Bioengineered Nanozymes for Orthopedic Degenerative Diseases: From Catalytic Mechanisms to Multimodal Therapy
Lei Peng, Chen Yan, and Honghao Song contributed equally to this study.
ABSTRACT
Orthopedic degenerative diseases, particularly osteoarthritis (OA) and intervertebral disc degeneration (IDD), represent a growing global health crisis. Current clinical management relying on analgesics and anti-inflammatory drugs provides only symptomatic relief, while surgical interventions, though temporarily effective for advanced cases, carry inherent risks of adjacent segment degeneration and surgical complications. Synthetic nanozymes, which possess intrinsic anti-inflammatory and antioxidant properties, demonstrate significant therapeutic advantages in the treatment of orthopedic degenerative diseases. This review summarizes recent advances in nanonzyme-based therapeutics, with a focus on their regulatory roles in mitigating orthopedic degenerative diseases, particularly IDD and OA. Key mechanisms include anti-inflammatory effects, extracellular matrix remodeling, attenuation of cellular senescence and death, and antioxidative stress activities. We systematically analyze nanonzymes categorized by their diverse enzymatic activities and chemical compositions. Furthermore, we explore emerging combinatorial strategies employing nanonzyme delivery systems to achieve synergistic therapeutic outcomes and enhanced efficacy. The comprehensive discussion highlights the transformative potential of nanonzymes in advancing IDD and OA treatment paradigms, offering novel perspectives for future research and clinical applications.
1 Introduction
Intervertebral disc degeneration (IDD) and osteoarthritis (OA) are age-related degenerative disorders with substantial global burden. IDD progresses with age, affecting 30%–70% of adults through combined genetic and modifiable risks (biomechanical stress, obesity, smoking) [1]. OA prevalence exceeds 10%–15% in adults > 60 years, showing 3:2 female predominance, with obesity, joint injury, and genetic predisposition as key drivers [2]. Currently, the egenerative osteoarticular diseases's primary treatments are symptomatic and include analgesics, anti-inflammatory drugs, and rehabilitation through physical therapy. These interventions primarily function to postpone or conceal the progression. Surgical options are considered last-resort treatments and carry increasing risks of complications [3]. Emerging regenerative approaches, such as stem cell therapy, gene therapy, and molecular treatments, have created new avenues for the restoration of intervertebral disc function. However, these approaches also have their limitations [4, 5], making it critical to find more effective strategies for treating regenerative osteoarticular diseases.
Orthopedic degenerative diseases, encompassing IDD and OA, are pathologically characterized by inflammation-driven tissue deterioration. The intervertebral disc (IVD), comprising the nucleus pulposus (NP), annulus fibrosus (AF), and cartilaginous endplates (CEP), functions as a spinal shock absorber [6]. The NP, acting as the core pressure-absorbing structure, forms a high-pressure microenvironment with chondrocyte-like cells, collagen-II fibers, elastin, and hydrophilic proteoglycans, maintained by its extracellular matrix (ECM) [7] to ensure hydration and structural integrity. The AF features inner (collagen-II/proteoglycan-rich) and outer (collagen-I-dominated) layers [8, 9]. The CEP, a thin cartilaginous layer covering the IVD's surfaces, facilitates nutrient supply and pressure distribution [10]. Developmentally, AF and CEP cells derive from the somite, while NP cells originate from the notochord [11], whose regression correlates with IVD aging. Mechanical stress, injury, infection, genetics, and inflammation can trigger or accelerate IDD [12]. The pathogenesis of OA involves multifactorial interactions. Cartilage degeneration originates from ECM breakdown, where matrix metalloproteinases (MMPs) and ADAM metallopeptidases with thrombospondin motifs (ADAMTS) excessively degrade type II collagen and aggrecan. Anabolic/catabolic imbalance in chondrocytes leads to apoptosis and phenotypic abnormalities. Synovial inflammation is triggered by cartilage debris-activated macrophages, with pro-inflammatory cytokines promoting synovial hyperplasia and destructive mediator release, establishing an inflammation-catabolism vicious cycle [13]. Aberrant bone remodeling manifests as subchondral bone sclerosis and osteophyte formation, where abnormal mechanical loading and RANKL/OPG imbalance drive osteoclast activation, with resultant abnormal bone architecture exacerbating cartilage stress injury [13]. Both IDD and OA exhibit the main pathological mechanism, which is dominated by inflammatory response.
Nanomaterials exhibiting natural enzyme-like activities are commonly referred to as “nanozymes.” A significant milestone in the development of nanozymes occurred in 2004 and 2007, when gold (Au) and Fe3O4 nanoparticles were found to possess catalytic-mimiking activity [14]. Based on their catalytic behavior, nanozymes can be classified into various groups, including redox enzymes such as superoxide dismutase (SOD), peroxidase (POD), and nitrate reductase, as well as hydrolases like esterases, nucleases, and silicateases [14]. Since then, a wide range of materials such as cyclodextrins, metal complexes, porphyrins, polymers, dendrimers, and biomolecules have been widely studied for their potential to replicate the structure and function of naturally occurring enzymes. In addition, materials with a variety of enzymatic activities are gradually emerging [15]. For example, an artificial antioxidant enzyme designed by Cheng et al. can simultaneously possess enzyme activities such as CAT/SOD/GPx/NOx [15]. With the advent of nanotechnology, nanozymes have increasingly been enriched with diverse materials, including but not limited to metal oxides, noble metals, carbon materials/polymers, metal-organic frameworks (MOFs), composite nanozymes, and single-atom nanozymes, which combine the benefits of each component and feature active centers with atomic distribution [16-21]. Compared to natural enzymes, nanozymes have unique benefits, including enhanced stability for long-term storage, lower production costs, multifunctional catalytic engineering, and performance under a broader range of conditions [22, 23], which provides a new idea for the treatment of orthopedic degenerative diseases.
In this review, we provide a thorough overview of the pathogenesis of IDD and OA, highlighting its underlying molecular mechanisms including inflammation, CS and death, ECM degradation and oxidative stress. Additionally, we classify the various types of nanomaterials currently being explored for orthopedic degenerative diseases treatment, including nanozymes and nano-enzyme delivery systems (NDDSs). Besides, we discuss how different nanomaterials are tailored to target specific pathogenic mechanisms of egenerative osteoarticular diseases. Furthermore, our critical analysis of nanomaterials' clinical challenges in osteoarticular therapeutics offers strategic pathways to accelerate bench-to-bedside translation.
2 Pathophysiology of Orthopedic Degenerative Diseases
2.1 Similarities Between IDD and OA
Both IDD and OA share overlapping molecular mechanisms driving tissue degeneration. Inflammatory responses play a central role, with macrophages contributing to chronic low-grade inflammation. In OA, synovial macrophages secrete pro-inflammatory cytokines, while in IDD, macrophage-like cells infiltrate degenerated disks, amplifying NF-κB and MAPK signaling to promote ECM degradation [24]. ECM dysregulation is another hallmark: upregulated matrix metalloproteinases (MMPs-3/13) and ADAMTS-4/5 degrade collagen II and aggrecan [24], while aberrant fibrotic remodeling (collagen I/III deposition) disrupts tissue integrity [25]. Cellular senescence and death further link these conditions. Senescent chondrocytes (OA) and nucleus pulposus cells (IDD) exhibit senescence-associated secretory phenotypes (SASP) [26], releasing proteases and cytokines that perpetuate inflammation. Both diseases involve oxidative stress, where ROS overproduction overwhelms antioxidant defenses, leading to DNA damage and lipid peroxidation. Collectively, these shared pathways highlight potential therapeutic targets, such as macrophage polarization modulation or SASP inhibition, to mitigate degenerative progression [27] (Figure 1).
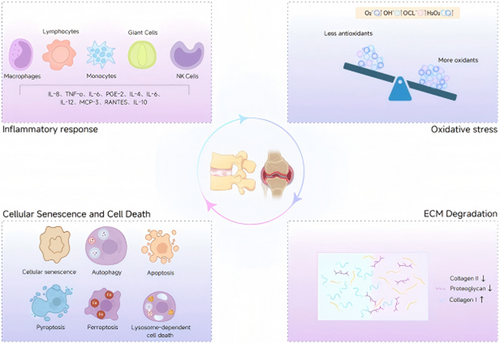
2.1.1 Inflammatory Response
Immune-privileged organs are protected by specialized physical barriers and various molecular mechanisms that create immune cell exclusion zones [28]. A close relationship exists between orthopedic degenerative diseases and the disruption of immune privilege [29]. Harmful factors such as aging [30], high physiological load or overload [31], and nutrient and oxygen deficiencies [32, 33] can result in a greater number and severity of disc cracks and tears, which in turn cause orthopedic degenerative diseases, disrupt immune privilege, and mediate primary immune responses.
2.1.1.1 Macrophages
CD68+ macrophages mediate inflammatory responses in IDD [34]. Postmortem studies detect CD68 in degenerated disks, reflecting activated macrophages/phagocytes [35]. Infiltration levels correlate with endplate degeneration severity, indicating macrophage-driven inflammation [36]. They exhibit plasticity by contributing to herniated disc resorption [36]. IVD macrophages display M1 polarization and distinct morphology from normal endplate cells, suggesting migratory origins [37-40]. CCL3-CCR1 signaling drives macrophage infiltration [41]. Macrophage coculture upregulates IL-8, TNF-α, IL-6, and PGE-2, alleviating mechanical pain in models [42]. Similarly, synovial macrophages dominate OA-associated inflammation, forming multinucleated giant cells (MGCs) that phagocytose cartilage debris. They secrete IL-1β, TNF-α, and MMPs, driving cartilage catabolism and synovial hyperplasia [43, 44]. In summary, the number of macrophages is directly linked to the level of inflammation. Due to the plasticity of macrophage, it has broad potential in orthopedic degenerative diseases treatment.
2.1.1.2 Lymphocytes
Th17 lymphocytes, abundant in degenerated intervertebral disks (IVDs), secrete IL-17 to upregulate pro-inflammatory cytokines (IL-4, IL-12, IL-6, IFN-γ) and recruit monocytes/neutrophils, implicating them in IDD pathogenesis [35, 45]. Activated T lymphocytes produce FasL, a TNF-family protein that induces apoptosis in Fas-positive immune cells. Reduced FasL expression during IVD degeneration reflects declining T-cell activity [46-48]. In herniated tissues, TH1 cells elevate IL-12/IFN-γ, while TH2 CD4+ cells exacerbate degeneration via IL-4 upregulation [35]. However, CD4+ lymphocytes are sparse in IDD lesions [35], and during chronic IDD (> 21 days), they constitute < 25% of immune infiltrates [49, 50], warranting further mechanistic studies. As for OA, T-cells infiltrate synovial tissues, promoting Th1/Th17 polarization to amplify IL-6, IL-17, and RANKL production, exacerbating synovitis and osteoclast activation [43].
2.1.1.3 Monocytes
Monocytes are present in IVD. A chemokine profile of monocyte and macrophage infiltration in disc herniation has revealed that cytokines such as MCP-3, IP-10, TNF-αand RANTES are secreted by monocytes and have a prominent role in the spontaneous herniated disks’ resolution [51]. Enriched peripheral blood-derived mononuclear cells (PBMC) can differentiate into CD14+ and CD206 + M2 macrophages in vitro, which have anti-inflammatory and moderate tissue repair effects and was able to control IDD progression and attenuate pro-inflammatory cytokines in the affected disks [52]. Furthermore, specific monocyte/macrophage subtypes can be used to assess the degenerative stage of IVD [53]. As for OA, monocytes recruited from circulation differentiate into pro-inflammatory macrophages under cytokine stimulation (CCL2, CCL5), perpetuating synovial inflammation [54].
2.1.1.4 Giant Cells
Giant cells are not typically present in IVD. Studies have indicated that, along with macrophages, mast cells are abundant in the granulomatous tissue area of IDDs, highlighting their critical involvement in the healing of damaged AF and the ensuing disc degeneration process [55]. Additionally, Zhu et al. demonstrated that the concentration of activated mast cells is significantly higher in IDD [56]. OA-associated MGCs exhibit distinct CD68 + /CD163+ phenotypes, facilitating cartilage fragment clearance but concurrently releasing catabolic enzymes [43]. In conclusion, the giant cells play a key role in the repair of the subsequent degeneration process.
2.1.1.5 Natural Killer (NK) Cells
NK cells help regulate immune responses by monitoring and eliminating damaged cells. Kunihiko et al. found that even undegraded autologous NP cells can be recognized by NK cells, indicating that NK cells are of great importance in the early stages of disc herniation [57]. During IDD, NK cells could release cytokines such as TGF-β and MMPs to promote tissue regeneration [58]. Activated by DAMPs (e.g., S100 proteins), NK cells secrete IFN-γand granzymes, inducing chondrocyte apoptosis and amplifying inflammatory loops [59].
2.1.2 CS and Cell Death
CS, autophagy, apoptosis, pyroptosis, ferroptosis, and lysosome-dependent cell death are key contributors to orthopedic degenerative diseases, highlighting potential therapeutic targets to mitigate its progression (Figure 2).
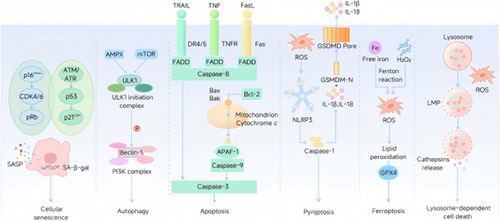
2.1.2.1 CS
CS involves progressive loss of cellular function/proliferation, driven by DNA replication errors (genomic instability, telomere attrition, epigenetic changes) [60]. In IDD, outer annulus fibrosus collagen shifts from fibrous to cartilaginous types, compromising structural integrity, while senescence-associated secretory phenotype (SASP) transitions to catabolic/inflammatory states [26]. CS markers (p16INK4A [26], SA-β-gal [30, 61, 62]) increase in aged disks. TNF-α suppresses IVD cell anabolism, linking inflammation to CS [63]. Therapeutic targeting of senescence pathways shows potential: SIRT1 silencing via Akt-FoxO1 axis mitigates oxidative stress-induced CS [64], while p16-Rb and p53-p21-Rb pathways (activated by ERK signaling [65]) critically regulate IVD aging [66]. p16 deletion alleviates IDD by restoring cell cycle progression and suppressing SASP, oxidative stress, and catabolism [67]. Preclinical strategies targeting SIRT1/Akt-FoxO1 and p16-Rb/p53-p21-Rb networks demonstrate efficacy in reducing CS-driven disc degeneration.
2.1.2.2 Autophagy
Autophagy is a cellular process that breaks down and reuses damaged components, playing a vital role in maintaining cell survival under stressful conditions [68]. Research has demonstrated that autophagy is present and active in both NP and AF cells. In compression-induced degeneration, ROS-mediated autophagy is activated in rat NP cells [69]. Specific factors can influence autophagy. Glucosamine has been shown to induce autophagy by inhibiting mTOR and p70S6K phosphorylation, thus protecting NP cells [70]. Additionally, overactivation of RHOA-PKN, through phosphorylation of KRT8 at Ser43, disrupts the transport of the small GTPase RAB33B, impairing autophagosome initiation and damaging NP cells, contributing to IDD [31]. Chen et al. suggested that reducing autophagy significantly decreases the apoptosis rate [71]. HIF1A alleviates apoptosis in NP-derived stem cells by upregulating autophagy [72]. Remarkably, regarding AF cells, studies indicate that IL-1β enhances autophagy in AF cells in a dose-dependent manner under serum deprivation conditions, thereby weakening their self-protection mechanisms [73]. In OA, impaired autophagy (reduced LC3-II/Beclin-1) leads to damaged organelle accumulation, activating mTOR and accelerating senescence [74, 75].
2.1.2.3 Apoptosis
Apoptosis in IDD [76] is regulated by IL-1β/NF-κB pathways, oxidative stress (via p65-mediated calcification [77]), and proteasomal LRP1 degradation (inhibiting NF-κB to reduce ECM apoptosis [78, 79]). The PI3K/Akt pathway counteracts IL-1β-induced NP cell apoptosis [80]. Autophagy crosstalk modulates apoptotic responses: HIF1A upregulates autophagy to mitigate compression-induced apoptosis [72], while SIRT1-autophagy signaling suppresses disc degeneration [81]. Oxidative stress exacerbates chondrocyte apoptosis dose-dependently [77], and IL-1β drives inflammatory cytokine production [82]. TNF-α/FasL activates caspase-3/8, while mitochondrial ROS opens permeability transition pores, releasing cytochrome c [83, 84]. Collectively, apoptosis involves multi-pathway interplay (e.g., IL-1β/NF-κB, PI3K/Akt, LRP1), with autophagy mechanisms (HIF1A/SIRT1-dependent) serving as critical safeguards against ECM degradation and cell death.
2.1.2.4 Pyroptosis
Pyroptosis, a gasdermin (GSDM)-driven programmed cell death linked to innate immunity, contributes to IDD via NLRP3 inflammasome activation [85]. ROS-induced pyroptosis in NP cells involves NLRP3/PYCARD signaling, counteracted by autophagy and NFE2L2 [86]. NLRP3 inhibitors (e.g., MCC950 [87]) and acid-sensing ion channel modulators [88] attenuate pyroptosis. Antioxidant strategies like mitochondrial-targeted SS-31 suppress ROS-mediated pyroptosis [89], while TXNIP inhibition (via saffron pigment) blocks caspase-1/NLRP3/IL-1β [90, 91]. PDGF dose-dependently suppresses pyroptosis mediators [92], and MSC-derived miR-26a-5p exosomes inhibit methyltransferase-like 14/NLRP3 pathways [93]. Bromodomain inhibitor JQ1 and NF-κB blocker Bay11-7082 reduce NLRP3-driven pyroptosis [94], whereas maltol delays IDD via NLRP3 suppression [95]. In OA, NLRP3 inflammasome activation cleaves gasdermin D, forming membrane pores for IL-1β release, linking cell death to inflammation [54]. These findings highlight pyroptosis as a key mechanism regulated by NLRP3, ROS, TXNIP, and NF-κB pathways.
2.1.2.5 Ferroptosis
Ferroptosis, an iron-dependent cell death driven by lipid peroxidation, contributes to IDD [96]. Dysregulated iron transport (e.g., FPN/SLC40A1) induces iron overload and extracellular matrix imbalance in NP cells [97, 98]. Key regulators like ATF3 promote ferroptosis by inhibiting SLC7A11/SOD2, enhancing ROS production [97, 98]. HO-1 upregulation and iron deposition occur in IDD models, though mechanisms remain unclear [99]. G3BP1-mediated autophagy protects against mechanical stress-induced ferroptosis [100]. Epigenetic modifications (e.g., ncRNAs like Circ-0072464/miR-431, miR-672-3p, lncGm36569) and GPX4 methylation drive ferroptosis via oxidative stress pathways [101-104]. Cynarin inhibits ferroptosis by upregulating GPX4/NRF2, reducing iron accumulation and lipid peroxidation [105]. In OA, lipid peroxidation (via GPX4 downregulation) and iron overload (transferrin receptor upregulation) induce iron-dependent death [106].
2.1.2.6 Lysosome-Dependent Cell Death
Lysosome-dependent cell death is essential in mediating the death of other cells, which is characterized by lysosomal membrane permeabilization (LMP). Partial and selective LMPS are able to trigger apoptosis and pyroptosis, which can lead to cell necrosis when completely ruptured [107, 108]. In OA, cathepsin B leakage from lysosomes activates caspases and MMPs, exacerbating ECM degradation [25].
2.1.3 ECM Degradation
The ECM is a intricate three-dimensional structure of approximately 300 proteins that offer structural support to tissues [109]. In the NP, cells interact with the ECM, influencing cellular behavior [110]. A range of genetic and environmental factors, including nutritional deficiency, mechanical overload, diabetes, smoking, and infections, can impair NP cell function and disrupt ECM homeostasis [111]. The depletion or phenotypic alteration of notochord-like NP cells results in an imbalance of the ECM, reducing the production of proteoglycans and type II collagen. This ECM composition' alternation, particularly the stiffening of the matrix [112], further affects NP cell behavior and is strongly linked to IDD [113]. Notably, changes in ECM composition modulate cell-ECM interactions, independent of mechanical stress, and influence the development of IDD [114].
In OA, proteolytic enzymes such as IL-1β and TNF-α drive ECM degradation by activating NF-κB and MAPK signaling pathways, which upregulate the expression of collagenase MMP-13 and aggrecanases ADAMTS4/5–enzymes responsible for cleaving type II collagen and aggrecan in articular cartilage [24, 115, 116]. Furthermore, matrix fragments generated during this process, including fibronectin and cartilage oligomeric matrix protein (COMP), perpetuate cartilage destruction by binding to integrin receptors (α5β1, αvβ3), thereby reactivating NF-κB and MAPK pathways to induce additional protease production in a self-amplifying cycle [25]. This degradative cascade is synergistically enhanced by adipokines such as leptin and adiponectin, which link metabolic dysfunction to ECM catabolism through JAK-STAT and PI3K-Akt signaling pathways, ultimately amplifying the production of MMPs and inflammatory mediators like NOS2 [117, 118].
2.1.4 Oxidative Stress
ROS are free radicals that contain oxygen and peroxides easily formed during cellular metabolism, which include superoxide anion (O2⁻) [119], hydroxyl radicals (OH−) [120], hypochlorite ions(OCl−) [121] and hydrogen peroxide (H2O2) [122]. The equilibrium between ROS and antioxidants is essential for preserving the proper function and viability of cells [123, 124]. ROS primarily affect the mitochondria, and disruptions in ROS metabolism can generate superoxides or hydrogen peroxide [125], triggering positive feedback loops that exacerbate mitochondrial damage [126]. In turn, mitochondrial dysfunction further accelerates ROS production [127]. ROS are implicated in IDD and OA through various signaling pathways, including the Keap1-Nrf2-ARE, NF-κB, MAPK, and PI3K-Akt pathways (Figure 3).
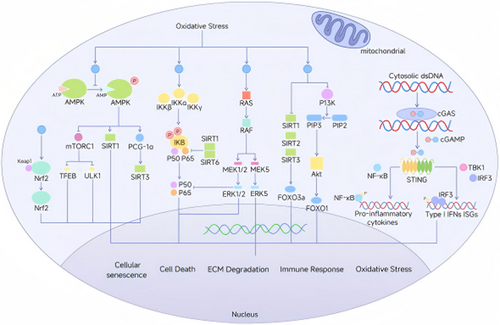
2.1.4.1 Keap1-Nrf2-ARE Signaling
The Keap1-Nrf2-ARE pathway, involving Kelch-like ECH-associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), and the antioxidant response element (ARE), is central to cellular defense against oxidative stress. Studies have shown that enhancing the expression of autophagy-related 7 (Atg7) and the Keap1-Nrf2-p62 autophagic pathway [128] alleviates oxidative stress-induced IVD degeneration [129]. During IDD, Nrf2 expression decreases [128], and its knockdown intensifies ROS-induced mitochondrial dysfunction and apoptosis [130]. Elevated ROS levels lead to the upregulation of Nrf2 and autophagic responses, which may mitigate ferroptosis in NPCs [86]. In OA, persistent ROS inactivates Keap1, enabling Nrf2 nuclear translocation to upregulate antioxidant genes (HO-1, NQO1). OA chondrocytes exhibit Nrf2 dysfunction, impairing adaptive responses [74, 75].
2.1.4.2 NF-κB Signaling
The NF-κB family, consisting of five members (RelA, RelB, c-Rel, NF-κB1, and NF-κB2) [131], is critical in inflammation and cellular responses to stress. ROS, such as H2O2, activates NF-κB signaling by inducing IκBα phosphorylation and degradation [132], and by triggering IKKβ S-glutathionylation [133]. In IDD, the activation of the NF-κB pathway correlates with oxidative stress and the extent of disc degeneration [134]. In AF cells, ROS-induced NF-κB activation contributes to the progression of IVD degeneration [135]. In OA, ROS oxidize IκB kinase (IKK), triggering IκB degradation and NF-κB nuclear translocation, amplifying IL-1β/MMP-13 expression [24].
2.1.4.3 MAPK Signaling
The MAPK family consists of extracellular signal-regulated kinase (ERK), p38, and c-Jun NH2-terminal kinase (JNK) [136]. Low concentrations of H2O2 could rapidly increase ROS levels in NP cells and activate p38, ERK, JNK, and Akt signaling pathways [137]. These pathways not only mediate ROS-induced apoptosis but also accelerate chondrocyte death and calcification of the cartilage endplates [77]. The activation of MAPK pathways creates a positive feedback loop with TNF-α, which further exacerbates IDD [138]. In OA, oxidative stress activates p38/JNK, phosphorylating c-Jun to drive proapoptotic (Bax) and catabolic (MMP-13) gene transcription [115, 116].
2.1.4.4 PI3K-Akt Signaling
The PI3K-Akt pathway is a key player in a range of diseases, including cancer [139], and neurodegenerative disorders [140]. Research has shown that activation of the PI3K/Akt pathway reduces apoptosis and mitochondrial damage in NP-derived mesenchymal stem cells [141]. Additionally, using PTEN inhibitors like VO-OHpic can prevent NP degeneration by suppressing oxidative stress and promoting cell proliferation [142]. Moreover, activation of PI3K/Akt signaling through ROS alleviates mitochondrial dysfunction and apoptosis in NP cells, indicating its possible therapeutic role in IDD [143]. In OA, redox imbalance inactivates PTEN, hyperactivating Akt to suppress FOXO3a-mediated autophagy and promote senescence [117, 118].
2.1.4.5 STING Signaling
The STING pathway is an important way for the body to recognize and respond to endogenous DNA damage or viral infection. It plays an immunomodulatory role by activating downstream interferons and inflammatory responses. For example, the Nrf2/STING/NF-κB pathway has been shown to be associated with the vicious cycle between M1 macrophages and senescent NP cells [144]. In addition, the cGAS/Sting/NLRP3 pathway is related to NP cell apoptosis and inflammation [145]. Chen et al. found that 2'3'-cGAMP could activate the STING pathway, resulting in significantly lower autophagic degradation in senescent NP cells and IVD than normal cells, suggesting STING as a potential way to treat IDD [146]. Cytosolic mtDNA released during oxidative stress activates STING-IRF3, inducing type I interferons and pro-inflammatory cytokines [147].
2.2 Differences Between IDD and OA
IDD and OA exhibit distinct anatomical and microenvironmental pathologies. IDD involves degeneration of the vertebral endplate and AF. Endplate calcification disrupts nutrient diffusion to the avascular nucleus pulposus, while annulus fissures permit herniation. The disc's hypoxic niche (regulated by HIF-1α) contrasts with OA's synovial fluid-nourished cartilage [27]. In contrast, OA primarily affects articular cartilage, subchondral bone, and synovium [27]. Synovial inflammation is unique to OA, characterized by macrophage-driven hyperplasia, fibrosis, and vascular invasion, which accelerates cartilage erosion [27]. These anatomical differences also make the pathogenesis of IDD and OA different.
3 Catalytic Mechanisms of Nanozymes for IDD and OA
3.1 Catalytic Nanozymes Classified by Enzymatic Activities
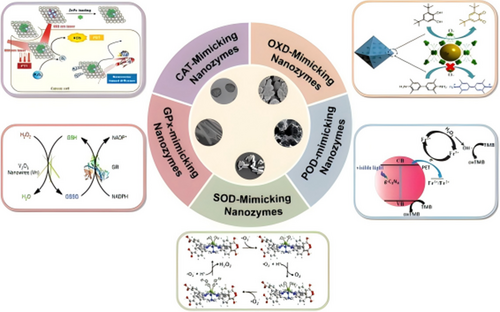
Nanozymes can generally be categorized into several major subclasses based on their enzymatic activities: POD, oxidase (OXD), SOD, catalase (CAT), Glutathione Peroxidase (GPx) and others (Figure 4 and Table 1).
| Enzyme activity | Function | Example | Reference |
|---|---|---|---|
| POD |
|
|
[149] |
| OXD |
|
|
[153] |
| SOD |
|
|
[143] |
| CAT |
|
|
[145] |
| GPx |
|
|
[162] |
3.1.1 POD-Like Activity
In 2007, Gao et al. reported that iron oxide nanoparticles exhibit inherent POD-mimicking activity, representing the first discovery of nanozymes exhibiting POD-like properties [148]. The principal chemical reactions of peroxidases typically involve the reduction of H2O2 and the oxidation of electron donor substrates, playing a critical role in breaking down H2O2 into nontoxic by-products like water [149]. Recently, the development of a protein/MOF hybrid nanozyme, Cyt c@PCN-222 NP, through nanoconfinement of cytochrome c within PCN-222 nanoparticles, significantly enhances POD-mimicking activity and H2O2 detection sensitivity, offering a potential strategy for designing highly active nanozymes [150]. In addition, Wu et al. prepared a microneedle patch incorporating dual nanozymes confined in porous silicon, which gives the biamonase to work with a type of POD and glutathione oxidase (GSHOx) activity [151]. In summary, the catalytic evolution of POD-mimicking nanozymes has progressed from the seminal discovery of iron oxide nanoparticles to advanced nanoarchitectures for precision biosensing and biomedical applications.
3.1.2 OXD-Like Nanozymes
Oxidases are enzymes that catalyze redox reactions using O2 as the electron acceptor to produce either H2O or H2O2 as by-products, including cytochrome c oxidase, glucose oxidase, monoamine oxidase, cytochrome P450 oxidases, and NADPH oxidase [152]. Zhang et al. designed the dual-site enhanced porphyrin-based metal-organic framework nanozymes and nano-/bioenzyme confined catalysis, which provides conceptual guidance for designing a nanozyme/biological enzyme limited catalytic system [153]. However, the development of highly specific nanozymes remains a challenge [154].
3.1.3 SOD-Like Nanozymes
Superoxide radicals, as products of oxygen metabolism, need to be constantly broken down into harmless oxygen forms to prevent cellular damage. SOD enzymes are highly efficient antioxidants that catalyze the conversion of superoxide radicals into oxygen and hydrogen peroxide via a ping-pong mechanism [155-157].
3.1.4 CAT-Like Nanozymes
Catalase enzymes catalyze the disproportionation of H₂O₂ into H₂O and O₂, which are crutial for protecting cells from oxidative damage caused by ROS [158]. Catalase enzymes catalyze the disproportionation of H₂O₂ into H₂O and O₂, playing an essential role in protecting cells from oxidative damage caused by ROS [159, 160].
3.1.5 GPx-Like Nanozymes
The GPX family consists of eight members (GPX1–GPX8), with distinct structural features. GPX1-4 and GPX6 use selenocysteine at their active sites to catalyze the reduction of H₂O₂ and organic peroxides. GPX5 and GPX7-GPX8 contain cysteine residues instead of selenocysteine, with GPX7 and GPX8 localized in the endoplasmic reticulum [161]. Around 2005, there was a gradual increase in the number of studies on nanomaterials mimicking GPx functions. V2O5 nanowires (Vn) mimic the antioxidant enzyme GPx, showing strong enzyme-like activity by scavenging ROS in various mammalian cells [162].
3.2 Catalytic Nanozymes Classified by Composition
3.2.1 Nanozymes Derived From Metal Nanoparticles
Metals can mimic the role of natural enzyme cofactors, found in the catalytic core of nanozymes, and are important in electron transfer while providing the structural foundation for nanomaterials [163]. Metal nanoparticles, including Au, platinum (Pt), silver (Ag), zirconium (Zr), and palladium (Pd), are widely used in nanozymes due to their ease of synthesis and flexibility for modification [164, 165].
3.2.2 Nanozymes Derived From Metal Oxide
Nanozymes derived from metal oxide exhibit peroxidase, catalase, or oxidase-like activities and include materials such as manganese dioxide, copper oxide, iron oxide, vanadium pentoxide, and nanoceria. These materials offer several advantages, including solubility, biodegradability, and tunable mechanical properties [166].
3.2.3 Nanozymes Derived From Carbon Nanostructures
Carbon-based nanozymes are derived from carbon nanoparticles such as graphene, carbon dots, carbon nanotubes, carbon nanospheres, and fullerenes, and exhibit POD-mimicking and SOD-mimicking activities. They represent one of the most effective categories of enzyme-mimicking nanozymes [167]. Nanozymes derived from carbon are derived from carbon nanoparticles such as graphene, carbon dots(CDs), carbon nanotubes, carbon nanospheres, and fullerenes, and exhibit POD-like and SOD-like activities. They represent one of the most efficient categories of enzyme-mimicking nanozymes [168].
3.3 NDD
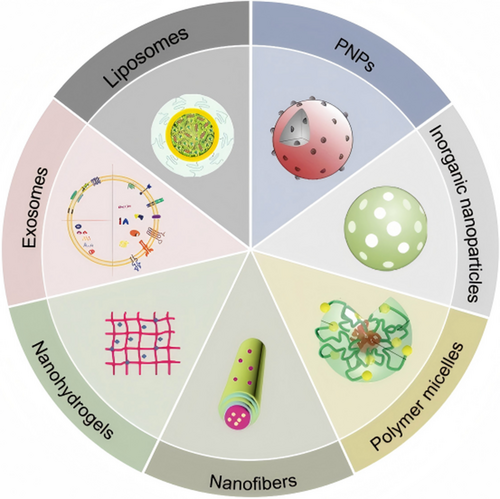
NDDs are a type of technology that uses nanoscale materials (typically ranging in size from 1 to 100 nanometers) as carriers to precisely deliver drugs, genes, or other therapeutic molecules to target locations (Figure 5 and Table 2).
| NDD Type | Size | Composition | Key features | Applications | Reference |
|---|---|---|---|---|---|
| Liposomes | 50 nm–5 μm | Lipid bilayer structure with hydrophilic and hydrophobic layers |
|
Cardiovascular diseases, cancers, neurodegenerative diseases therapy | [179, 180, 182-184, 197] |
| PNPs | 1–1000 nm | Natural or synthetic polymers such as PLA, PLGA, PEG, chitosan, collagen |
|
Cancer therapy, neurodegenerative diseases, diabetes, tissue engineering, and cell-targeted delivery. | [170] |
| Inorganic Nanoparticles | 1–100 nm | Metal nanoparticles, carbon-based nanoparticles, silica, calcium, ceramic nanoparticles, quantum dots |
|
Imaging, cancer therapy and diagnostic applications. | [171] |
| Polymer Micelles | Varied | Amphiphilic block copolymers forming core-shell structures |
|
Cancer therapy, gene therapy, and targeted unsoluable drug delivery. | [172] |
| Nanofibers | Varied | Synthetic or natural biodegradable polymers like PLA, PCL |
|
Tissue engineering scaffolds | [174, 194] |
| Nanohydrogels | 5–100 nm | Three-dimensional polymer networks formed by chemical or physical crosslinking |
|
Targeted therapy and tissue-specific applications. | [175, 195] |
| Exosomes | 30–150 nm | Natural extracellular vesicles secreted by cells |
|
Cancer, cardiovascular diseases and neurodegenerative diseases therapy, and immune modulation. | [173, 196] |
3.3.1 Liposomes
Liposomes are lipid-based spherical vesicular systems, with hydrophobic bilayers, sandwiched between two hydrophilic layers. As carriers for small molecules, peptides, genes, and monoclonal antibodies in drug delivery systems, liposomes were among the first nanoparticles translated to clinical applications [176-180]. The primary advantages of liposomes lie in their high biocompatibility, flexibility in tuning physiochemical properties such as reactivity, strength, surface area, and stability, and their well-established preparation methods that allow customization based on specific applications [169, 181]. Liposomal formulations employed to treat various diseases, such as cardiovascular diseases, neurodegenerative disorders, diabetes, and cancers. Su et al. developed an injectable DHA-loaded liposomal formulation designed as a plaque-targeting nanodrug for intravenous administration to prevent cardiovascular events related to atherosclerosis [182]. ROS-sensitive bio-liposomes containing geniposide and rhein demonstrated synergistic dual-cell therapy for atherosclerosis regression [183]. Moreover, brain-targeting liposomes containing monoclonal antibodies reduced α-synuclein aggregation and improved behavioral symptoms in Parkinson's disease models [184]. Liposomal hybrid nanovesicles have also been explored as dual-gene delivery systems for Alzheimer's disease gene therapy [185]. For wound healing in diabetic conditions, ROS-clearing liposomal nanoparticles carrying mRNA formulations accelerated the healing process [186]. Liposome-based combination therapies have been designed to deliver immunogens to tumor cells, thereby activating immunogenic cell death [187]. Chen et al. reported that chemically programmed STING-activating liposomal nanovesicles enhanced anticancer immunity [188].
3.3.2 Polymeric Nanoparticles (PNPs)
PNPs are typically homogeneous spherical structures at the nanoscale, encased by a distinctive polymer membrane made from either natural or synthetic polymers [189]. These nanoparticles can be produced using a range of polymers, such as poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), polycaprolactone, and polyethylene glycol (PEG), as well as from natural polymers like alginate, chitosan, fibrin, gelatin, collagen, and albumin [190, 191]. PNPs are often utilized in conjunction with ECM or cell-targeting strategies for enhanced therapeutic delivery [192], ensuring drug delivery to its site of action to maximize the pharmacological desired effects of the drug [170].
3.3.3 Inorganic Nanoparticles
Inorganic nanoparticles come in various forms and sizes, ranging from 1 to 100 nm, from inorganic materials such as metal nanoparticles, nanoparticles derived from carbon, silica nanoparticles, calcium nanoparticles, ceramic nanoparticles, and quantum dots [171]. These nanoparticles can be modified through various interactions, such as electrostatic, hydrophobic, and enzyme-sensitive covalent bonds, to achieve responsive release and enhanced therapeutic efficacy.
3.3.4 Polymer Micelles
Polymer micelles are created by the self-assembly of amphiphilic block copolymers in water, leading to the formation of a core-shell structure, where the hydrophobic core encapsulates insoluble drugs and the hydrophilic shell faces the aqueous medium. First proposed in 1992 by Yokoyama et al. [193], polymer micelles have since gained significant attention as drug delivery carriers, especially for insoluble drugs, serving as “nano-reactors” with unique properties for improving the bioavailability and targeting of therapeutics [172].
3.3.5 Nanofibers
Nanofibers are an important class of biomaterials characterized by high surface-to-volume ratios, high porosity, and low density. Most nanofibers are composed of biodegradable synthetic and natural polymers. In the biomedical field, nanofibers are primarily employed as scaffolds in tissue engineering. Recently, their application has emerged as an important new development, leveraging their ECM-like structural properties to improve interactions between cells and drugs [174, 194].
3.3.6 Nanohydrogels
Nanohydrogels [140-142], three-dimensional polymer structures formed through chemical or physical crosslinking of hydrogels and nanomaterials [175]. These nanohydrogels possess distinct characteristics derived from both hydrogels and nanoparticles, allowing them to encapsulate drugs within their 3D network structures and protect them from external environmental degradation. Furthermore, their smaller volume, high permeability, long plasma half-life, and enhanced surface modification capabilities have made them a focus of extensive research for in vivo delivery, especially in IVD applications [195].
3.3.7 Exosomes
Exosomes are nanoscale extracellular vesicles (EVs) secreted by different cell types in both healthy and disease states [173]. As natural nanocarriers, exosomes can be engineered to enhance their drug delivery properties for many diseases, including cancer, cardiovascular diseases, and neurodegenerative diseases. Their ability to naturally cross biological barriers and target specific cells makes them promising candidates for next-generation nanomedicine [196].
4 Nanozymes in Multimodal Therapy for IDD and OA
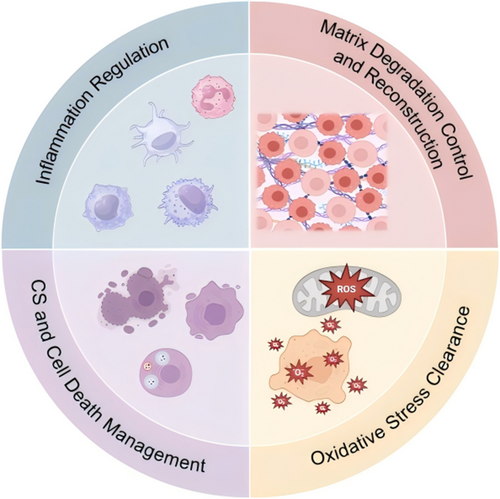
Repair and regeneration of orthopedic degenerative diseases involve various strategies, primarily including the use of nanocatalysts and NDDS that carry therapeutic agents. These strategies target CS and death by modulating inflammatory pathways, oxidative stress, and ECM alterations, ultimately aiming to treat orthopedic degenerative diseases (Figure 6 and Table 3).
| Formulation | Therapeutic agents | Mechanisms | Dosage | Outcome | Year | Reference |
|---|---|---|---|---|---|---|
| Nanoparticles | Peptide receptor-1 antagonist conjugated fullerene | Inflammation/NLRP3 regulation | 10 nmol per 20 g mice | ∙ Attenuated the secretion of pro-inflammatory cytokine | 2017 | [198] |
| Fullerol | 10 µM 100 µM |
∙ Counteracted pain sensitization and the inflammatory cascade | 2017 | [199] | ||
| LN and fullerol combination | Fullerol and LN | Inflammatory factors inhibition | Fullerol: 1 μM. LN: 10 ng/mL |
∙ Promoted matrix production ∙ Anti-inflammatory and anti-catabolic effects on AF cells. |
2018 | [200] |
| Microspheres | h-MnO2 | NA | ∙ Regulation of cytokine expression ∙ Slow drug release capability |
2022 | [202] | |
| Exosomes | AF cells | 100 μg/mL | ∙ Promoted HUVECs migration and inflammatory factor expression | 2021 | [203] | |
| TMNP@SR | Rapamycin | Inflammation/GFAP c-FOS signaling |
5 µL (80 µg/mL) | ∙ Mitigated the pro-inflammatory microenvironment | 2024 | [205] |
| PDA@CNO | Ni3+/Ni2+and Co3+/Co2+ redox couples | Direct ROS scavenging | 0.5 μg 5 μg |
∙ Reduced intracellular ROS ∙ Protected NP cells ∙ Slowed IDD progression |
2024 | [239] |
| MHSGN | siGrem1 | NA | ∙ Facilitated ECM production in the NP | 2024 | [229] | |
| PBNPs | SOD1 POD CAT |
Redox-related signaling pathways | 2 μg 0.2 μg |
∙ Increased SOD1 activity ∙ Improved mitochondrial function |
2022 | [242] |
| H-MnO2 nanoparticles | TGF-β3 | 20 µL | ∙ Prevented degeneration ∙ Promoted self-regeneration |
2022 | [202] | |
| MS@MCL | MnO2 nanozymes LOX enzyme |
Regulation of oxidative stress via immune and inflammatory pathway | 0.4 mg/kg MnO2 | ∙ Reduced local lactate accumulation ∙ Decreased inflammatory responses ∙ Promoted extracellular matrix metabolism and cell survival |
2022 | [244] |
| Injectable ROS-scavenging hydrogel | Rapamycin | NA | ∙ Reduced ROS levels ∙ Promoted M2 macrophage polarization |
2019 | [245] | |
| Polyplex Nanomicelles | Luc2 mRNA and Runx1 mRNA-loaded Polyplex Nanomicelles | 6 µL | ∙ Improved disc height and ECM remaining | 2022 | [212, 213] | |
| Hydrogel Scaffolds | Self-Assembling Peptides RADA16-I Functionalized with BMP7 Motifs | NA | ∙ Enhanced hdNPCs' proliferation, migration, and ECM secretion | 2015 | [214] | |
| PLGA nanoparticles | TGF-β3 MSCs |
1000 μg/mL | ∙ Induce MSCs to NP-like cells ∙ Promote ECM-related biosynthesis |
2016 | [216] | |
PEG hydrogel synthesized via Ag-S coordination Injectable and self-healing hydrogel system |
Agomir874 | NA | ∙ Restoration of IVD to normal height ∙ Improved MRI water content scores ∙ Histological sections indicated improved collagen levels and type II collagen expression |
2019 | [218] | |
Injectable HAMA microspheres DOTAP/Chol/DOPE cationic liposomes |
circSTC2 silencing genes-loaded lipoplexes | NA | ∙ Promotion of ECM synthesis and restoration of NP tissue after 8 weeks ∙ Maintenance of ECM metabolism balance |
2022 | [219] | |
| USC exosomes | MATN3 protein | 2 μL per injection | ∙ Reduced senescence and increased proliferation of NPCs ∙ Enhanced ECM synthesis, including COL2 and ACAN ∙ Alleviation of IVD degeneration in rats(CT, MRI, and histological analyses) |
2021 | [224] | |
IGK@SeNP Based on diselenide block copolymers |
IGK | Cellular senescence management | 2 μL per injection | ∙ Scavenged ROS and enhanced autophagy in NPCs ∙ Protected NPCs from apoptosis and ECM degradation |
2023 | [226] |
| Liposomal siRNA delivery system | siRNA against Caspase 3 and ADAMTS5 genes | Autophagy regulation | 0.2 μmol/mL | ∙ Downregulation of target genes | 2018 | [228] |
| OMT-LIP | OMT | Apoptosis regulation | 100 μg/μL | ∙ Increased drug accumulation in the intervertebral disc ∙ Reduced NP cell apoptosis ∙ Decreased expression of MMP3/9 and IL-6, and decreased degradation of type II collagen |
2020 | [197] |
| dECM@exo | ADSC-derived exosomes | 1 µg of exosomes per 100 µL | ∙ Maintained early IVD microenvironment homeostasis ∙ Ameliorated IVD |
2021 | [232] | |
| MCDs | SOD CAT POD |
Pyroptosis regulation | 2 μg 4 μg |
∙ Restored needle-triggered IVD degeneration ∙ Suppressed pyroptosis and maintained ECM homeostasis |
2024 | [233] |
| BMSC-EVs | Circ_0072464 | 200 μg | ∙ Upregulation of circ_0072464 and NRF2 ∙ Downregulation of miR-431 in treated mice ∙ Inhibition of NPC ferroptosis ∙ Promotion of matrix synthesis and proliferation of NPCs |
2022 | [235] | |
| PDA NPs | PDA NPs for reactive ROS depletion, iron ions chelation, and GPX4 ubiquitination suppression | Ferroptosis | 0.25 mL−1 concentrations 1 μg mL−1 concentrations |
∙ Mitigated IVD in vivo ∙ Reduced ferroptosis |
2023 | [236] |
| FDA-TA combining FDA with TA | FDA-TA hydrogel for targeted adsorption of ex-iron | 20 μL | ∙ Reduced ferroptosis in NPCs ∙ Inhibited NCOA4-mediated ferritinophagy ∙ Activation of the PI3K-AKT pathway |
2024 | [236] |
4.1 Anti-Inflammatory Therapy
Nanoparticles modulate multiple inflammatory mechanisms to alleviate inflammation and pain associated with IDD and OA. Among them, fullerenes is indispensable. Xiao et al. discovered that fullerene nanoparticles, conjugated as a novel formyl peptide receptor-1 antagonist, specifically bind to this receptor on activated macrophages, offering a potential targeted therapy for degenerative disc diseases [198]. Similarly, Jin et al. reported that fullerene-based nanoparticles could alleviate inflammation induced by IDD via NLRP3 inflammasome modulation and neuropeptide release, providing relief from radiculopathy [199]. Yeh et al. also observed that the simultaneous application of fibronectin N-terminal peptide (LN) and fullerene effectively inhibited the IL-1α-induced elevation of pro-inflammatory mediators (such as IL-6 and COX-2) and matrix metalloproteinases (including MMP-1, −2, −9, and −13) [200]. Besides, MnO2 not only gradually decomposes into Mn2+, downregulating inflammatory cytokine production at the gene level to reduce inflammation [201], but also synergizes with proteins to degrade pro-inflammatory factors [202]. When decellularized AF matrix (DAFM)/poly(ether-carbonate-urethane) urea (PECUU) composite electrospun scaffolds were transplanted into rabbits, inflammation surrounding DAFM/PECUU scaffolds was significantly lower compared to that surrounding PECUU scaffolds [203]. AF-derived exosomes (AF-exo) promoted angiogenesis in human umbilical vein endothelial cells (HUVECs) by enhancing cell migration and expression of inflammatory factors such as IL-6, TNF-α, MMP-3, MMP-13, and VEGF [204]. Li et al. recently developed structured biomimetic nanoparticles encapsulating TrkA-overexpressing macrophage membranes (TMNP@SR) to reduce mechanical allodynia and heat hypersensitivity in a rat IDD model by downregulating CGRP and substance P expressions and modulating GFAP and c-FOS signaling [205].
Liposomal drug delivery platforms demonstrate superior therapeutic advantages through sustained pharmacological action and reduced off-target toxicity. As a representative example, rapamycin encapsulated in liposomal nanocarriers exhibits dose-dependent suppression of pro-inflammatory cytokines (notably IL-6), while substantially mitigating systemic toxicity associated with free drug administration, thereby enhancing its therapeutic index in osteoarthritis management [206]. Notably, synovium-targeted liposomes loaded with clodronate achieve localized depletion of pro-inflammatory macrophages through selective phagocytosis inhibition, showing remarkable efficacy in ameliorating obesity-associated osteoarthritis progression in diet-induced obese murine models. Furthermore, the acid-activated curcumin polymer (ACP) nanocarrier system demonstrates pH-responsive release profiles that enable site-specific accumulation in inflamed synovium, effectively downregulating TNF-α and interleukin IL-1β expression levels. This targeted intervention significantly preserved cartilage integrity and subchondral bone architecture in experimental arthritis models [207].
4.2 ECM Repair
Currently, nanomaterials primarily improve IDD through two main ECM mechanisms. On one hand, the degradation of the ECM is reduced by modulating factors. Teixeira et al. used Chitosan/Poly-γ-glutamic acid (Ch/c-PGA) nanoparticles to reduce ECM degradation by downregulating MMP-1 and MMP-3 and upregulating collagen II and proteoglycan production [208]. On the other hand, IDD can be alleviated by ECM regeneration through several pathways. To enhance NP cell ECM production in vitro, Liang et al. constructed a nanostructured 3D poly(lactic-co-glycolic acid) (PLGA) scaffold. This scaffold, loaded with dexamethasone (DEX) and growth factor-encapsulated heparin/poly(l-lysine) nanoparticles, effectively promoted rat mesenchymal stem cell (rMSC) growth and differentiation into NP-like cells [209]. Subsequently, for the restoration of proteoglycan accumulation in IDD, they implanted ADSC-seeded PLGA microspheres into IVDs in rats, resulting in ~63% and ~76% restoration of disc height after 24 weeks for PLGA microsphere (PM) and ADSC-treated groups, respectively, with corresponding MRI signal intensity recovery [210]. In 2014, Jin et al. found that nanoparticles based on fullerene could enhance the levels of water and proteoglycans in IDD, inhibiting ectopic bone formation and effectively preventing IDD [211]. Nanomicelles delivering Runx1 mRNA increased IVD hydration and ECM production, effectively preventing fibrosis at degenerating disc sites [212, 213]. A scaffold with nanofibrous structure can not only stimulate the proliferation, migration, and collagen and SOX-99 secretion in vitro, but also biocompatibility in the body [214]. Feng et al. transfected IDD cells with Nuclear Receptor 4A1(NR4A1) plasmid DNA to form nanofibrous spongy microspheres (NF-SMS), reversing fibrotic degeneration and supporting disc regeneration [215]. TGF-β3-loaded PLGA nanoparticles, evenly incorporated into hydrogels, induced MSC differentiation into NP-like cells and promoted ECM biosynthesis [216]. Injectable nanostructured colloidal gels, similar to natural NP, serve as carriers for mesenchymal stem cells (MSCs), promoting their differentiation into NP cells [217]. Chen et al. found that gene-hydrogel microenvironments regulated ECM metabolism, using PEG nanohydrogels with excellent agomir loading to locally deliver agomir-874 to IVDs, regulating ECM catabolism and improving the NP tissue microenvironment [218]. A nanohydrogel microsphere loaded with circRNA (circSTC2)-silencing genes was used to silence pro-IDD genes in NP cells, regulating ECM metabolic balance [219].
Current advancements in OA-targeted nanotherapeutics emphasize stimulus-responsive drug release and cartilage-specific biodistribution. For instance, a matrix MMP-13-responsive nanomicelle system enables pH-dependent sustained drug release within the acidic microenvironment of osteoarthritic joints, thereby optimizing therapeutic bioavailability [220]. Furthermore, self-assembled core–shell PEGylated kartogenin (PEG/KGN) micelles, engineered through covalent crosslinking between hydrophilic poly(ethylene glycol) and hydrophobic kartogenin components, demonstrate exceptional biocompatibility alongside prolonged drug release kinetics. This platform significantly attenuates OA progression in rodent models via enhanced cartilage-targeting efficiency, as evidenced by histological and biomechanical assessments [221]. Notably, a cartilage-penetrating nanovehicle achieved depth-dependent payload delivery with 60% reduction in cartilage lesion width and 80% suppression of osteophyte formation at 4-week postimplantation, validated through contrast-enhanced micro-MRI and synchrotron radiation phase-contrast imaging, highlighting its potential for intra-articular precision therapy [222].
4.3 CS and Cell Death
4.3.1 CS
Lin et al. were the first to demonstrate that targeted delivery of senolytic drugs can effectively treat IDD caused by CS, providing a therapeutic avenue for other types of CS-associated degenerative diseases [223]. Moreover, urine-derived stem cell (USC) exosomes, MATN3, have shown antiaging effects in improving disc degeneration [224]. These findings highlight the potential of targeting CS as a viable therapeutic approach.
4.3.2 Autophagy
Two distinct strategies have been employed to modulate autophagy pathways for improving intervertebral disc degeneration: one involves inhibiting autophagy to slow the progression of degeneration, while the other activates autophagy to promote cellular repair and delay degeneration. Zheng et al. [225] identified a thermo-sensitive, ROS-responsive hydrogel encapsulating MR409, which alleviated rat IDD by inhibiting secretory autophagy pathways. An isoginkgetin-loaded ROS-responsive delivery system based on diselenide block copolymers (IGK@SeNP) was proven to promote autophagy in NPCs, preventing ECM breakdown and cell apoptosis, leading to notable therapeutic outcomes [226].
In the context of OA therapeutics, a carrier-free nanoplatform co-delivering dual therapeutic agents (bilirubin and rapamycin) has been developed through surface modification with type II collagen-targeting peptide (WYRGRL motif). This self-assembled PP/BRRP nanoparticle system synergistically amplifies anti-inflammatory and chondroprotective effects by mechanistically coupling autophagy activation with mTOR signaling pathway inhibition, thereby achieving coordinated suppression of synovial inflammation and apoptosis in preclinical OA models [227].
4.3.3 Apoptosis
Banala et al. developed a lipid nanoparticle-based small interfering RNA (siRNA) targeting the caspase 3 gene, which plays a pivotal role in apoptosis, and injected it into an IDD rabbit model. The results indicated that lipid nanoparticle-siRNA significantly downregulated the expression levels of proapoptotic markers, offering promising potential for noninvasive treatment of IDD [228]. Additionally, Oxymatrine liposomes (OMT-LIP) were shown to reduce apoptosis in NP cells [197]. Recent studies have also demonstrated that an intelligent microgel-based gene delivery system can effectively silence Grem1, thereby alleviating cellular apoptosis [229]. Furthermore, TMNP@SR promotes the transition of macrophages from the M1 to M2 phenotype, mitigating the chronic activation of inflammation in degenerated disks and subsequently preventing NP cell apoptosis [205]. These studies have shown that regulating apoptosis-related genes, cytokines and inflammatory response can effectively reduce the occurrence of apoptosis in the process of IDD.
Emerging nanotherapeutic strategies for OA management demonstrate targeted cellular modulation capabilities. Quercetin-loaded nanoparticles (Q-NPs) selectively clear senescent chondrocytes through apoptosis induction, thereby interrupting the SASP that drives OA progression [230]. Concurrently, an injectable hydrogel platform engineered with catalase-mimicking nanozyme activity achieves dual therapeutic effects: it attenuates chondrocyte apoptosis via ROS scavenging while reprogramming synovial macrophages toward anti-inflammatory M2 polarization, synergistically ameliorating OA pathogenesis [231].
4.3.4 Pyroptosis
It is possible to effectively mitigate cell damage induced by pyroptosis by regulating pyroptosis-related pathways, particularly through the inhibition of NLRP3 inflammasome activation, ROS production, and the expression of associated proteins. Xing et al. employed a thermosensitive decellularized ECM hydrogel conjugated with adipose-derived mesenchymal stem cell (ADSC)-derived exosomes (dECM@exo), which continuously released matrix MMPs to regulate ECM degradation. This approach was shown to inhibit nucleated cell pyroptosis by downregulating NLRP3, cleaved caspase-1, NT-GSDMD, and IL-1β protein levels [232]. In addition, Shi et al. developed MCDs as biocatalysts to scavenge ROS, effectively suppressing pyroptosis in NP cells and alleviating IDD [233]. As for OA treatment, the injection of FP-GNP-DPPC into intra-articular joints in rats can effectively inhibit apoptosis [234].
4.3.5 Ferroptosis
The activation of ferroptosis is closely related to the excessive iron accumulation. Therefore, intervening in iron metabolism and inhibiting ferroptosis can effectively slow down or reverse intervertebral disc degeneration. Yu et al. proposed that extracellular vesicle- (EV-) extracted from mouse BMSCs (BMSC-EVs) could suppress ferroptosis in NP cells, thereby alleviating disc degeneration [235]. Yang et al. used polydopamine nanoparticles (PDA NPs) to scavengers ROS and chelate Fe2+ to mitigate ferroptosis of NP cells in vitro [236]. Furthermore, in an analysis of IDD tissue, Chen et al. observed a positive correlation between the extracellular concentration of ex-iron and the severity of ferroptosis. Inspired by the magnetic attraction of metals, they combined polyether F127 diacrylate (FDA) with tannic acid (TA) to construct a magnetic-responsive hydrogel (FDA-TA). This hydrogel exhibited a remarkable ability to adsorb iron and remodel cellular iron metabolism, exhibiting excellent durability and self-repair capabilities. Notably, it activated the PI3K-AKT pathway, inhibiting ferritinophagy mediated by nuclear receptor coactivator 4 (NCOA4) under iron-rich conditions [237]. In addition, the platinum-loaded nanozyme-incorporated silk fibroin/amylopectin hydrogel alleviates OA via ferroptosis [238].
4.4 Antioxidant Therapy
Various strategies targeting oxidative stress pathways have shown significant potential in IDD's treatment, including direct ROS scavenging, modulation of redox signaling pathways, immune system regulation, and macrophage polarization. As for direct ROS scavenging and antioxidant enzyme activity enhancement, Wang et al. built a core-shell nanostructure enzyme system with NiO nanoparticles (CNO) as the core, encapsulated in a PDA shell, named PDA@CNO. This system effectively converts intracellular ROS in NP cells to H2O and O2, protecting NP cells from stagnated proliferation and metabolic dysfunction [239]. Likewise, Guo et al. observed that a microgel system (MHSGN) with phenolic hydroxyl groups could clear ROS [229]. Furthermore, glutathione-doped carbon dot nanozymes (GSH-CDs) were shown to rescue NP cells from the oxidative stress microenvironment, alleviating disc degeneration [240]. Besides, N-acetylcysteine-derived carbon dots (NAC-CDs) exhibited excellent biocompatibility and strong SOD, POD, CAT activity, as well as total antioxidant capacity, effectively rescuing IDD from ROS-induced NPC aging [241]. As for regulation of redox-related signaling pathways, Zhou et al. discovered that Prussian blue nanoparticles (PBNP) promoted development under magnetic resonance T1, increased the activity of antioxidant enzymes such as SOD1, ultimately improving mitochondrial structure and boosting antioxidant capabilities [242]. Under mildly acidic conditions, MnO₂ breaks down into manganese ions, serving as an intermediary for drug-enhanced disc degeneration alleviation. Proteins enclosed within hollow MnO₂ (h-MnO₂) microspheres were administered to enable controlled drug delivery, thereby alleviating oxidative stress in vivo [202]. As for regulation of oxidative stress via immune and inflammatory pathways, the ROS-reactive MR409-loaded hydrogels applied locally to the IVD suppressed autophagic pathways and related IL-1β secretion, resulting in reduced IDD in a rat model [225]. What's more, studies have shown that after TGF-β3 was loaded onto h-MnO2 nanoparticles, the expression of iNOS was significantly reduced during an 8-week posttreatment period, a phenomenon not observed in the TGF-β3 and MnO2 groups [243]. Shen et al. grafted MnO2-LOX composite nanocatalysts onto microfluidic hydroxyethyl methacrylate (HAMA) microspheres, creating injectable microspheres (MS@MCL) for localized lactate depletion, which effectively alleviated oxidative stress damage [244]. As for promotion of macrophage polarization and Inflammatory Mediators, Bai et al. designed ROS-scavenging nanohydrogel scaffolds for controlled rapamycin release, which reduced ROS levels and promoted M2 macrophage polarization, thereby slowing down the advancement of IDD [245].
Nanomaterials combat oxidative stress in osteoarthritic joints through three principal ROS-scavenging mechanisms: enzyme-mimetic catalysis, electron transfer, and proton donation. For instance, hollow Prussian blue nanozymes (HPBzymes) demonstrate dual catalase (CAT)-like and superoxide dismutase (SOD)-mimetic activities to neutralize hydrogen peroxide and superoxide anions, effectively reducing ROS burden in degenerated cartilage [246]. Meanwhile, silk fibroin nanoparticles co-encapsulating celecoxib and curcumin achieve ROS elimination via electron transfer-mediated redox cycling, synergistically protecting chondrocytes from oxidative damage [247]. Furthermore, p47phox siRNA-loaded PLGA nanoparticles (siRNA-p47phox/PLGA NPs) specifically target NADPH oxidase complexes, suppressing ROS overproduction at the transcriptional level. This strategy significantly attenuates chondrocyte apoptosis, as evidenced by in vitro oxidative stress models and in vivo experimental osteoarthritis studies [248].
5 Conclusions and Perspectives
5.1 Current Clinical Transformation of Nanomaterials
While nanomaterials show promise in treating generative osteoarticular diseases, their clinical translation for IDD and OA remains in early stages: Biomimetic peptide-based nanomaterials, designed to mimic ECM components, promote nucleus pulposus stem cell survival and mitigate disc degeneration in preclinical studies. Catalytic nanodots (MCD) combined with d-mannose have shown potential in restoring redox balance and slowing degeneration in rodent models [233]. However, human trials are yet to commence. As for OA, cartilage-targeted therapies, such as chondrocyte membrane-coated drug-loaded nanoparticles (CM-NPs), have demonstrated efficacy in animal models. These systems prolong intra-articular drug retention (≥ 34 days) and inhibit cartilage degradation [249], with clinical trials pending.
Additionally, an important factor limiting the clinical application of nanomaterials is the gap between experimental animal models and humans. In experiment models (Table 4), such as rodents, the absorption, distribution, metabolism, and elimination of nanomaterials can vary considerably due to species-specific factors. The metabolic processes also differ, with rodents often having faster clearance rates for nanomaterials, while human metabolism can lead to accumulation and longer retention in tissues, posing potential long-term toxicity. Appropriate animal models similar to humans including baboons, macaques and baboons cannot be widely used due to ethical issues [250].
| In vivo model tested | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Rats |
|
|
[236] |
| Rabbits |
|
|
[228] |
| Mice |
|
|
[198, 199, 214] |
| Canine |
|
|
[249] |
5.2 The Shortage of Nanomaterials
Nanomaterials exhibit significant limitations in biomedical applications, primarily due to unresolved biosafety concerns and technical complexities. Their small size (< 100 nm) and high surface reactivity enable unintended biological interactions, including cellular membrane penetration, DNA damage, and organ-specific toxicity from prolonged accumulation. Immunological risks arise from protein corona formation in blood, altering targeting efficiency and triggering inflammatory responses [251]. While size- and charge-dependent targeting strategies show promise, dynamic physiological environments often cause nanoparticle destabilization through pH/enzymatic degradation or shear forces, leading to premature drug release [252]. Manufacturing hurdles include stringent requirements for batch consistency during surface functionalization and high production costs [252].
5.3 Prospectives
In this review, we systematically review the principal mechanisms underlying current nanomaterial-based therapies for IDD and OA, with particular emphasis on four predominant pathological mechanisms: inflammation regulation, ECM remodeling, cellular senescence and cell death, and oxidative stress mitigation. Additionally, we discuss the different classifications of nanomaterials and the progress of research targeting these mechanisms for the treatment [231-234]. Furthermore, we evaluate the preclinical applications of these nanotherapeutic strategies. Through this comprehensive analysis, we aim to establish a scientific foundation for future clinical translation of nanomaterials while providing deeper insights into the pathophysiological mechanisms underlying osteochondral degenerative diseases, including IDD and OA.
The treatment of IDD and OA remains challenging due to the complex and multifactorial nature of its pathogenesis [11], including inflammation, ECM, CS and cell death and oxidative stress. While current therapeutic options primarily focus on symptom management [3], they often fail to address the underlying causes of degeneration. Nanomaterials exhibit enhanced drug targeting and controlled release properties, along with superior biocompatibility. Meanwhile, nanozymes demonstrate advantages including low-cost production, multifunctionality, reduced surface leakage of nanoparticles, and broad-spectrum antioxidant capabilities. The design of specific nanomaterials targeting particular biological sites enables precise therapeutic interventions, thereby facilitating the development of precision medicine and offerring a promising direction for advancing IDD and OA treatment. However, several critical aspects warrant further consideration in this field.
First, current paradigms in nanomaterial design remain constrained by oversimplified mechanistic approaches that deconstruct complex pathological processes into isolated therapeutic targets. However, IDD/OA pathogenesis inherently entails multilevel network interactions spanning mechanobiology, epigenetic regulation, and metabolomic adaptation. For example, conventional unidirectional targeting strategies, while effective in modulating specific macrophage phenotypes, risk perturbing the dynamic M1/M2 equilibrium essential for maintaining osteoarthritic joint homeostasis [253]. Thus, it's necessary to adopt the complex systems theory for next-generation therapeutic material development.
Second, treatment strategies for IDD and OA vary at different stages. For example, immunotherapy and other approaches are typically used to repair and regenerate disks in the early stages of degeneration (Pfirrmann grades I, II, III), but are insufficient for treating advanced stages (Pfirrmann grades IV–V) [254], where surgery remains the last choice. Therefore, precision-driven nanotherapeutic design should incorporate stage-adaptive strategies to address the pathophysiological evolution of osteochondral degenerative diseases across distinct clinical phases.
Third, the clinical translation of nanomaterials remains a critical challenge. Current animal models exhibit significant discrepancies from human physiology, while safety concerns persist due to environmental variations in nanomaterial behavior. Additionally, determining optimal administration routes and dosages for clinical trials requires systematic investigation in subsequent research. In a summary, we hope our research will provide a scientific foundation for advancing nanomaterial applications in treating egenerative osteoarticular diseases.
Author Contributions
Kaiqiang Sun and Jiangang Shi proposed the concept of the review and built the preliminary framework. Lei Peng, Chen Yan and Honghao Song refined the framework of the article and were responsible for writing it. Lei Peng also made figures and tables of the review. Kaiqiang Sun and Jiangang Shi corrected the reviews together. All the authors confirmed the final version. All authors have read and approved the final manuscript.
Acknowledgments
We sincerely thank Biorender and PPT software for their graphic support of this paper. Additionally, we also thank Deepseek for polishing the language of the article. This study was supported by the National Natural Science Foundation of China (Grant No. 82302760) and Shanghai Sailing Program (Grant No. 23YF1459100).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




