Management of peripheral nerve injuries using natural based biomaterials and their derivatives: Advances and prospective
Abstract
The management of peripheral nerve injuries is an important concern due to the their incidence of nerve lesions and inappropriate regeneration that follows severe injuries, which ultimately reduces the lives of patients with this condition. Different strategies have been investigated to repair severe nerve injuries with the improvement of motor and sensory regeneration. Although autograft remains the gold standard technique, an emerging number of research articles concerning nerve conduit use have been reported in the last few years. Nerve conduits aim to overcome autograft disadvantages, but they satisfy some requirements to be suitable for nerve repair. A universal ideal conduit does not exist since conduit properties have to be evaluated case by case; nevertheless, because of their high biocompatibility and biodegradability, natural-based biomaterials have great potential to be used to produce nerve guides. Although they have many characteristics with synthetic biomaterials, natural-based biomaterials are preferable because of their extraction sources; indeed, these biomaterials are obtained from different renewable sources or food waste, thus reducing environmental impact and enhancing sustainability in comparison to synthetic ones. This review highlights the recent progress in the development of natural-based biomaterials and their derivatives for the management of peripheral nerve injuries.
1 INTRODUCTION
Peripheral nerve injury (PNI) affects between 13 and 23 people out of every 100,000 people annually in developed nations, and it can cause either a complete or partial impairment of the neurological, sensory, motor, and autonomic nervous systems in the impacted body parts.1 Peripheral axons can repair themselves after being damaged by a nerve, and if the right pathway is available, they can reconnect with the point of origin.2, 3 Improved functional recovery may result from therapeutic interventions that hasten axonal regrowth.3 Various publications have suggested that the success rate of surgical procedures on peripheral nerves can vary between 36% and 51.6%, depending on the specific nature of the damage and the procedure performed.4 To facilitate the healing process following surgery, immobilization for a period of up to 6 weeks may be required, depending on the severity of the injury.5 The outcome of nerve restoration is primarily dependent, to an extent of up to 50%, on the patient's age as well as the time of therapy. This phenomenon arises due to alterations in the reactivity of macrophages, Schwann cells (SCs), axons, and neurons that transpire with the progression of age.6 The outcome of nerve restoration is mostly dependent 50% on the patient's age as well as the timing of therapy.7 In addition, neuronal scarring and the aging of repair stem cells might develop as a consequence of persistent nerve injuries that have been treated for a prolonged period.8, 9 Peripheral injuries that involve nerve stitching frequently lead to restricted motor recovery as a consequence of a limited capacity for regeneration and time limits to reach the target organ.10 When treating severe nerve injury, autologous nerve grafting is the treatment of choice, especially in cases where there are large gaps or extensive scars that prevent the proximal nerve stump from regenerating and being innervated.11 However, functional recovery during allogeneic nerve grafting is frequently restricted due to the absence of effective reinnervation that occurs after the procedure.12, 13 Many synthetic nerve guidance conduits (NGCs) have been made from polymeric materials such as poly(l-lactide), poly (lactic glycolic acid), poly(-caprolactone), polyamides, and others. Biocompatibility, non-immunogenicity, and biodegradability are a few of the conditions that materials for NGCs need to fulfill before they can be used.14-16 Accelerating and improving the quality of axonal development during nerve regeneration has been shown to improve functional recovery.17 Axon regeneration can be improved in some ways. Several strategies have been identified to enhance axon cross-coaptation, modify the progression of Wallerian degeneration, and reduce the duration of the degeneration in muscles.18 The insertion of a biological material scaffold at the location of a nerve injury has the potential to deliver a sustained local supply of immuno-suppressants as well as growth factors while simultaneously encouraging the directed development of axons via the lesion cavity.18, 19
Polysaccharide-based biomaterials have emerged as a highly versatile and promising class of materials with a wide range of applications in the field of biomedicine.20 These natural polymers, derived from carbohydrates, are gaining recognition for their unique properties. The biodegradability of many polysaccharides further enhances their value, eliminating the need for surgical removal after their intended use. Some polysaccharides possess immunomodulatory properties, making them useful in immune-related therapies.20 Moreover, their cost-effectiveness and the ability to chemically modify them for specific functions make them a sustainable and adaptable choice for a wide range of biomedical applications.20 Synthetic polymers like poly (lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyethylene glycol (PEG) are frequently utilized in nerve guidance conduits, offering controlled degradation and structural support.16 Collagen, a natural protein found in connective tissues, creates a biocompatible environment in nerve scaffolds and conduits.18 Gelatin, derived from collagen, can be customized for enhanced bioactivity and cell growth support. Silk-based biomaterials, derived from silkworms or spiders, present suitable mechanical properties and are fashioned into conduits, films, or coatings. Hydrogels, such as agarose, alginate, and hyaluronic acid, provide a three-dimensional (3D) matrix that mimics the extracellular environment. Electrospun nanofibrous scaffolds, carbon nanotubes, decellularized tissue scaffolds, and bioactive glasses also play essential roles in nerve repair.19 Natural polysaccharides and their derivatives have some drawbacks that prevent them from being utilized in the pharmaceutical and medical industries.21 These drawbacks include challenges associated with the extraction and purification processes, unpredictable mechanical qualities (such as viscosity and hydration rate), microbial contamination, and variability from batch to batch.22 Other disadvantages associated with the use of polysaccharides include high polydispersity, fluctuating chemical composition, particularly in situations where several sources are involved, and poor solubility.23 However, these limitations can be greatly reduced by chemically and structurally manipulating polysaccharides as well as combining them with other natural, semi-synthetic, and synthetic polymers. Chitosan, hyaluronic acid, and alginate were used to regenerate peripheral nerves. However, these analyses mostly include the use of polysaccharide-based biomaterials for the treatment of muscle injuries rather than nerve damage.24
Biomaterials emerge as integral components in the realm of nerve regeneration, playing a pivotal role in providing a supportive framework for damaged nerves.16 Characteristics of biomaterial for the management of peripheral nerve injuries (1) polymeric biomaterials, crafted to mimic the natural extracellular matrix, create an environment conducive to cell adhesion and growth, thus fostering nerve regeneration.16 Among these biomaterials, biodegradable scaffolds offer temporary structural support and gradually degrade, aligning with the healing process. This innovation stands at the forefront of peripheral nerve injury management, promising more effective and targeted interventions. (2) Additionally, derivatives such as neurotrophic factors and extracellular matrix components significantly enhance the regenerative potential of nerve tissues, stimulating growth and survival.9 This sophisticated approach represents a leap in peripheral nerve injury treatment, showcasing the potential for accelerated and robust regenerative outcomes.9 (3) Precision medicine revolutionizes the field by customizing biomaterials based on individual patient characteristics, considering age, genetics, and the specific nature of the nerve injury.21 (4) Integrating localized drug delivery systems into biomaterials allows for targeted administration, minimizing systemic side effects and enhancing the patient experience. Nanotechnology emerged as a key player, creating biomaterials with a lasting impact on nerve regeneration.20 By incorporating nanoscale features, researchers achieve sustained support over time, emphasizing the importance of long-term solutions in peripheral nerve injury management.20 Advances in 3D printing contribute further, enabling the creation of complex structures. This personalized medicine approach marks a significant stride towards optimizing treatment outcomes by accounting for the individualized nature of peripheral nerve injuries.11 Delving into the intrinsic properties of these biomaterials, including their biocompatibility, bioactivity, and regenerative potential, the review segment comprehensively outlines the rich spectrum of natural-based biomaterials and their applicability in nerve regeneration. The subsequent sections meticulously dissect recent advancements in peripheral nerve injury management, emphasizing breakthroughs, clinical trials, and innovative technologies that showcase the efficacy of these biomaterials in promoting nerve repair.
2 PERIPHERAL NERVE INNERVATION (PNI) THERAPY UTILIZING POLYSACCHARIDE-BASED BIOMATERIALS
Physical trauma, compression, and disease are a few of the causes of injuries to the peripheral nerves in the body, which can impair motor and sensory functions in those having experience.15 This pioneering approach seeks to redefine the landscape of nerve regeneration by harnessing the unique properties of polysaccharides, ensuring a harmonious interplay between biology and materials science. Non-immunogenicity takes center stage in this exploration, as polysaccharide-based biomaterials exhibit a remarkable ability to navigate the immune response.25 This intrinsic characteristic ensures sustained therapeutic efficacy, allowing for long-term implantation without triggering adverse reactions. The therapeutic journey becomes a harmonious symphony, where the immune system and biomaterials collaborate in orchestrating a healing process that is both effective and enduring. Aligned with the natural healing mechanisms, these materials gradually degrade over time, mirroring the pace of tissue regeneration. Tissue engineering involves the creation of functional tissues and organs by combining cells, biomaterials, and biochemical factors. This interdisciplinary field aims to develop innovative strategies to repair, replace, or regenerate damaged or diseased tissues and organs. By harnessing the principles of biology, engineering, and medicine, tissue engineering holds great promise for addressing the limitations of traditional therapeutic approaches. Biomaterials based on polysaccharides have shown promise due to their biocompatibility, biodegradability, and capacity to promote tissue regeneration. Recent advances in the treatment of peripheral neuronal injuries are highlighted, and the potential of polysaccharide-based biomaterials is explored.25 The biocompatibility of polysaccharides makes them amenable to modification to yield derivatives with the requisite physicochemical qualities for nerve regeneration. Polysaccharide-based materials, including NGCs, hydrogels, and films, are discussed here as potential solutions for healing nerve damage. In summary, this exploration into PNI therapy using polysaccharide-based biomaterials encapsulates a paradigm shift in the approach to nerve regeneration. By harmonizing biocompatibility, nonimmunogenicity, and biodegradability, these biomaterials stand as beacons of hope for individuals facing peripheral nerve injuries.15
3 SUPPORTIVE BIOMATERIALS FOR SOLID NEURONAL GLIAL CELLS
While inflammation is a normal response to the introduction of a foreign object into the body, its severity varies according to the structure and physicochemical qualities of the implanted object.26 In this context, recent years have seen a concentration of effort on developing novel implantations in the form of NGCs for the rejuvenation of peripheral nerves. Minimal tissue irritation is caused by biodegradable prostheses like NGCs Acute inflammation facilitates the generation of enzymatic molecules that facilitate the degradation of the inserted materials. However, if the inflammatory response is too strong, vital tissues at the implantation site are destroyed, and regeneration is stunted. Natural biopolymers (particularly proteins and polysaccharides) are now the focus of a significant amount of research due to the potential that they play an integral part in the development of NGCs.27 These polysaccharides are immune-modulatory, biocompatible, biodegradable, nontoxic, and beneficial to tissue regeneration.
3.1 Chitosan-based supportive biomaterials for solid neuronal glial cells
The most common method for obtaining chitosan involves processing crab tendon to remove proteins and calcium phosphates. In addition to being extremely compatible with bacteria, microbicidal, environmentally friendly, and biodegradable by lysozyme, the polysaccharide also possesses biocidal properties.28 The impact of deacetylated chitin, with molecular masses ranging from 50 to 400 kDa, on the regeneration of nerves has been demonstrated to be substantial as a result of its robust bioadhesive properties.29 Histological tests showed that chitosan NGCs improved axonal growth after sciatic nerve injury repair, and patients recovered faster than those in the control group.30-32 Zhang et al.33 utilized the Sprague-Dawley rats' model of sciatic nerve injuries to assess the feasibility of synthesizing chitosan NGCs for prosthetic nerves across the body. When compared to the effects of simple conduits, the outcomes of chitosan NGCs with reinforcing fibers utilized to connect a gap of 10 mm in the nerve tissue were superior. Furthermore, electro electro-fabrication technique allowed the one-step fabrication of chitosan NGCs with adjustable mechanical characteristics, confirming adjustable, scalability, and biocompatibility in vitro and displaying positive effects on nerve regeneration at the autografting level.34, 35 Chitosan and graphene oxide were combined to generate a graft that improves the characteristics of chitosan NGCs.36 A composite NGCs made of polyacrylamide and chitosan that was customized with mechanical and topographical guidance cues was used to correct a sciatic nerve deficit that measured 15 mm in rabbits.37 Intriguingly, wet-weight ratio, electrophysiological, and neuronal histomorphometry tests also revealed that the polyacrylamide/chitosan NGCs using 8.4 kPa and a surface depression with a 30 m range enhanced the regeneration of nerves with homologous nerve grafting.38 These were discovered by employing a technique known as nerve histomorphometry. When it comes to the process of nerve regeneration, the electrical characteristics of the therapeutic materials play an important role. To improve the conductivity of NGCs, a double-layered chitosan-based NGCs was developed and manufactured. Chitosan was utilized in the construction of the NGCs primary framework, while pluronic and modified carboxymethyl chitosan were utilized in the production of the luminal conductive hydrogel, both of which included polyaniline and aldehyde functional groups.38 The NGC, which had increased conductivity properties, was able to support sciatic nerve regeneration in rats following a 10 mm gap injury, and it performed similarly to autologous nerve grafting in terms of efficacy.
3.2 Hyaluronic acid based supportive biomaterials for solid neuronal glial cells
When HA and collagen were mixed with poly(-caprolactone) nanofibers, they made a better material for stem cell growth and the development and guidance of axons.39 In vitro, tests demonstrated that nanocomposite NGCs of HA comprised of nanotubes made of carbon and mesoporous silicon dioxide NPs had better stiffness and electrical permittivity because it helped SCs hold together and live long. A composite made of HA and collagen, when combined with poly(-caprolactone) nanofibers, provided superior support for stem cell proliferation as well as axonal development and guidance.39 The enhanced attachment and viability of stem cells in a laboratory setting demonstrated that nanocomposite NGCs made of HA-containing carbon nanotubes and mesoporous silica nanoparticles (NPs) exhibited superior mechanical strength and electrical conductivity.
4 HYDROGELS-BASED SUPPORTIVE BIOMATERIALS FOR SOLID NEURONAL GLIAL CELLS
The mechanisms by which hydrogels crosslink, as well as the density of crosslinking, have a direct impact on the final features of polysaccharide hydrogels, including regeneration of tissues, assurance, resilience, resorption dynamics, the rate at which bioactive substances are released, and more.40 Therefore, the numerous approaches such as (physical and chemical) of crosslinking these hydrogels are of interest for nerve regeneration and repair. Although some hydrogels are physically and chemically crosslinked and might be discussed in either area, they are addressed in two separate subsections below.
4.1 Crosslinked hydrogels via chemical reactions for supportive biomaterials for solid neuronal glial cells
Rickett et al. reported that a chitosan-based, photo-cross-linkable hydrogel could be used to connect nerves in the periphery.41, 42 In addition to being nontoxic, cytocompatible, and mechanically sound, the samples showed promise as bioadhesives for application in peripheral neurosurgery procedures. Hydrogels have been produced from CMCS using radiation-induced crosslinking with an electron beam dosage of 25 kGy43; to facilitate peripheral regeneration of nerves. Furthermore, the CMCS hydrogel was delivered through a naturally occurring biodegradable NGC composed of a combination of trimethylene carbonate (TMC) and a substance called polylactic acid (PLA).43 The gel has been demonstrated to be nontoxic through both in vitro testing using L-929 fibroblasts from mice and in vivo testing with subcutaneous implants in male Wistar rats weighing more than 250 g. Although CMCS NGCs have shown promise in treating nerve defects in animal models, more research is required to fully assess its efficacy in this setting. According to Wu et al.44 methacrylate gelatin, 4-arm poly (ethylene glycol) acrylate, and HA were used to create cryogel-based NGCs using free radical copolymerization. To conduct in vivo studies with the hybrid cryogel, an incision of 10 mm across the rat sciatic nerve was performed in adult Sprague-Dawley rats. At the 16-week mark, the cryogel has shown a capacity to facilitate the process of axonal regrowth and remyelination. Particularly, the cryogel demonstrated myelination width and nerve fiber concentration that were equivalent to those reported in the autograft category.45 The cell walls of certain strains of bacteria and brown algae (often known as seaweed) contain a naturally occurring polymer called alginate. Alginate can also be extracted from certain types of algae. It can be easily tailored to specific applications by Modifying the ratio of subunits and the molecular mass of the polymer material chain. to alter its physical and rheological properties, and it degrades naturally and sterilizes without degrading.46 However, alginate has a few drawbacks, including poor stability and temperature sensitivity and rather weak mechanical characteristics. The use of NGCs, or hydrogels based on alginate, for nerve regeneration had positive results.45
4.2 Crosslinked hydrogels via physical reactions for supportive biomaterials for solid neuronal glial cells
Physically crosslinked hydrogels as drug carriers are increasing.46 To achieve the hydrogel's basic features water content, soft and flexible nature, and biocompatibility-crosslinked hydrogels are formed as interconnected networks of polymer chains in three dimensions held together by chemical crosslinks.47 They have a broad variety of applications in the pharmaceutical industry, including not just the fields of drug administration and tissue engineering, but also some other fields as well. Hydrogen bonding and hydrophobic interactions also play important roles in the stability of both polyelectrolyte polymers. These electrostatic and dipole–dipole associations can be reversed. In contrast to hydrogels that undergo chemical-based crosslinking, mechanically cross-linked hydrogels are frequently characterized by their nontoxic nature, high tolerance, and biocompatibility. Because of the potentially harmful nature of many synthetic crosslinkers, hydrogels have been developed that bind carbohydrates via ionic crosslinking with multivalent interactions such as electrostatic interactions between polyelectrolytes with opposing electrical charges, hydrophobic interactions, and hydrogen bonding.48, 49 Even in the absence of an external electrical stimulus, a considerable number of studies have demonstrated that electrically conductive hydrogels can promote neurite have been covalently crosslinked and typically call for extra purification processes.47 The vast majority of synthetic polymers that have been chemically crosslinked can provoke an immune response.48
5 THE CURRENT STATE OF POLYSACCHARIDE-BASED DRUG DELIVERY SYSTEMS FOR REPAIRING INJURIES TO NERVE TISSUE
Naturally occurring polysaccharides are being studied as potential drug delivery systems for promoting nerve regeneration due to their biological suitability, being biodegradable, as well as their capacity to encapsulate and release therapeutic chemicals in a regulated manner. Polysaccharides can improve the transport of neurodegenerative medications to injured nerves, which eventually helps facilitate nerve repair and regrowth. Nerve regeneration is a complex process, and employing polysaccharides as carriers for neurodegenerative drugs can improve the administration of these therapeutic agents. These naturally occurring polysaccharides can encapsulate many therapeutic molecules, such as growth factors, neurotrophic factors, anti-inflammatory drugs, and other medicinal compounds that stimulate neuron regeneration. Drug delivery systems can provide continuous support for nerve healing over an extended period because of their ability to adjust the rate at which these agents are released.
5.1 Chitosan-based polysaccharide drug delivery systems for repairing injuries to nerve tissue
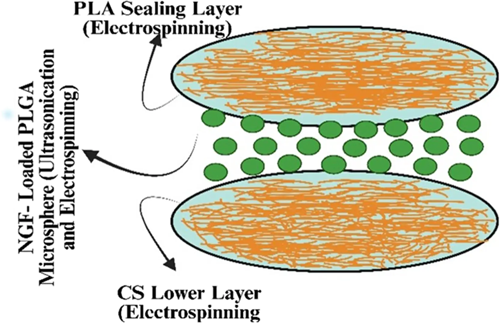
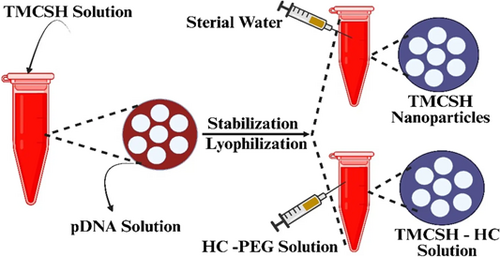
Chitosan (CS) is a potential carbohydrate that is produced by the deacetylation of chitin, which is derived from the shells of crustaceans.50, 51 CS has been extensively utilized in the field of brain tissue engineering. Hydrogels and nanoscale drug delivery systems (DDSs) have been proposed in the last decade as potential therapeutic approaches for addressing injuries to the neuronal.30 Heparin, a well-known anionic glycosaminoglycan that stops blood from clotting reduces inflammation, and has a high binding affinity for growth factor receptors, was used to bind CS ionically. The methodology exhibited a high level of biocompatibility with the neural stem cell lineage and demonstrated ease in its binding to fibroblast growth factor, a critical element for the sustenance of neural stem cells.52 Wu et al.53 used a combination of the chemical ferulic acid (FA) and glycolic CS (a CS precursor that acts as a water-soluble) to create neuroprotective nanoparticles (Nps). The combination of glycol CS and FA has the potential to generate hydrophobically self-assembled Nps. These nanoparticles will have a core composed of FA that is hydrophobic and a hydrophilic exterior composed of glycol CS. Systemic administration of Nps was also used to assess their bioavailability pharmaceutical kinetics, and recovery of function in a rat model of spinal cord puncture injuries. The administration of FA-glycol CS Nps led to a considerable increase in the repair of axons and neuron cells at the site of injury while simultaneously reducing the number of activated astrocytes and macrophages. These neuroprotective characteristics eventually helped with functional recovery after a PNI.53 Song et al.54 developed a CS scaffold to treat PNI injuries. Preparation of a sandwich-structured composite system using electrospray and electrospinning allowed for the sustained, controlled release of NGF over a 2-month period, which is crucial during the healing phase of PNI. Figure 1 illustrates a simplified model of the composite scaffolding.55 In their study, scientists have examined a distinct combination between chondroitin sulfate (CS) and a medication. They accomplished these by creating CS nanoparticles (Nps) to deliver methylprednisolone (MD), a corticosteroid commonly employed in the management of acute PNI to minimize inflammation and reduce the amount of neuronal impairment resulting from the injury. Ni and coworkers investigated the use of prolonged administration of chondroitinase ABC (chABS), an enzyme that can break the glycosaminoglycan chains of chondroitin sulfate proteoglycans (CSPGs), which have been shown to prevent axonal regeneration after spinal cord injury. Figure 2 shows the steps that were taken to make BDNF-loaded Nps.56 To make TMSCH-HC Nps, a plasmid expressing tetanus neurotoxin was added to the TMCSH solution as the HC-PEG solution. Tetanus neurotoxin can alter the retrograde transport of nanoparticles following peripheral injection. Kwiecien and his partners produced a hydrogel made of CS-collagen that was serpine, also known as Serp-1, which is an inhibitor of serine proteases and is loaded with the compound. Serpine is a biological medication that modulates the immune system. This hydrogel was designed to treat crush-induced PNI. Serp-1 was injected into Rats with simulated PNI caused by dorsal column compression at both low (10 g) and high (100 g) concentrations using CS-collagen hydrogels. During these investigations, an analysis of histopathology was performed, as well as an evaluation of locomotor functioning.57 The scientists demonstrated that Serp-1-loaded hydrogel improved motor recovery and decreased neurological impairments more than low-Serp-1-loaded hydrogel or unloaded hydrogel. In addition, a Serp-1-induced decrease in apoptosis was attributed to the reduction of brain damage that was detected when using the high-dose-loaded hydrogel.57 This reduction was observed alongside a reduction in the severity of neural injury. The valproic acid release from CS Nps was studied by Wang and his associates. It was shown that valproic acid can protect microglia from damage and can also lessen the inflammation brought on by abrasions to the nervous system.58
5.2 Alginate (ALG) based polysaccharide drug delivery systems for repairing injuries to nerve tissue
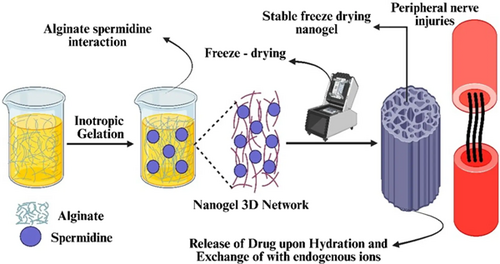
ALG is a naturally occurring longitudinal anionic in nature polysaccharide composed of recurrent units of (1-4)-d-mannuronic acid and a -l-guluronic acid building block.59 In particular, alginate (ALG) has been extensively employed in the domain of neurological cells as well as tissue engineering to create a diverse array of innovative drug delivery systems (DDSs). Alginate is employed in the context of peripheral nerve injuries (PNI) due to its excellent biocompatibility, which minimizes immunogenic responses. Alginate's ease of processing and utility in research contribute to its significance in PNI, though material selection depends on specific injury parameters. The DDSs include implantable hydrogels, as well as non-injectable hydrogels derived from hydro microfibers. Additionally, more sophisticated composite frameworks with characteristics promote neuroprotection as well as neurodegeneration.60, 61 (Table 1) provides an overview of all of the ALG and dextran-based DDSs that have been reported. Downing et al.68 used ALG to create a rolipram-containing microfibrous drug delivery device. Rolipram is an anti-inflammatory medication with numerous beneficial qualities for the treatment of injury related to either peripheral or central nervous and peripheral nerve injury (PNI). The method created used a microfibrous substrate made of poly(l-lactide) electrospun and covered with a CaCl2-cross-linked ALG hydrogel layer, enabling rolipram distribution that was both regulated and localized. The capabilities of the platform to govern the distribution of the drug have been demonstrated by showing a sudden release of about 40% of the rolipram during the initial 18 h of the experiment and then followed by a steady release that endured even after 1.5 days. Rats that had a C5 hemisection lesion were given therapy, and it was seen that the therapy improved axon regeneration as well as functional and anatomical recovery.68 Nanogels were produced by cross-linking ALG with spermidine (SP) to provide cutting-edge instruments for the Rejuvenation of nerves located in the periphery of the body spermidine is a bioamine that may be found in nature and has been shown to possess neuroprotective properties. It also possesses a cationic character, which makes it possible for it to interact with the anionic ALG. The synthesis of nano- and microgel dispersions was achieved by cross-linking ALG at high and medium viscosity using the ionotropic gel formation approach with SP at varying concentrations (Figure 3).69 To optimize the mean hydrodynamic diameter, a DoE method was used to determine the optimal combination of the two variables. The use of measurements of viscosity and solid-state characterization, specifically through FT-IR analysis, facilitated our research endeavors.
| Dds-based therapies | Synthesis process | Pharmacological compound | Applications | In vitro Model | In vivo model | References |
|---|---|---|---|---|---|---|
| Alginate | ||||||
| CaCl2 cross-linked ALG hydrogel-coated PLA microfibers | Microfiber spin-off, hydrogel cross-linking | Rolipram | Peripheral nerve injury | Lesioned C5 hemisection rats | [62] | |
| CaCl2-crosslinked PLGA microsphere-ALG-fibrinogen hydrogel | Solvent evaporation | Glial cell-derived neurotrophic factor | Peripheral nerve injury | The PC-12 cell line | Model-based on the hemisection of the spinal cord of a rat | [63] |
| ALG cross-linked by CaCl2 | Hydrogel formation through CaCl2 cross-linking; data unavailable for microspheres and Nps | Glial cell-derived neurotrophic factor | Peripheral nerve injury | SH-SY5Y and NIH-3T3 cells. | spinal cord hemisection model in rat. | [64] |
| ALG/ALG-S complex | Lyophilization | MH and PCX | Peripheral nerve injury | Experimental rat model of left lateral hemisection | [62] | |
| Cross-linked acryloyl glycine-spermidine hydrogel | Freeze-drying/ionotropic gelation | Spermidine | Peripheral nerve injury | Schwann cells | [63] | |
| Dextran | ||||||
| Nps Ibuprofen | Dx hydroxyl and ibuprofen carboxylic acid esterification triggered by N, N-carbonyl diimidazole | Methylprednisolone | Peripheral nerve injury | BV-12 microglia | A peripheral nerve injury rat model intraperitoneal injection | [65] |
| Dx acetalating Nps P | Microprecipitation modality | PCX | Peripheral nerve injury | Modeling mechanical peripheral nerve injury in rats | [66] | |
| Nanostructured acetylated-Dx Nps microspheres | A device that focuses on microfluidic flow | Methylprednisolone | Peripheral nerve injury | Modeling mechanical peripheral nerve injury in rats | [67] | |
Des Rieux and his team used hydrogels made from fibrinogen to move vascular endothelial growth factor (VEGF).64 VEGF is a protein that protects nerve cells and has been shown to help the spinal cord heal. VEGF was used in either its native form or in the form of nanoparticles or microscopic spheres of synthetic polylactic acid (PLGA).64 Human neuron-like SH-SY5Y cells proliferated modestly in the presence of free VEGF-loaded hydrogel, but NIH-3T3 cells that have characteristics similar to murine fibroblasts did not proliferate at all, even after being given fibrinogen as a supplement. Therefore, the VEGF-free hydrogel that was filled with hydrogel was able to cause neurite formation in dorsal root ganglia cells grown outside of the body, but it didn't have the same effect when tested in a model of a rat spinal cord that had been cut in half.64 First, the growth factor release profile from VEGF Nps or microspheres was characterized. Both systems released the growth factor more slowly than the free VEGF-loaded hydrogel, but the release from VEGF microspheres was too slow. Subsequently, the technique was employed in a rat model of spinal cord hemisection, where a combination of unbound VEGF and VEGF Nps was supplied to achieve a dual objective of facilitating prompt and prolonged protein release.64 Historically, VEGF has been shown to induce neurite outgrowth and angiogenesis at the lesion site. Unfortunately, the VEGF-loaded hydrogel failed to facilitate a more rapid restoration of function in the rats.64
5.3 Dextran (Dx) based polysaccharide drug delivery systems for repairing injuries to nerve tissue
Dextran is often produced via a variety of Gram-positive, anaerobic cocci, including strains of Streptococcus and Leuconostoc strains.70 In addition, Dx has found widespread application in nanomedicine as well as for the delivery of drugs; due to its neutral charge, it is an ideal choice for Nps production since it helps increase nonspecific cellular absorption.71 To facilitate the delivery of PCX, Liu et al. synthesized Dx-loaded Nps. The microprecipitation technique was used to synthesize acetylated-DX Nps for their claimed neuroprotective effect. It was determined that a ratio of 1:5 PCX to acetylated-Dx was optimal for the effectiveness of encapsulation, the degree of the loading process, and the PCX delivery pattern. The administration of loaded Nps resulted in improved locomotor recovery, neuronal regeneration, and neuroprotection in rats.66 To achieve this result, PCX was injected at the location and then gradually released over 7 days.66 Dx was utilized in the synthesis of DDSs, which were designed for the healing of spinal cord injury by intravenous injection.71 The combination of diclofenac and Dx was employed. in the form of Nps to facilitate the administration of methylprednisolone (MP).71 The combination of ibuprofen and dextran occurred due to an esterification reaction between the hydroxyl groups of Dx and the groups of carboxylic acids of ibuprofen.66 This esterification was then accelerated using N-carbonyl diimidazole. The confirmation of the structure's biological compatibility was achieved by in vitro experiments conducted on BV-12 cells containing microglial cells. In a PNI of rat model, the MP content in the blood plasma was measured again subsequent intraperitoneal delivery through the comparison of MP-loaded nanoparticles (NPs) against a standard MP solution.67 The administration of diclofenac-loaded NPs has been seen to facilitate the restoration of both locomotor function and neurological impairments, as well as the repair of nerve functioning following PNI. In addition, acetylated-Dx was utilized by Li and his colleagues to generate microspheres loaded with Nps. These microspheres were designed for sustained MP release in the event of SCI.
5.4 Agarose (AG) based polysaccharide drug delivery systems for repairing injuries to nerve tissue
AG is a naturally biodegradable polysaccharide that is water-soluble and was derived from marine red algae. and is constructed by repeating units of this sugar.72 Aside from its high water-uptake capacity, which is capable of stimulating cell growth, differentiation, and proliferation,73 AG has special characteristics and can self-gel at 37°C without the addition of the cross-linking agents, hence avoiding their ultimate toxicity. Additionally, this marine polysaccharide displays significant bioactivity, extraordinary mechanical qualities, and a precise resemblance to the natural extracellular matrix (ECM). As a result of these factors, AG has recently garnered a lot of attention as a biomaterial for the establishment of complicated carriers for controlled DDSs. As a consequence of this, AG has broad application in the fields of biological engineering and pharmaceutical delivery systems for the treatment of a broad spectrum of conditions, including the regeneration of cartilage as well as bone, cardiovascular disorders, skin wounds, and the therapy of problems affecting the brain and nervous system.72 PNI research has focused on a wide range of systems, such as in situ gelling systems and hydrogels with complicated shapes that can host other systems like nanoparticles and microtubes with therapeutic agents.
To localize MD release, Chvatal et al. created an AG hydrogel. More specifically, MD was put inside PLGA nanoparticles that were made using the procedure of multiple emulsions.73 Because of the combination of hydrogel and nanoparticles, it was able to successfully recognize an extended release of the drug over 6 days. Additionally, it has been demonstrated that this type of system, when applied topically in vivo in a spinal cord injury rat model, was able to encourage the reduction of the injury size as well as the decrease in ensuing trauma-related inflammatory processes over 7 days.74 To accomplish their goal of delivering ChABC, an enzymatic agent that can break CSPGs and is responsible for the regulation of axon formation during SCI, Lee et al. employed the use of AG in the production of lipid microtubes, which were then encased in a hydrogel that's composed of AG that can be used for delivery. These microtubes were then immersed in an AG-based hydrogel. Trehalose was used to stabilize ChABC because of the protein's sensitivity to heat at 37°C before it was incorporated into lipid microtubes. After that, the system was implanted in a rat model of spinal cord damage. There, it set up a steady flow of ChABC, which kept the rat's ability to break down CSPGs for 2 weeks after the damage. As a result of the action of the thermally stabilized ChABC, axonal development and functional recovery in the employed rat model were also seen.75 Gao et al. came up with the idea for a sophisticated AG scaffold that is made up of microchannels that are arranged in a honeycomb pattern. To be more specific, the framework of the scaffold was comprised of an AG platform, which distinguished out from other platforms due to the presence of internal micro-channel guides measuring 166 m. These guides were derived from fiber bundles that were composed of hexagonally packed (honeycomb architecture) polystyrene fibers that were arranged in a linear orientation. Bone marrow stem cells from a syngeneic donor were used to enhance the system's BDNF expression. These cells were generated using the viral vector that included encoding for the completely human BNDF. In an in vivo model, the researchers demonstrated that their bioengineered scaffold can both support and guide the development of axons, and this development of axons is done by cutting the spinal cord of a rat in half. For neuroprotection in SCI, Cox et al.78 made PLGA Nps which is full of estrogen (E2).76 A rat model of spinal cord injury that ranged from mild to severe was utilized to assess the effectiveness of the scaffold. To create the framework, the E2-loaded PLGA Nps that were produced via the nanoprecipitation process were combined with an AG hydrogel.76 A broad spectrum of cytokine profiles was observed, and it was demonstrated that the plasma level of E2 was twice as high as the normal level.76 Wang et al. investigated the neurological characteristic of methylhistamine (MH), which could target the recurrent process that is activated during an injury, to identify a potential therapeutic intervention for the treatment of PNI. To produce a drug with a sustained release, a complex was produced of the pharmaceutical with Dx sulfate by using an interaction that was supported by metal ions.77
5.5 Cellulose (CL) based polysaccharide drug delivery systems for repairing injuries to nerve tissue
In addition to its localization inside the cell membranes of plants, cellulose can also be synthesized by mammals, fungal organisms, and microorganisms. In terms of its structural composition, it is mostly consisting of d-glucopyranose rings that are connected by −1,4-glycosidic linkages. These ring units are then organized in chains to create fibrillary units, which are then put together to form microfibrils.78 CL has been used for many pharmacological reasons because it can respond to chemical, physical, and mechanical signals and is bioactive and biocompatible without breaking down. According to a study from 2022, the use of bacterial CL for the repair of damaged neural tissue has become popular.79 For example, Stumpf and his colleagues used biosynthesized cellulose (BC) made by Gluconacetobacter hansenii to make nerve guides that helped neurons grow back after a PNI. Based on the growth of G. hansenii cells at 3, 6, 9, 12, 16, 18, and 22 days, BC tubes (BCTs) with different BC levels were developed. BCTs could be identified by their dimensions, which included a diameter in the center of 3.53 mm, an outer diameter of length of 5 cm, and a width of 0.75 mm. and mechanical characteristics that were comparable to those of natural neuronal tissue. Neurite stimulation was provided by NGF-loaded BCTs.80 BCTs that had been loaded with NGF had good mechanical characteristics; in particular, BCTs that had been cultured for 22 days (BCTs22) had a Young's Modulus that was comparable to that of the spinal cord. NGF also remained bioactive on PC12 cells following BC tube removal.80 A biocompatible, biodegradable, and processable soy protein isolate was employed. SCs and PQQ, a neurotrophic factor that boosts SC proliferation and migration, were planted in tubes. For in vivo studies, a model consisting of rats was employed. A “gold standard” autograft nerve was used as a positive control. When compared with the control category, the scaffold improved the regeneration of nerves, especially when it was implanted with fibroblasts and PQQ.81
5.6 Gelan gum (GG) based polysaccharide drug delivery systems for repairing injuries to nerve tissue
Gelan gum is a longitudinal anion-like exopolysaccharide that is produced by Sphingomonas paucimobilis during the fermentation process. It is composed of repeated units of tetrasaccharide, each of which has one carboxyl lateral group.82 GG doesn't deteriorate or get brittle when subjected to extreme heat or acidity.83 The fact that GG has mechanical characteristics that are equivalent to the elastic modulus of typical tissues is one of its most attractive qualities, which makes it an excellent material for biological engineering and the production of DDS.84 Table 2 summary of the cellulose and GG based data distribution systems used for Peripheral nerve injury. Provides an overview of all of the ALG-based DDSs that have been reported. GG is also noncytotoxic, biocompatible, and structurally similar to native glycosaminoglycans. Because of its characteristics, it is a potentially useful material in the construction of a wide variety of DDSs, such as particles, films, fibers, and hydrogels. Along with its pH-dependent swelling and stability, GG's anionic nature, which enables it to gel when mixed with monovalent or divalent cations,84 is a desired property for the DDS formulation process. Vigani et al.90 used gelan gum to develop an integrated approach for the administration of RC-33, a therapeutic potential that has previously been utilized by identical authors. Due to its positive charge, GG is capable of cross-linking with anionic polymers, as seen in Figure 4.
| Dds-based therapies | Synthesis process | Pharmacological compound | Applications | In vitro model | In vivo model | Reference |
|---|---|---|---|---|---|---|
| Gellan gum (GG) | ||||||
| Nanofibers of gelatinous glycoprotein that have been CaCl2 cross-linked and implanted in a freeze-dried matrix supplied with gelatinous glycoprotein. | Nanofibers produced by electrospinning, | RC-33 | Peripheral nerve injuries | - | - | [85] |
| Polymethyl methacrylate-intercalated xanthan pulp and guar gum-based hydrogel tubes. | Inter cross-linking via thermal ionization | Bioactive substances and bovine serum albumin (BSA) | Peripheral nerve injuries | - | - | [86] |
| Thiol-modified GG hydrogel15 | In situ gel is formed by pouring a 60°C solution into a mold. | Collagen and nerve growth factor | Peripheral nerve injuries | NSCs from a rat brain | [87] | |
| Nanofibrils of GG and GL | Electrospinning | Spermidine | Peripheral nerve injuries | Axons of Schwann | [16] | |
| Cellulose-based | ||||||
| Bio cellulose (BC) tubes | Silicon tube-molded custom bioreactor | NGF | Peripheral nerve injuries | PC12 cells | [88] | |
| Embryonic stem cell-seeded CL-soy protein tubes | The tubular mold | Pyroso- quinolinequinone | Peripheral nerve injuries | A rat model with a severed sciatic nerve | [89] | |
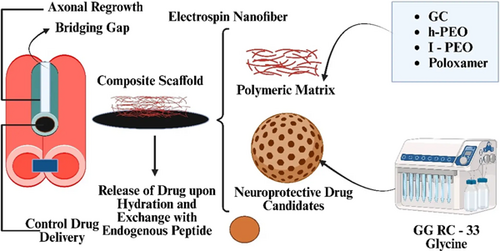
Calcium chloride across interconnected gelan nanofibers to produce by combining two different forms of poly (ethylene oxide) (PEO), such as a high concentration of (h-PEO) and low concentration of (l-PEO) molecular mass with the poloxamer (P407) to produce GG more readily electro spinnable. Electrospinning resulted in the production of nanofibers, which were then encased in a freeze-dried external framework consisting of a combination of RC-33/GG interface products and glycine, which serves as the cryogenic protection agent.90 Within this matrix, various quantities of the drug candidate were injected. Dialysis equilibrium experiments were used to determine the maximal binding capacity of GG for RC-33 and determine the RC-33/GG interaction product.90 The previously mentioned investigations were conducted to characterize the interaction. Four different loaded matrices were created by altering the concentration of RC-33, as determined by the hemodialysis state of equilibrium results of the analysis.90 The matrices were responsible for providing the exterior structure of the composite system. subsequently then emerged that the matrix structure with a higher concentration of RC-33, which interacts with 40% of GG sites of binding, was more effective in terms of biomechanical and hydrating properties. In the last step, nanofibers that had been cross-linked were put into a solution with 40% RC-33/GG and then freeze-dried to make the final composite system, which had better mechanical properties.90 To make nanofibers containing SP and gelatin (GL) for the treatment of PNI, the same authors employed GG as a base. Due to its inherent positive electrical charge, SP serves dual purposes as an agent for cross-linking and as an active ingredient for activation in the regeneration of peripheral nerve treatment. This resulted in a beneficial outcome. To study the GG/SP interaction, mixtures were produced with varying GG concentrations and rising SP concentrations, ranging from 0% to 0.125% by weight.91 It was shown that the incorporation of GL improved the biomimetic features of the system. Electrospinning was used to create cross-linked nanofibers from the optimal combination of GG, SP, and GL (Figure 5).

After being submerged in water for 24 h, these fibers were examined to determine their shape and mechanical characteristics. This confirmed the creation of an interaction product, which was expected given that nanofibers are insoluble in aqueous media. Evaluation of nanofiber biocompatibility using SCs revealed that the addition of GL was essential to improving cell compatibility.92
5.7 Silk fibroin as a natural biomaterial for peripheral management of nerve regeneration
Natural biomaterials are integral to the field of regenerative medicine, particularly in the context of managing peripheral nerve regeneration, where the intricate interplay of various biological and mechanical factors is crucial. Among these biomaterials, silk fibroin has gained prominence due to its unique attributes. Extracted from silk fibers, silk fibroin exhibits exceptional biocompatibility, minimizing the risk of adverse reactions within the biological system.88 This property is essential for ensuring that the material is well-tolerated by the body, facilitating its integration into the regenerative process. As a biomaterial gradually breaks down within the body, it aligns with the natural healing and remodeling mechanisms.89 This controlled degradation is vital for allowing the gradual formation and maturation of regenerated tissue. In the specific context of peripheral nerve regeneration, silk fibroin's ability to provide mechanical support is of paramount importance. The mechanical strength of silk fibroin enables the creation of robust scaffolds and conduits, offering the structural integrity necessary for guiding the regrowth of nerves across injury sites.93 Researchers are actively exploring methods to incorporate silk fibroin into nerve conduits and scaffolds, tailoring the material to create a microenvironment that is conducive to cellular activities critical for regeneration.94 This includes promoting cell adhesion, proliferation, and differentiation, all of which are essential processes in the intricate journey of nerve regeneration.95 In conclusion, silk fibroin emerges as a promising natural biomaterial for peripheral nerve regeneration, offering a harmonious blend of biocompatibility, biodegradability, and mechanical strength.96
5.8 Collagen as a natural biomaterial for peripheral management of nerve regeneration
Collagen, a fibrous protein abundant in the extracellular matrix of various tissues, has garnered attention as a natural biomaterial for the peripheral management of nerve regeneration.97 Its widespread presence in connective tissues throughout the body, coupled with its biocompatibility, biodegradability, and low immunogenicity, makes collagen an appealing candidate for regenerative medicine applications. One of the key advantages of collagen in nerve regeneration lies in its ability to mimic the native extracellular matrix. The extracellular matrix provides structural support and cues for cell adhesion, migration, and differentiation—essential processes for successful nerve regeneration.98 Collagen-based scaffolds or conduits can be designed to closely resemble the natural environment, facilitating cell interactions and promoting the regeneration of damaged nerves.98 Collagen's biocompatibility ensures that it is well-tolerated by the body, minimizing the risk of adverse reactions. This property is crucial for creating a supportive environment for nerve cells and other relevant cell types involved in the regeneration process. Additionally, collagen's biodegradability allows for gradual breakdown over time, aligning with the pace of tissue regeneration and remodeling. As the collagen matrix degrades, it can be replaced by newly formed tissue, promoting a seamless integration of the regenerated nerve. Researchers have explored various strategies to enhance the regenerative potential of collagen, such as incorporating growth factors or combining collagen with other biomaterials to create composite scaffolds.99 These modifications aim to further optimize the microenvironment for nerve regeneration, promoting increased cellular activity and more efficient recovery. While collagen-based materials show promise in peripheral nerve regeneration, challenges persist. Fine-tuning the mechanical properties of collagen scaffolds, addressing issues of rapid degradation, and ensuring sustained release of bioactive molecules are ongoing areas of research.100, 101
5.9 Modified polysaccharides (GelMA)
Modified polysaccharides, particularly gelatin methacryloyl (GelMA), have emerged as versatile and promising natural biomaterials for peripheral nerve regeneration. GelMA, derived from gelatin through methacryloyl modification, possesses a unique combination of properties that makes it well-suited for tissue engineering applications. The incorporation of methacryloyl groups allows GelMA to undergo photo-crosslinking, transforming it into a hydrogel with tunable mechanical and biochemical characteristics.102 This photo-crosslinking ability facilitates the creation of three-dimensional (3D) structures that mimic the extracellular matrix, providing a supportive environment for cellular activities critical to nerve regeneration.103 The mechanical properties of the hydrogel can be precisely adjusted to match the needs of nerve tissues, ensuring optimal support and guidance for regenerating nerves.104 The porous structure of GelMA further facilitates the diffusion of nutrients and the removal of waste products, fostering an environment conducive to cell survival and tissue regeneration.105 In addition to its physical attributes, GelMA's biofunctionalization capabilities make it a versatile platform for enhancing peripheral nerve regeneration. Researchers can introduce bioactive molecules, growth factors, or other signaling cues into the GelMA matrix to actively promote cellular interactions.106 This biofunctionalization not only supports the attachment, proliferation, and differentiation of nerve cells but also guides the alignment of regenerating nerve fibers, crucial for functional recovery. The application of GelMA in peripheral nerve regeneration extends to the design of conduits and scaffolds that serve as guides for regenerating nerves across injury sites.106 These structures provide mechanical support and create a permissive microenvironment for nerve cells to grow and reconnect. Moreover, GelMA has demonstrated efficacy in promoting axonal outgrowth, a critical aspect of nerve regeneration. The hydrogel's ability to support the extension of nerve fibers is essential for bridging the gaps created by nerve injuries.107 This property positions GelMA as a promising biomaterial for addressing challenges associated with nerve defects, where promoting axonal growth is paramount for functional recovery.107 Researchers are exploring ways to combine GelMA with other biomaterials, creating composite structures that harness the strengths of each component.108 This approach aims to address limitations and further optimize the regenerative potential of GelMA-based constructs.108 Its unique combination of tunable properties, biofunctionalization capabilities, and support for axonal outgrowth positions it at the forefront of biomaterials research in the realm of regenerative medicine.109
6 IN VITRO MODELS AND ANIMAL MODELS UTILIZED IN THE DEVELOPMENT OF INNOVATIVE NGCS
In the development of NGCs, various animal models are employed to assess the efficacy and safety of these innovative devices in a living organism. Table 3 summarized the In vitro models and Animal models are commonly employed in the process of developing novel NGCs. Animal models play a crucial role in assessing the safety, biocompatibility, and efficacy of NGCs, providing valuable data before advancing to human clinical trials. Ethical considerations and adherence to guidelines for the humane use of animals in research are essential aspects of conducting studies involving animal models in NGC development.
| Animal model | Description | Applications in NGC development | Reference |
|---|---|---|---|
| Animal models | |||
| Rodents (mice, rats) | Small size, cost-effective; used for initial biocompatibility and efficacy assessments | Preliminary testing of NGCs for basic functionality, biocompatibility, and early-stage observations of nerve regeneration. | [110] |
| Rabbits | Larger nerve anatomy than rodents; suitable for more detailed assessments of NGC performance. | Allows for studying NGCs in larger nerve structures, providing insights into performance in more complex anatomies. | [111] |
| Pigs | Closer anatomical resemblance to humans; used in preclinical studies before human trials. | Provides a bridge between smaller animal models and humans, offering insights into NGC performance in larger, more complex systems. | [112] |
| Nonhuman primates | Closer physiological and anatomical similarities to humans; used for specific research needs. | Applied in studies requiring a higher degree of anatomical and physiological relevance to humans, especially for advanced preclinical trials. | [111] |
| In vitro models | |||
| Cell culture | Nerve cells (neurons) grown in controlled environments; are used to study cell behavior and NGC interactions | Initial screening of NGC materials, assessment of biocompatibility, and understanding of cellular responses to different conduit formulations. | [110] |
| Organotypic cultures | Nerve tissues cultured in three-dimensional arrangements; provide a more realistic environment for testing. | Mimics the in vivo environment more closely, allowing for detailed studies on NGC integration, tissue interaction, and overall performance. | [113] |
| Microfluidic devices | Devices that mimic the microenvironment of nerves; allow precise control over conditions during NGC testing. | Enables researchers to study NGCs in controlled microenvironments, providing insights into how conduits perform in conditions resembling natural nerve environments. | [113] |
| 3D bioprinting | Creating three-dimensional nerve tissue models for studying NGC integration and performance in a controlled setting. | Allows for the fabrication of complex nerve structures, offering a platform to assess the integration and functionality of NGCs in a more intricate 3D environment. | [114] |
7 SYNTHETIC NGCS BIOMATERIALS FOR PERIPHERAL MANAGEMENT OF NERVE REGENERATION
7.1 Poly(l-lactide) (PLLA)
Synthetic NGCs have seen significant advancements in their design, with polymeric materials playing a crucial role in their construction. Among these materials, poly(l-lactide) (PLLA) has emerged as a particularly valuable option. PLLA, a biodegradable polyester, possesses excellent biocompatibility and has been extensively employed in the biomedical field.115 One notable characteristic of PLLA is its ability to gradually degrade into nontoxic byproducts, aligning with the principles of controlled degradation essential for NGCs.116 When used in NGC fabrication, PLLA serves as a structural framework, offering mechanical support for regenerating nerves. Its controlled degradation ensures a synchronized breakdown with the pace of natural healing processes, minimizing the risk of inflammatory responses. PLLA's biocompatibility and controlled degradation make it an ideal candidate for creating NGCs, providing a conducive environment for nerve regeneration while maintaining structural integrity during the critical stages of healing.117
7.2 Poly (lactic-co-glycolic acid) (PLGA)
Synthetic NGCs have made substantial strides in the realm of nerve regeneration, with polymeric materials, particularly PLGA, taking a forefront role in their composition. PLGA, a copolymer of lactic acid and glycolic acid, is characterized by its biodegradability, making it a compelling choice for biomedical applications.118 The controlled degradation of PLGA is a key attribute, allowing for a gradual breakdown into nontoxic byproducts, which corresponds well with the temporal requirements of nerve regeneration.119 This controlled degradation ensures that the NGC provides mechanical support during the critical stages of healing while gradually yielding to natural tissue repair processes. PLGA's biocompatibility is another crucial factor contributing to its widespread use in NGC design. The copolymer creates a structural scaffold within the conduit, offering physical support for regenerating nerves.120 The incorporation of glycolic acid into the polymer enhances the hydrophilicity of the material, facilitating improved interactions with surrounding cells and tissues. This feature is particularly advantageous in promoting cell adhesion and migration within the NGC, fostering an environment conducive to successful nerve regeneration. PLGA-based NGCs have demonstrated efficacy in preclinical and clinical settings, showing promise in providing a supportive environment for nerve regeneration.121
7.3 Poly(caprolactone) (PCL)
The fabrication of synthetic NGCs has witnessed significant progress, with polymeric materials like poly(caprolactone) (PCL) playing a crucial role in their development. PCL is a biodegradable polyester known for its favorable mechanical properties, biocompatibility, and controlled degradation characteristics, making it well-suited for applications in neural tissue engineering.122 PCL's biodegradability ensures that the conduit gradually breaks down into nontoxic byproducts over time, aligning with the temporal requirements of nerve regeneration. This controlled degradation allows the NGC to provide structural support during the critical stages of nerve repair while facilitating seamless integration with the body's natural healing processes. The mechanical properties of PCL, including its flexibility and strength, make it an excellent choice for constructing NGCs.123 The polymer can be processed into various forms, such as tubes or fibrous scaffolds, providing a supportive architecture for regenerating nerves. The versatility of PCL allows researchers to tailor the physical characteristics of the NGCs based on the specific requirements of the nerve injury being addressed. Additionally, PCL's biocompatibility ensures minimal adverse reactions when implanted in the body, promoting a favorable environment for nerve regeneration.124 The material's ability to support cell adhesion and migration contributes to its efficacy in fostering the growth of new nerve tissue. Overall, PCL-based NGCs hold promise in promoting successful nerve regeneration due to their biodegradability, mechanical properties, and biocompatibility.
7.4 Polyamides
The development of synthetic NGCs has incorporated various polymeric materials, and polyamides have emerged as noteworthy candidates in this regard. Polyamides, including nylon, offer unique properties that make them suitable for creating NGCs for nerve regeneration.125 Polyamides are characterized by their robust mechanical strength and biocompatibility, making them attractive for applications in neural tissue engineering. These polymers provide a stable and supportive framework for regenerating nerves, aiding in maintaining the structural integrity of the NGC during the critical phases of nerve repair.123, 125 The inherent flexibility and versatility of polyamides allow for the fabrication of NGCs in different forms, such as tubes or fibers, providing options for tailoring the conduit's structure based on specific requirements. Biocompatibility is a crucial factor in the success of NGCs, and polyamides' compatibility with biological systems ensures minimal adverse reactions when implanted in the body.126 This characteristic promotes a conducive environment for nerve regeneration, allowing for the integration of the synthetic conduit with the surrounding tissues. Researchers continue to explore the potential of polyamides in NGC design, experimenting with different formulations and fabrication techniques to optimize their performance.
8 CHALLENGE AND FUTURE PROSPECTUS
The management of peripheral nerve injuries using biomaterials and their derivatives presents a multifaceted set of challenges at the intersection of materials science, medicine, and engineering.127, 128 One pivotal challenge lies in ensuring the biocompatibility and long-term safety of implanted biomaterials, necessitating careful consideration of immunological responses and the potential for adverse reactions. Designing biomaterials that effectively promote nerve regeneration and functional recovery is another hurdle, requiring a delicate balance in terms of material selection, mechanical properties, and integration with existing nerve tissue.129 Achieving long-term stability and addressing the translational gap from laboratory success to clinical application pose additional complexities, involving regulatory, ethical, and scalability considerations. Patient-specific approaches, acknowledging individual variations in age, overall health, and injury specifics, are essential for tailored interventions. Moreover, the cost-effectiveness of biomaterial-based therapies and fostering multidisciplinary collaboration between researchers, clinicians, and industry professionals are critical elements in advancing the field.113 Tackling these challenges collectively will pave the way for transformative advances in the management of peripheral nerve injuries, offering hope for improved clinical outcomes and widespread accessibility.
Technological progress is expected to yield more sophisticated biomaterials, including bioactive scaffolds and nerve conduits, tailored for enhanced nerve regeneration.130 Precision medicine approaches, considering individual patient characteristics and genetic factors, may become more prevalent in nerve injury management.131 The integration of biomaterials with emerging technologies like neurostimulation could offer synergistic benefits for nerve repair.132 Additionally, gene therapy may play a pivotal role in manipulating genes to promote nerve growth and repair.133 As research progresses, there will likely be a focus on translating these innovations into clinically applicable therapies, with a keen eye on regulatory and ethical considerations. The integration of digital health tools and increased international collaboration is anticipated to contribute to the robustness of research findings and accelerate progress.134 Future outcomes may not only measure physiological recovery but also emphasize the overall impact on patient quality of life, including functional recovery, pain management, and psychological well-being. Education and training for healthcare professionals in the application of these novel therapies are also expected to be integral components of the evolving landscape in peripheral nerve injury management.135
9 CONCLUSIONS
The management of peripheral nerve injuries using biomaterials and their derivatives represents a rapidly advancing frontier in regenerative medicine. This field is filled with exciting prospects and innovative approaches that hold great promise for the treatment and recovery of individuals with nerve injuries. These advances have the potential to revolutionize the way we address peripheral nerve damage caused by trauma, disease, or other factors. Through the application of biomaterials, researchers and clinicians aim to promote enhanced nerve regeneration by providing structural support, guiding axonal growth, and creating an environment conducive to nerve cell repair. This not only has the potential to reduce functional deficits but also significantly enhance motor and sensory recovery, ultimately improving the quality of life for affected patients. Furthermore, the versatility of biomaterials and their derivatives is a significant advantage, offering diverse applications that range from surgical implants and drug delivery systems to tissue engineering and nerve conduits. This adaptability enables tailored solutions based on the specific characteristics and severity of the nerve injury. The field is marked by ongoing research and innovation, with scientists and clinicians continuously exploring new materials, delivery methods, and therapeutic strategies to optimize outcomes and address the limitations of current approaches. As the field evolves, the potential for personalized medicine in nerve injury management emerges, allowing treatments to be customized to individual patient needs, genetics, and injury profiles. In conclusion, the management of peripheral nerve injuries using biomaterials and their derivatives is a promising and multifaceted domain that offers the prospect of transforming the way we treat and rehabilitate patients with nerve injuries, ultimately enhancing their quality of life and overall well-being. Sustained research and development are crucial for realizing the full potential of these innovative therapies.
AUTHOR CONTRIBUTIONS
Suraj Kumar: Data curation (equal); formal analysis (equal); methodology (equal); writing—original draft (equal). Rishabha Malviya: Conceptualization (lead); supervision (lead); writing—review & editing (equal). Sonali Sundram: Data curation (equal); formal analysis (equal); investigation (equal); writing—original draft (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable. The study did not qualify for any particular funds from any of the financing organizations that operate in governmental, private, and nonprofit organizations.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




