Simultaneous detection of dual targets Escherichia coli and Salmonella enteritidis using enzyme-free strand displacement reaction
Shixin Yan, Yuling Xiao, and Ruijuan Shen contributed equally to this study.
Abstract
Escherichia coli (E. coli) and Salmonella enteritidis (S. enteritidis) are common food-borne pathogens, which pose a very significant threat to the healthcare environment. The rapid detection of relevant bacteria can help control their rapid spread, while the traditional bacterial culture detection method is time-consuming and not conducive to the rapid detection of pathogens. Recently, new detection methods for related pathogenic bacteria have emerged, but these methods are relatively complex, and few methods can detect two bacteria at the same time. Therefore, there is an urgent need to develop multi-target, convenient, and fast pathogen detection methods. This method successfully constructed an enzyme-free fluorescent biosensor based on the adapter-mediated strand displacement reaction to detect E. coli ATCC25922 and S. enteritidis ATCC13076. This method had an ultrasensitive detection limit of 0.7 CFU/mL and 0.61 CFU/mL within 20 min, with a broad linear range of 34–105 CFU/mL of E. coli and 17–106 CFU/mL of S. enteritidis, respectively. Importantly, the spiked recovery of the three clinical fluid samples performed well, which proved that this method had the potential to detect E. coli and S. enteritidis in clinical samples. The sensor constructed by this method can detect dual targets at the same time, increasing the possibility of large-scale clinical use.
1 INTRODUCTION
Contamination of food with pathogens is a major problem, and foodborne disease outbreaks resulting from the consumption of contaminated food are a major public health issue worldwide. Such illnesses cause an estimated 1.9 million deaths globally each year.1 Bacteria are the most common cause of foodborne illness and exist in a variety of shapes, types, and characteristics.2 Of these, more than half of global bacteria-caused deaths are caused by a variety of important bacterial pathogens, including Escherichia coli and Salmonella enteritidis.3 Rapid and accurate detection of these pathogens is critical for effective prevention of foodborne pathogen outbreaks before they occur and for significantly reducing the number of hospitalizations and deaths due to foodborne illness.4
Several methods have emerged for the identification and detection of pathogenic bacteria, such as direct smear microscopy, nucleic acid hybridization, gene chips, polymerase chain reaction (PCR), gas chromatography, and high-performance liquid chromatography (HPLC).5 At present, the gold method for the detection of foodborne pathogens commonly used in the clinic is still the microbial cultivation method, but the events required for the operation of this method are usually longer, and it requires high technical requirements for the equipment and the operators, which are disadvantages that determine that it is not suitable for the point-of-care testing (POCT) of pathogenic bacteria.6, 7 Therefore, the development of a new rapid and accurate method for the detection of foodborne pathogens has become an urgent problem.
The nucleic acid isothermal signal amplification strategy is a powerful tool to improve the detection sensitivity of analytical methods with strong signal amplification efficiency; in particular, the process of isothermal nucleic acid signal amplification does not require expensive equipment and can be realized at appropriate temperatures compared to other signal amplification strategies.8 Toehold-mediated strand displacement (TMSD) reactions are versatile and stable and can be used to construct DNA nanomachines, complex DNA loops, and neural networks. TMSD has the advantages of a simple and convenient method, low cost, high detection accuracy, and is a reaction that does not require the involvement of enzymes. Therefore, they are widely used in biosensing. In recent years, aptamer-binding TMSD amplification strategies have received increasing attention and focus from researchers as an effective tool for bacterial analysis.9, 10 TMSD is triggered by complementary single-stranded structural domains, where branch migration during toe end binding causes nucleic acid strands with some complementarity to hybridize with each other, replacing one or more pre-hybridized nucleic acid strands.11 The amplification efficiency of TMSD depends strongly on the sequence and length of the complementary part. Theoretically, the chain substitution reaction efficiency of TMSD can reach 106-fold.12
In general, enzyme-assisted amplification has been shown to have higher detection sensitivity in DNA-mediated signal amplification than isothermal nucleic acid amplification techniques that do not use enzymes.8 However, enzyme-assisted amplification methods, such as rolling circle amplification,8 strand displacement amplification,13 and loop-mediated isothermal amplification,14 have the disadvantages of being costly, difficult to store, and having complex reaction conditions.15 Compared with other enzyme-free amplification methods, including hybridization chain reaction, DNA enzyme, and entropy-driven circuit, strand displacement reaction (SDR) has higher catalytic efficiency, lower background signal, and simpler and more stable reaction system.16, 17 As a simple and versatile isothermal nucleic acid amplification strategy, SDR has become a satisfactory alternative for biomarker determination.
Studies have shown that SDR can be adapted to a variety of analytical formats, such as fluorescence, electrochemistry, colorimetry, surface plasmon resonance, and electrophoresis.18 Although the entity of SDR is nucleic acid chain displacement, the detection target is not limited to nucleic acids.19 Many other analytes, including metal ions, proteins, enzymes, and even cancer cells and a variety of bacteria can be measured based on SDR.20
In recent years, several biosensors have been developed for the detection of foodborne pathogens.21 However, almost all of these studies only detected one target pathogen through a single sample injection. In addition, foodborne pathogens can only be detected at very high bacterial concentrations. Detecting foodborne pathogens at very low bacterial concentrations remains a challenge. This study used adapter and enzyme-free SDR amplification methods to simultaneously detect two important foodborne pathogens, namely E. coli and S. enteritidis, with ultra sensitivity and convenience. Under optimized conditions, the average fluorescence intensity of the target substance was strongly linearly correlated with its concentration. The linear range of the developed biosensor for E. coli and S. enteritidis was 34–105 CFU/mL and 17–106 CFU/mL, respectively. The proposed biosensor had an ultrasensitive limit of detection (LOD) of 0.7 CFU/mL and 0.61 CFU/mL for E. coli and S. enteritidis, respectively. Compared with other reported adapter-based biosensors for detecting E. coli and S. enteritidis, this value shows significantly higher detection sensitivity. According to the experimental results, this method has good specificity.
2 RESULTS
2.1 Methods of this study
The principle of a biosensor based on the chain substitution reaction for the detection of E. coli and S. enteritidis is shown in Scheme 1. In this biosensor, the protection probe C1/C2 and the probe that captures biomarkers are combined to design a complex in which the main part of the capture probe is an aptamer of E. coli/S. enteritidis for recognizing the bacteria of the dual target, as well as the remaining part to trigger and protect the binding of the probe. When a target is present in the system, a portion of the double-stranded P1T1/P2T2 will be turned on by the competing sequence C1/C2, and P1/P2 with the fluorescent moiety Cy5/FAM will bind to C1/C2. As the fluorescent/quenched structure is destroyed, the system will release some of the fluorescence. The compeleted off-free single chain T1/T2 will participate in the SDR reaction and bind to the exposed toe chain at the 3′ and 5′ ends of A1A2 in the system. The released A1/A2 will continue to compete with P1T1/P2T2, destroying the fluorescence quenching structure and releasing an enhanced fluorescence signal.
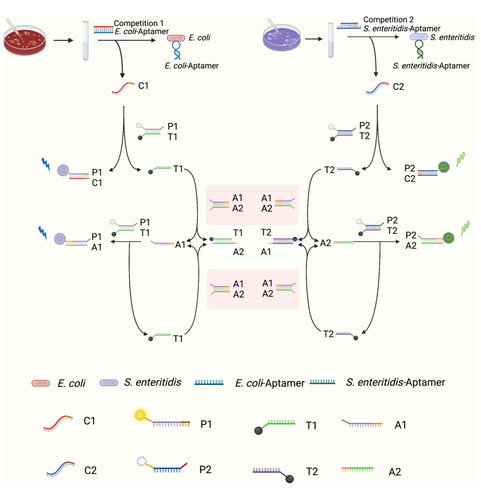
2.2 Feasibility analysis
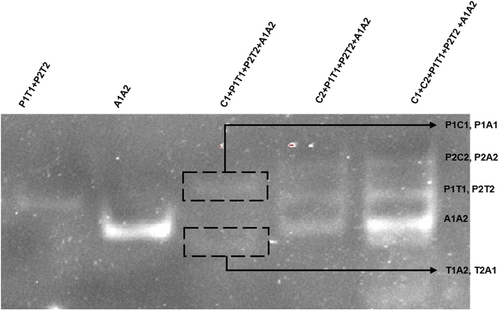
In the presence of E. coli ATCC25922 strain or S. enteritidis ATCC13076, multiple double-stranded DNA can be generated through several strand displacement reactions, among which P1C1, P1A1, P2C2, and P2A2 can release fluorescent signals and be detected in an enzyme-free manner. To verify whether these double strands can be generated, that is, whether the reaction system can operate normally, this study added the final reaction solution to the electrophoresis lane for intuitive characterization. As shown in Figure 1, when the initiating sequences C1 and C2 were not added, only the annealed P1T1, P2T2, and A1A2 double-stranded DNA existed in the solution; After the addition of trigger sequences C1 and C2, as shown in lane 3 and lane 4, P1T1 (P2T2) and A1A2 are reduced to varying degrees, and new bands are generated in other locations of the lane. These are the product DNA produced by the reaction. Among them, P1A1, P1C1, P2A2, and P2C2 can be excited by fluorescence spectra to release fluorescence, which is used for qualitative and quantitative determination of the target object. In addition, both C1 and C2 sequences can trigger the reaction of the system, which is the basis of dual-target detection. Comparing the fifth lane with the third and fourth lanes, it can be seen that the reaction system containing C2 is more efficient than that containing C1, which is also consistent with the results of our follow-up experiments. The above results prove the correctness of the reaction system. In the presence of E. coli ATCC25922 and S. enteritidis, new double-stranded DNA can be generated through SDR cyclic amplification. This is a new attempt to develop an enzyme-free dual-target assay that provides a novel sensing technology that can meet the needs of not only bacterial pathogens but also a wide range of biomarkers.
2.3 Verifying the identification capabilities and specificity of biosensors
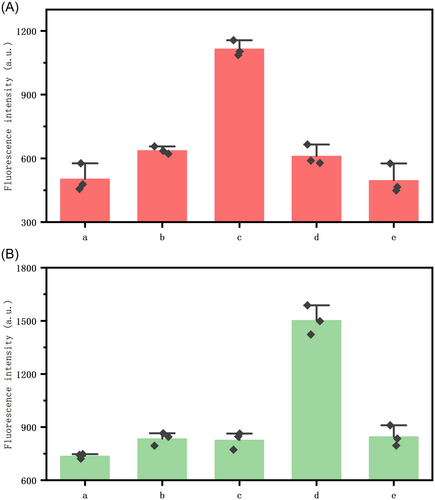
Second, to demonstrate the usability of the method, we added clinically collected S. enteritidis strains and standard strains of E. coli as targets and performed controlled experiments. As shown in Figure 2(a–e) of the x-axis are blank control groups, inactivated E. coli, healthy E. coli, healthy S. enteritidis, and inactivated S. enteritidis, respectively. Each bacterium was added at a concentration of 1 × 103 CFU/mL. Figure 2A,B represents the corresponding fluorescence in the presence of healthy E. coli and healthy S. enteritidis, respectively. In Figure 2A, the fluorescence shown is the fluorescence intensity displayed by the blank control, in the absence of the target bacterium, most of the two fluorescent probes of the system are burst by the corresponding burst probes, and no fluorescence is emitted. Meanwhile, the fluorescence of Figures 2A(b) and 2B(e) is enhanced relative to the reformed control, which we guessed to be due to incomplete inactivation, but it is obvious that the fluorescence produced by the inactivated dual bacteria added to the system is much smaller than that produced by normal healthy bacteria. When healthy E. coli and S. enteritidis were used as targets, as shown in Figures 2A(c) and 2B(d), the corresponding fluorescence intensities of both groups increased significantly, proving that the method can be used for the detection of these two bacterial microorganisms. At the same time, any one target does not cause an increase in the corresponding fluorescence of the other target, which proves that there is no obvious signal crosstalk in the dual-targeting strategy, which proves that the method has good stability and can be usable for dual-targeting detection.
2.4 Method optimization
We optimized some parameters of the method that affect the sensor performance, mainly: (a) the ratio of the three pairs of probe duplexes; (b) the reaction time; and (c) the reaction temperature, as shown in Figures S1–S3. Briefly, the sensor had the best performance under the following experimental conditions: (a) the ratio of probes P1T1:P2T2:A1A2 was 1:1:2; (b) the optimal reaction time was 10 min; and (c) the optimal reaction temperature was 37°C.
2.5 Detectability
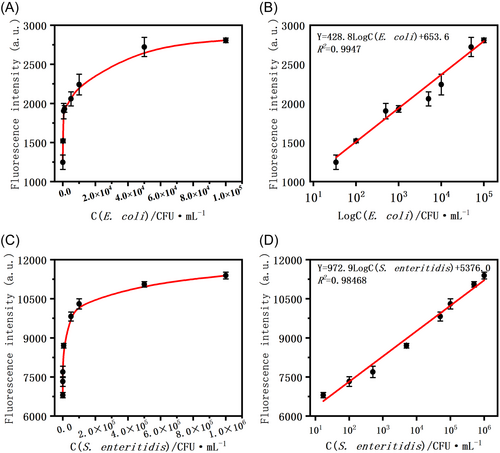
To test the performance of the sensor, we used clinical cultures of E. coli and S. enteritidis for incubation and then added 10 –106 different concentrations of the two strains for the experiments and tested their two fluorescence intensities at 10 and 20 min after the reaction, respectively. As shown in Figure 3, the fluorescence intensity of the system increased with increasing concentrations of E. coli (34–105 CFU/mL) and S. enteritidis (17–106 CFU/mL), indicating that fluorescence production is highly correlated with the concentration of the target. These results validate the performance of the biosensor designed in this study in which the dual target binds to the aptamer and releases the trigger sequence, thereby initiating the chain displacement reaction and generating fluorescence. Figure 3B,D also showed the linear relationship between the fluorescence intensity of the system and the concentration of the two targets (Figure 3B, E. coli ATCC25922 and Figure 3D, S. enteritidis ATCC13076). Under the optimized conditions, as shown in Figure 3B,D, a very clear linear relationship was exhibited between the average fluorescence intensity of the target and the concentration of the target, with a linear range of 34–105 CFU/mL for E. coli and 17–106 CFU/mL for S. enteritidis. The LOD of the developed biosensor was determined to be 0.7 CFU/mL (n = 3) for E. coli and 0.61 CFU/mL (n = 3) for S. enteritidis. Compared to some other bacterial detection biosensors based on aptamers as well as DNA amplification and amplification methods, the present method has higher detection sensitivity (Table 1).16, 22-26 This high sensitivity and wide detection range can be attributed to the stability of the amplification of the DNA strand generated by the target-induced triggering sequence cyclic and the intentional amplification efficiency. In particular, the biosensor of the present study has the advantage of being enzyme-free, that is, it does not require enzymatic involvement in the reaction to maintain excellent efficiency. Thus, the biosensors developed in this study are sensitive, accurate, and economical for the ultra-sensitive detection of E. coli and S. enteritidis.
| Detection method | Materials used | LOD (CFU/mL) | Linear range (CFU/mL) | Reference |
|---|---|---|---|---|
| Fluorescence | Aptamer, Cu NPs | 25 | 50–104 | 15 |
| Fluorescence | Aptamer, GO | 25 | 102 to 107 | 22 |
| SERS-based lateral flow | AuMBA@Ag NPs, antibody | 27 | 27–2.7 × 106 | 23 |
| Fluorescence | Aptamer, GO | 40 | 40–4 × 109 | 27 |
| Fluorescence | G4 | 60 | 102 to 107 | 25 |
| Fluorescence | Aptamer, Ag NC | 50 | 102 to 107 | 28 |
| Fluorescence | Aptamer | 0.61 | 10–106 | This work |
- Abbreviation: LOD, limit of detection.
2.6 Selectivity and specificity
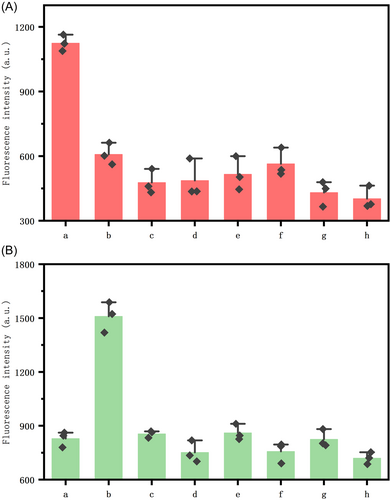
The specificity of the biosensor was explored by involving seven bacteria as targets for sensing, including E. coli, S. enteritidis, Staphylococcus aureus, Listeria monocytogene, Streptococcus enteritis, E. coli BL21, E. coli DH5α and background control in the same condition of 105 CFU/mL. As shown in Figure 4, except for E. coli and S. enteritidis, the fluorescence intensity of the system did not increase significantly after the addition of the above bacteria compared to the negative background control. Also, as shown in Figures 4A(a) and 4B(b), E. coli and S. enteritidis released higher fluorescence intensity only for probes that were able to participate in the reaction. This high specificity may be due to the high affinity of the aptamer for dual bacteria. In addition, the design of the probe protection sequences and the precise base complementary strand migration mechanism increased the specificity of this protocol. All these results demonstrate the very excellent selectivity and specificity of the biosensor we developed for targeting dual bacteria.
2.7 Precision and repeatability
Precision evaluation is an important evaluation index to illustrate the applicability of a method, so this index was experimentally measured in this study. First, 1 × 103 CFU/mL of E. coli and S. enteritidis were measured consecutively and the intra-batch precision was determined to be 4.12% for E. coli and 7.03% for S. enteritidis (n = 5). The RSD values between samples were 6.22% for E. coli and 8.95% for S. enteritidis when the samples were re-examined after 72 h under the same reaction conditions (n = 5). Subsequently, three parallel experiments were conducted on the same batch of 1 × 103 CFU/mL of E. coli and S. enteritidis to analyze the inter-batch precision, achieving the value of 5.42% for E. coli and 8.29% for S. enteritidis (n = 3).
2.8 Detection of S. enteritis in clinical normal human fluid samples
The possibility and potential of the designed biosensors to be used in clinical applications were also assessed by detecting E. coli and S. enteritidis in body fluid samples. These two culture strains were added to cerebrospinal fluid, pleural fluid, and ascites at a concentration of 1 × 105 CFU/mL; then, after a simple pretreatment of the samples, the proposed biosensor was applied to detect the bacteria. As shown in Table 2, the recoveries of the two-target bacterial standard samples in the three body fluids were very promising, it was shown that the presence of complex components in the body fluid samples did not significantly interfere with the detection of E. coli and S. enteritidis. This result can be attributed to the high affinity between the two bacteria and the adapter as well as the high efficiency of the DNA circular amplification method. More importantly, these results demonstrate the feasibility and application potential of the developed biosensor in clinical sample detection. In addition, the proposed biosensor can be developed into a versatile platform for the detection of any bacteria or even other species of biomarkers.
| Body fluid sample | Target added (CFU/mL) | Detected by the biosensor (CFU/mL) | Recovery of the biosensor (%) | RSD (%) (n = 3) | ||||
|---|---|---|---|---|---|---|---|---|
| E. coli | S. enteritidis | E. coli | S. enteritidis | E. coli | S. enteritidis | E. coli | S. enteritidis | |
| Hydrothorax | 1 × 105 | 1 × 105 | 0.92 × 105 | 0.94 × 105 | 92 | 94 | 7.83 | 3.29 |
| Abdominal dropsy | 1 × 105 | 1 × 105 | 1.09 × 105 | 1.10 × 105 | 109 | 110 | 6.68 | 6.64 |
| Cerebrospinal fluid | 1 × 105 | 1 × 105 | 0.89 × 105 | 1.05 × 105 | 89 | 105 | 4.56 | 7.66 |
3 DISCUSSION
In this study, a biosensor based on aptamer and isothermal enzyme-free DNA circular amplification was developed for a dual-target detection strategy for E. coli and S. enteritidis. The biosensor is portable, rapid, inexpensive, and the enzyme-free and isothermal properties allow for ultra-rapid detection in POCT environments, suggesting that the sensor has the potential to be developed into a kit. The sensor's sensitivity, specificity, reproducibility, and immunity to interference make it potentially useful for clinical detection of E. coli and S. enteritidis. These features also indicate that the SDR cyclic amplification method constructed by this sensor performs well, thanks to the high affinity of the aptamer for the bacteria, the binding stability of the protection probe and aptamer sequences, and the high efficiency of the cyclic method approach. Therefore, this method is not limited to the detection of bacteria or a specific target; by changing the sequences of the aptamer and the protection probe and performing certain optimizations, the sensor has the potential to detect a wide range of other biomarkers, including proteins, nucleic acids, small molecules and so on.
In addition, the present method has the advantage of simultaneous detection of two to-be-assayed substances, which is quite advanced in the current bacterial detection and even biosensing field, and with the improvement and optimization of the sequences, the present method has also paved the way for subsequent research, based on which it is expected that the simultaneous detection strategy for more kinds of biomarkers will be developed in the future.
More importantly, the method was briefly investigated for its detection performance in complex samples using pleural fluid, ascites, and cerebrospinal fluid, and it was found that the method has excellent stability and reproducibility in human body fluid environment, which is crucial for the development of the method for clinical applications in the future. This is also a solid step toward the exploration of this method toward clinical detection. In addition, since the assay module in this study essentially consists of DNA primers and double-stranded DNA, it offers great advantages in terms of cost and preparation, which makes it possible to develop this protocol into a kit for POCT detection.
However, there are some limitations to this study. For example, the recognition scheme of this method for bacteria is achieved through the binding of aptamers to bacteria, which limits the study to detecting only a portion of the bacteria for which the developed aptamers can be stably paired, and may not be able to effectively detect some of the mutant strains in the clinic. This also limits the choice of biomarkers if the method is to be explored for a wider range of biomarkers than just bacteria. That is, the biomarker needs to have a proven aptamer for pairing. Also, the detection limit of the developed fluorescent biosensor may still be difficult for the detection of some biomarkers at lower concentrations, and further improvements are needed to achieve high throughput, faster and cheaper detection of live pathogens.
4 CONCLUSION
In this study, an enzyme-free dual-target microbial detection sensor is developed using aptamer-based target recognition and cyclic amplification. By innovating the use of aptamer-based target recognition, single-stranded DNA-mediated cyclic amplification and fluorescence amplification processes, this method realizes ultra-sensitive detection of E. coli and S. enteritidis, with a wide linear range. More importantly, this method can identify E. coli and S. enteritidis in various body fluids. This shows that the method is suitable for complex samples detection and may be developed into a clinically usable POCT test suite. Meanwhile, detecting two bacteria at the same time in this study is a novel and eye-opening method, which is expected to target more types of biomarkers, not just bacteria.
5 MATERIALS AND METHODS
5.1 Chemicals and materials
PBS buffer was purchased from Sangon Biotechnology. Ultrapure water (18.2 MΩ cm) was produced by a Millipore filtration device. N,N,N′,N′-tetramethylethylenediamine (TEMED), Ammonium persulfate (APS), Acrylamide (29:1), and TBE buffer were purchased from Sangon Biotechnology. HPLC purified and polyacrylamide gel electrophoresis (PAGE) purified oligonucleotides were synthesized by Sangon Biotechnology (Table S1).
5.2 Source of bacteria
E. coli ATCC25922, S. enteritidis ATCC13076, Listeria monocytogenes ATCC 19115, and S. enteritis are all clinically cultured strains. Staphylococcus aureus was purchased from Kuujia Co., E. coli BL21 and E. coli DH5alpha were purchased from Sangon Co.
5.3 Instrument
Fluorescence emission spectra were obtained on a fluorimeter F-7100 (Hitachi, Japan).
5.4 Preparation of DNA sequence
The aptamers (E. coli-Aptamer and S. enteritidis-Aptamer) (final concentration was 10 nM) and the competing sequences (Competition 1 and Competition 2, hereinafter referred to as C1 and C2) (final concentration 10 nM) were incubated at 95°C for 10 min and gradually cooled to room temperature in PBS buffer.
The two components after annealing are called Apt_C1 and Apt_C2, respectively.
Probe 1 (hereinafter referred to as P1) and Trigger 1 (hereinafter referred to as T1), Probe 2 (hereinafter referred to as P2) and Trigger 2 (hereinafter referred to as T2), and Amplification 1 (hereinafter referred to as A1) and Amplification 2 (hereinafter referred to as A2) were mixed (final concentration 100 nM), heated at 95°C for 10 min, and gradually cooled to room temperature in 100 μL of PBS buffer.
The three components after annealing are called P1T1, P2T2, and A1A2, respectively.
5.5 Bacterial and culture conditions
E. coli ATCC25922 and clinically collected S. enteritidis ATCC13076 were spread on blood plates and cultured at 37°C for 24 h. Monoclonal bacteria were selected from the blood plates, 10 mL of lysogeny broth (LB) medium was added, and the plates were shaken at 37°C for 16 h. The cultured bacterial solution was diluted 106 times and evenly spread on the LB solid plate (100 μL of diluted bacterial solution) with a coating rod, and incubated at 37°C for 12 h. The cultured colonies were counted, and the count of the cultured colonies was the number of viable bacteria in the bacterial solution, in CFU/mL. Incubate a mixture of 104 CFU/mL of E. coli and S. enteritidis enterica at 100°C for 15 min until they have been inactivated. Afterward, the heat-inactivated mixed bacterial solution was incubated on LB plates at 37°C for 2 days, and the inactivation step was successful if no colonies were eventually observed on the plates.
5.6 Dual bacteria detection
The two bacteria were mixed with Apt_C1 and Apt_C2, respectively, incubated at room temperature for 30 min, and the supernatant was collected after centrifugation (5 min, 3000g, 4°C). The collected supernatants are called Groups 1 and 2.
The annealed mixed solutions P1T1 and P2T2 (100 nM 20 μL each) were mixed, and 118 μL PBS buffer was added and incubated at 37°C for 30 min. Then, 20 μL 100 nM A1A2 was added, and 20 μL Group 1 was added, and the reaction was carried out at room temperature for 10 min. Then, the above system was placed in a fluorescence spectrophotometer for detection, with an excitation wavelength of 640 nm and an emission wavelength range of 660–700 nm, and the standard curve of E. coli was obtained. Then, 20 μL of Group 2 solution was added, and the reaction was carried out at room temperature for 10 min. The system was placed in a fluorescence spectrophotometer for detection with an excitation wavelength of 495 nm and an emission wavelength range of 515–550 nm to obtain a standard curve of S. enteritidis.
5.7 Bacterial availability and selectivity
For the purpose of analyzing the effect of other control strains with negative results such as non-E. coli and S. enteritidis on the biosensor system, S. aureus, Listeria monocytogenes, S. enteritidis, E. coli, S. enteritidis, inactivated E. coli, inactivated S. enteritidis were added in the system. The steps following the addition of bacteria to this experiment were the same as those described previously for the detection of bacteria.
5.8 Clinical stability validation
The samples of body fluid were added to test the stability and clinical availability of the method. Thoracic fluid, ascites, and cerebrospinal fluid collected from the clinic, 80 μL of each, were added into 10 μL of E. coli ATCC25922 and S. enteritidis ATCC13076, respectively, so that their final concentration reached 1 × 105 CFU/mL. Then, they were added as targets to the system for the reaction, and finally, their fluorescence signals were obtained in the F-7100.
AUTHOR CONTRIBUTIONS
Shixin Yan: Data curation (equal); formal analysis (equal); validation (equal); writing—original draft (equal). Yuling Xiao: Methodology (equal); validation (equal); writing—original draft (equal). Ruijuan Shen: Data curation (equal); validation (equal). Jiazhe Cheng: Validation (supporting). Yuling Zhang: Validation (supporting). Nan Wu: Validation (supporting). Jinhao Chen: Validation (supporting). Jie Chen: Writing—review and editing (supporting). Peng Zhang: Data curation (equal); methodology (equal); writing—original draft (equal); writing—review and editing (equal). Jia Geng: Project administration (equal); writing—review and editing (equal).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (82102509), Sichuan Science and Technology Program (2022YFS0096 and 2023NSFSC1225), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (2019HXFH051 and ZYYC23015), Young Taishan Scholar Program of Shandong Province (tsqn202306362), Shandong Provincial Natural Science Foundation (ZR2021QH064 and ZR2024MH215), Guangdong Basic and Applied Basic Research Foundation (2022A1515220041), and Shenzhen Science and Technology Program (JCYJ20220530150413029). Thanks to biorender and origin software for charting and manipulating data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Clinical body fluid samples were provided by West China Hospital of Sichuan University (Chengdu, China), and the clinical experiments involved in this study were approved by the Biomedical Ethics Committee of West China Hospital of Sichuan University (approval number, 20191116).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




