56373 | Toxin-antitoxin system |
| relE/parE | 56363. .56662 | Toxin-antitoxin system |
| gene_14 | Complement (56698. .57363) | Hypothetical protein |
| mcbG | Complement (57933. .58496) | Microcin B17 immunity protein, McbG |
| mcbF | Complement (58500. .59231) | Microcin B17 export protein, McbF |
| mcbE | Complement (59228. .59953) | Microcin B17 export protein, McbE |
| mcbD | Complement (59963. .61153) | Microcin B17 synthetase complex protein, McbD |
| mcbC | Complement (61134. .61952) | Microcin B17 synthetase complex protein, McbC |
| mcbB | Complement (61954. .62841) | Microcin B17 synthetase complex protein, McbB |
| mcbA | Complement (62868. .63077) | Microcin B17 precursor peptide |
| fdtC | 63607. .64059 | dTDP-3-amino-3,6-dideoxy-alpha-d-galactopyranose 3-N-acetyltransferase |
| stbB | Complement (64462. .64815) | Type IV partitioning system |
| stbA | Complement (64815. .65777) | Type IV partitioning system |
| gene_3 | 66289. .67215 | Hypothetical protein |
| yhdJ | 67600. .68283 | DNA adenine methyltransferase YhdJ |
| gene_1 | 68284. .68505 | Hypothetical protein |
| Gene_81 | 68519. .68950 | Hypothetical protein |
- Abbreviation: CDS, coding sequence.
Open Research
DATA AVAILABILITY STATEMENT
Plasmid genome sequences are available in GenBank with the following accession numbers: pMccB17, ON989342: https://www.ncbi.nlm.nih.gov/nuccore/ON989342; and pPY113, OR091272: https://www.ncbi.nlm.nih.gov/nuccore/OR091272