Exploring the potential of transethosomes in therapeutic delivery: A comprehensive review
Abstract
The growing need for innovative drug delivery systems has led to extensive research to address the limitations associated with conventional dosage forms. Lipid nanoparticles have become prominent as frontline nanocarriers for the delivery of drugs and vaccine formulations. However, the pursuit of new materials and modifications to improve lipid nanocarrier properties remains ongoing. In this context, transethosomes have gained attention as a promising solution, offering distinct advantages over traditional formulations. Transethosomes minimize plasma fluctuations, first-pass metabolism, organ toxicity, and poor bioavailability. This comprehensive review provides an in-depth exploration of transethosomes, starting with an overview of the impact of formulation components on their properties and effective targeting. This article delves into the production techniques and evaluation properties employed to ensure efficient drug delivery. A significant contribution of this review lies in the analysis of various routes of administration for transethosomes, including transdermal, transvaginal, pulmonary, and ocular delivery, showcasing the versatility of transethosome-loaded with drugs and their potential to target specific tissues to achieve controlled release. Furthermore, the potential of functionalization and photodynamic therapy approaches to enhance drug delivery efficacy are explored. Overall, this review emphasizes the significant potentiality of transethosomes as a promising drug delivery system addressing the challenges associated with conventional drug delivery approaches.
1 INTRODUCTION
The oral route is frequently chosen for drug delivery because of its ease of administration. Still, these formulations pose significant drawbacks like gastrointestinal discomfort, unpleasant taste, and decreased bioavailability due to first-pass metabolism.1, 2 An alternate strategy, continuous intravenous injection, is regarded as a high-dose drug management approach for avoiding hepatic ‘first pass’ metabolism and maintaining a long-lasting, consistent drug level. However, this necessitates hospitalization of patients and careful monitoring under medical help. Hence, the health sector currently reaps significant benefits from transdermal drug delivery technologies that reduce plasma drug level fluctuations for repeated treatment, avoid organ toxicity, and early metabolism, limit dose-based side effects, and prevent gastrointestinal discomfort and poor bioavailability.3-5 It also provides benefits such as controlled drug delivery, lower doses, and improved patient compliance.6-9
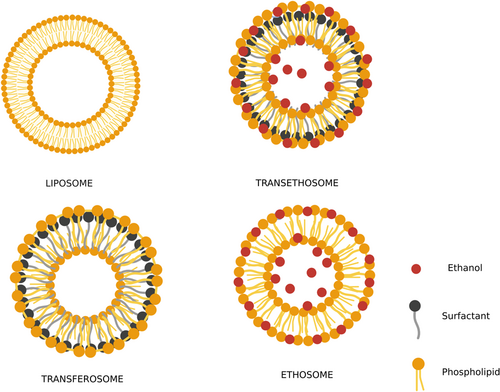
The tight junctions in the uppermost layer of the epidermis, stratum corneum (SC), continue to be a significant barrier to the free penetration of drugs into the body, hence reducing the bioavailability of transdermal drugs.10-12 Liposomes for topical administration of therapeutic agents have ushered in a new era of research in the drug delivery domain.13 However, their unstable nature of merging with the skin's lipids, dehydrating, and persisting near the skin surface resulted in poor skin permeability. Hence, they were only associated with topical drug delivery. To address the limitations of traditional liposomes, nanocarrier systems such as transfersomes and ethosomes were initiated.14, 15 Transferosomes were developed by Cevc et al.16 in the 1990s containing surfactant (SA) or edge activator (EA)17 to impart elasticity to the prepared vesicles. These systems can deform their shape but cannot permeate into deeper layers of the skin.18 On the other hand, ethosomal systems were introduced in 1998 containing high levels of ethanol in their formula, which resulted in improved drug absorption through the skin due to the result of ethanol mixing in lipid bilayer vesicles and enhancing saturation of SC lipids.19 To combine the synergistic effect of transfersomes and ethosomes, Song et al.20 introduced a new delivery system, that is, transethosomes (TE) (Figure 1) which combines both systems to conquer transdermal drug delivery systems and can also be administered through ophthalmic, transvaginal and pulmonary routes.21, 22
Transethosomes also enhance drug penetration across ocular barriers for targeted delivery to specific ocular tissues and improve bioavailability. They provide sustained drug release, reducing the frequency of administration and risk of adverse effects in other parts of the body. They are patient-friendly as it offers a convenient and noninvasive method for self-administration.23 In addition, TEs can be formulated for targeted delivery to specific regions of the pulmonary system. They enhance drug penetration across lung tissues, improving therapeutic efficacy. Inhalation of drugs allows for direct delivery to the lungs, where a large surface area and rich blood supply facilitate rapid absorption into the bloodstream.24 Furthermore, transvaginal drug delivery bypassed the gastrointestinal tract and hepatic metabolism, resulting in improved bioavailability and reduced systemic side effects. The vaginal mucosa is highly vascularized, allowing efficient drug absorption into the bloodstream. This route provides a noninvasive and convenient method of drug administration, with potential applications in hormone replacement therapy, contraception, and treatment of vaginal infections.1
These novel ultra-deformable vesicular systems have drawn much attention in recent years.25 TEs contain the essential components of ethosomes (phospholipids, ethanol, and water) and the SA/EA in their formula.26, 27 These nanocarrier systems showed enhanced penetration and biocompatibility, as their main component in the preparation is a phospholipid, the most abundant ingredient of eukaryotic cells.23, 28-31 The presence of ethanol together with surfactant provided softness and enhanced penetration, promoting their passage into the biological membrane as measured by deformability and permeation experiments.32, 33 The SA in its formulation leads to the transformation of SC lipids, thereby increasing its deformability into the biological membrane. Cholesterol can be added to enhance the vesicles' stability and control the drug release rate.34 TEs can trap hydrophilic and lipophilic drugs/agents in the aqueous compartment or the lipid bilayer. They can lower the concentration of drugs in other parts of the body, minimizing the risk of drug toxicity by delivering pharmaceuticals to the intended site of action. Additionally, TE systems have significant patient compliance, stability, and biocompatibility. However, its molecular size must be appropriate for a drug to be absorbed via the biological membrane. Notably, TEs may cause skin dermatitis like ethosomes since they have a high alcohol concentration.35 Moreover, this vesicular formation is easy to scale up and an excellent industry choice.
Following an overview of phospholipids, ethanol effect, edge activator effect on the properties of transethosomal formulation, and effective targeting, this review discusses the production and evaluation properties used to ensure the efficient delivery of drugs. Additionally, this article exclusively highlights the various approaches to delivering drugs through functionalization, photodynamic therapy, and multiple routes of administration. Furthermore, the therapeutic applications of transethosome-based drug delivery systems are also discussed.
2 THE IMPACT OF FORMULATION COMPONENTS ON TRANSETHOSOME PROPERTIES
To investigate the impact of formulation components on TE preparation, assimilating the role and influence of these components is crucial for designing effective transethosomal formulations that can optimize drug delivery across the biological barrier. This section delves into the key formulation components, such as phospholipids, ethanol, edge activator, and stabilizer, highlighting their impact on the physicochemical properties, stability, and performance of TEs.36 The main elements of TE are given in Table 1.
| Sl. no. | Component | Examples | Uses | References |
|---|---|---|---|---|
| 1. | Phospholipids | Lecithin, soybean lecithin, phosphatidylcholine, sunflower phospholipids, Lipoid S 100 Phospholipon 90G |
Vesicle forming agent | [24, 37-46] |
| 2. | Surfactant | Tweens, Spans, oleyl alcohol, Sodium deoxycholate, oleic acid, diacetyl phosphate, Cetyl Trimethyl Ammonium Bromide Dimethyl di-dodecyl ammonium bromide |
Edge activator | [1, 24, 26, 37, 45, 47, 48] |
| 3. | Alcohol | Ethanol, isopropyl alcohol | Penetration enhancer | [2, 32, 37, 41, 49-51] |
| 4. | Stabilizer | Hydroxypropyl-β-cyclodextrin | Imparts stability | [6, 28, 34, 37, 44, 51, 52] |
2.1 Phospholipids
Phospholipids are the key building blocks and vesicle-forming agents derived from different sources. Based on the sources, phospholipids can be divided into natural and synthetic phospholipids. Natural phospholipids can be found in various products, including soybeans, egg yolks, and sunflower seeds.53 Utilizing natural unsaturated phospholipids cause the SC to fluidize, allowing APIs to permeate deeper layers. However, using saturated (hydrogenated) phospholipids improves or restores the skin's barrier function, facilitating APIs to remain intact for longer. The phospholipids can be categorized as phosphatidylcholine (PC), phosphatidylinositol, phosphatidylserine, phosphatidylethanolamine, phosphatidic acid, and phosphatidylglycerol. Due to the unsaturated properties of the hydrocarbon chain, compared to synthetic phospholipids, natural phospholipids are less stable during liposomal production.54 Natural lipids can be utilized to make synthetic phospholipids, and an unlimited number of distinct and specified phospholipids can be produced by altering the non-polar and polar portions of phospholipid molecules.55 Synthetic lipids include dipalmitoyl phosphatidylcholine (DPPC), dimyristoyl phosphatidylcholine (DMPC), distearoyl phosphatidylcholine (DSPC), hydrogenated soy phosphatidylcholine, 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-distearoyl-sn-glycero-3-phospho-1′-rac-glycerol, and 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol.56 The type and concentration of phospholipid selection are essential criteria for preparing TEs because they influence vesicle properties such as entrapment efficiency (EE), size, ζ-potential, penetration properties, and stability. In transethosomal development, phospholipid concentrations typically range from 0.5%–5%. For instance, a study by Garg et al.57 investigated the effect of various concentrations of PC on the ketoprofen-loaded TEs properties. The study found that increasing PC concentration from 0.5% to 2% resulted in an increase in vesicle size from 174.1 to 264.2 nm and EE from 55.3% to 72.6%, while a further rise in PC concentration to 3% and 4% caused a decrease in vesicle size to 214.7 and 211.8 nm and EE to 69.3% and 65.9%, respectively. Ahmed et al.34 study results demonstrated that when the drug-to-phospholipid's molar dose increases, the particle size increases and forms multilamellar vesicles, significantly increasing penetration efficiency. Another study by Esposito et al.58 evaluated the impact of different types of phospholipids, including DPPC, DMPC, and DSPC, on the properties of quercetin-loaded TEs. The study found that DMPC and DSPC resulted in smaller vesicle sizes and higher entrapment efficiency than DPPC. Specifically, DMPC and DSPC led to vesicle sizes of 123.7 nm and 129.8 nm, respectively, compared with 228.7 nm for DPPC. Furthermore, DMPC and DSPC resulted in entrapment efficiencies of 54.3% and 58.4%, respectively, compared to 41.7% for DPPC. Moreover, the unsaturation degree of the phospholipid acyl chain can also impact the properties of TEs. A study by Fang et al.59 evaluated the influence of the degree of unsaturation of the phospholipid acyl chain on the properties of TEs loaded with curcumin. The results showed that TEs prepared with unsaturated phospholipids had higher EE and skin permeation than those prepared with saturated phospholipids. This relationship holds good until a specific concentration; further phospholipid concentration will later not affect EE.23
2.2 Ethanol
Ethanol provides softness to the vesicle membrane and acts as a penetration enhancer.32 Ethanol concentration influences vesicle size, zeta potential, stability, enhanced skin, mucosal permeability, and entrapment efficacy. The concentration of ethanol in the TE is 10%–50%. As per the reports of Salem et al.1 and Abdulbaqi et al.,6 the size of the vesicle decreases with the increase in ethanol concentration from 10% to 30% w/v and exceeds the optimum level, leading to a leaky bilayer with a slight increase in the size of the vesicle. Further increase in ethanol causes the phospholipid to easily dissolve in ethanol, with a significant decrease in entrapment efficacy; this might be due to the interaction between the lipid layer and ethanol. A study by Tamer et al.60 found that TEs containing 10%–20% ethanol showed higher stability and deformability compared to those containing 30%–50% ethanol. The study also showed that the addition of cholesterol to the TE formulation improved stability and deformability at higher ethanol concentrations. In another study, Abdallah et al.61 showed that TEs containing 30% ethanol significantly increased the skin permeation of 5-fluorouracil compared to a control solution. The study also found that increasing the concentration of PC in the TE formulation enhanced skin permeation. According to the study of Nayak et al.,18 high ethanol saturation functions as a negative charge on the surface of the vesicle, preventing the transethosomal system from converging as a result of electrostatic repulsion. Hence, during the preparation process, the ethanol concentration should be optimized.
2.3 Edge activator
The deformability and flexibility of phospholipid vesicles are imparted by adding an edge activator. The type and quantity of EA can alter the drug's permeability profile.18 Surfactants employed in the formation of TEs include Tween 20, sodium cholate, dipotassium glycyrrhizinate, bile salts, Span 80, oleic acid, and Tween 80.62 As per the study of Hui Song et al.63 the transethosomal systems containing tween and oleic acid resulted in increased size, and the sodium deoxycholate system remained virtually unchanged. Still, the zeta potential value is increased, indicating stability and a threefold increase in EE, which may be due to reduced polarity by combining deoxycholate anion and drug cation.37 Salem et al.1 and Khalid et al.38 study revealed that increasing surfactant concentration could cause a substantial decrease in the vesicle size due to interfacial tension being reduced by high surfactant levels covering the carrier surface. This Tween 80 effect results in its solubilizing property and inhibition of vesicle cohesion.28, 36 Due to the polyethylene oxide groups on the nanovesicle's surface, tween 20 prevents nanovesicle aggregation.24 Recently, Fe-chlorophyllin TEs were prepared using 0.1% and 0.3% w/v concentrations of span 20, tween 20, tween 80, and cremophor A25 as edge activators with phosphatidylcholine and 20% w/v ethanol. When these surfactants were employed at low concentrations, the mean vesicle sizes ranged from 456 to 685 nm. TEs containing 0.1% w/v cremophor A25 showed a deformability index of 26.2–3.8 mL/s.39 Surfactants at high concentrations may cause skin irritation and the disruption of the vesicular structure.
2.4 Stabilizer
Stabilizers are commonly used in the formulation of TEs to prevent their aggregation, maintain their size and structure, and improve their shelf-life. It imparts stability to the TE system. Cholesterol is the most commonly used stabilizer. A study by Tamer et al.60 found that the addition of cholesterol to the TE formulation improved stability and deformability at higher ethanol concentrations. The increase in cholesterol concentration declines the EE due to the low solubility of cholesterol.37 Liu et al.64 investigated the effect of different concentrations of a stabilizer, hydroxypropyl-β-cyclodextrin, on the stability and drug release properties of TEs loaded with a hydrophilic drug, metronidazole. The study found that the addition of hydroxypropyl-β-cyclodextrin significantly improved the stability and drug-release properties of the TEs. Moreover, the TEs containing a higher concentration of hydroxypropyl-β-cyclodextrin showed enhanced skin permeation of metronidazole. The choice of a stabilizer depends on the specific drug and formulation, and excessive amounts of a stabilizer can also negatively affect the stability and integrity of TEs. The effect of different concentrations of sodium deoxycholate was studied by Cao et al.65 on the stability and drug release properties of TEs loaded with ivermectin, an antiparasitic drug. The study found that high concentrations of sodium deoxycholate (above 1%) decreased the stability of the TEs and caused drug leakage, which could decrease their therapeutic efficacy.
2.5 Drug or active compound
The addition of a drug to the transethosomal system influences the vesicle's mean diameter and zeta potential without affecting the dispersity index. According to the study of Kabil et al.,24 plain Nanovesicles showed a negative charge on the surface. The vesicles displayed a positive charge on the surface upon adding the drug. The effect of drug loading on the stability and drug release properties of TEs loaded with a lipophilic drug, curcumin, was studied by Zhang et al.66 The study found that the addition of curcumin decreased the particle size and increased the zeta potential of TEs. Moreover, the TEs showed sustained drug release over 48 h and enhanced skin permeation of curcumin compared to a conventional cream. However, the addition of a drug can also negatively affect the stability and integrity of TEs, and excessive drug loading can cause aggregation and drug leakage. The investigations of Zhuang et al.67 on the effect of different drug-to-phospholipid ratios on the stability and drug release properties of TEs loaded with tacrolimus, an immunosuppressive drug. The findings showed that high drug-to-phospholipid ratios decreased the stability and drug-release properties of the TEs and caused drug leakage, which could decrease their therapeutic efficacy.
2.6 Surface functionalization of TE
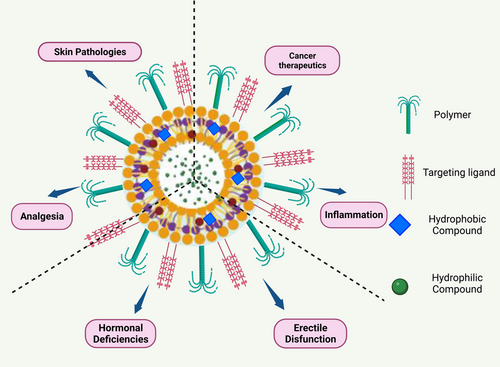
Functionalization of TEs can be achieved by modifying the surface of the vesicles with different chemical moieties, such as targeting ligands, peptides, and antibodies. These modifications can improve the specificity and selectivity of the TEs, allowing them to target specific cells or tissues.68 For example, the functionalization of TEs with folic acid has been shown to enhance their uptake by cancer cells that overexpress folate receptors. Similarly, the use of transferrin-conjugated TEs has been proved to enhance the uptake of drugs into cells that express transferrin receptors.69, 70 Several studies have investigated the impact of functionalization on the drug delivery efficiency of TEs. Ellis and Hicklin71 found a peptide that targets the vascular endothelial growth factor receptor (VEGFR). The VEGFR-targeted therapy was found to have higher cellular uptake and improved anticancer activity compared to non-functionalized therapy in the in vitro studies using breast cancer cells. In another study by Zhao et al.72 functionalization with a peptide that targets the αvβ3 integrin, which is overexpressed in several cancer cells. The αvβ3 integrin-targeted therapy was found to have enhanced cellular uptake and improved anticancer activity in in vitro studies using prostate cancer cells. Hui Song et al.63 developed a sinomenine hydrochloride-loaded TE decorated with an antioxidant surface that achieved targeted drug delivery and improved hydrophilic drug efficiency, deformability, and permeation efficiency. The antioxidant surface-coated TE is localized at the inflammation site through redox interactions in the presence of highly reactive oxygen species levels. The applications of surface functionalized TEs are given in Figure 2.
3 METHODS FOR PREPARATION AND THEIR EFFECT ON PROPERTIES OF TE
3.1 Cold method
The cold method refers to a technique where the formulation of TEs is performed at low temperatures.73 This method is convenient and extensively used, which involves preparing aqueous and organic phases separately. The organic phase is acquired by vigorously mixing phospholipid, penetration enhancer, and other lipid materials in organic solvents at room temperature in a closed vessel.74 Then, the aqueous phase, i.e., bidistilled water, standard saline solution, or buffer solution, is added dropwise at a continuous rate to the organic phase using a syringe pump. Using a magnetic stirrer, the mixture is stirred for 5–30 min at a 700–2000 rpm speed. Vivek Gupta et al.50 developed TE at different stirring rates, and the studies revealed that the size of the vesicle is altered by the stirring time. Depending on the physicochemical characteristics of the molecule, the drug incorporated into the TE system will dissolve in either an aqueous or an organic phase.20, 28 The final formulation is refrigerated and stored for further use.15 This approach avoids the thermal stress that can lead to drug degradation.32
3.2 Hot method
The hot method is a technique that involves the application of heat during the formulation process of TEs. This method is used to enhance lipid solubility, facilitate lipid fusion, and improve the EE of the drug withwithin.73 In this process, colloidal dispersion of the phospholipid is formed by dispersing in water at 40°C. In a different container, a mixture of polyol and ethanol is heated to 40°C concurrently.30 The organic phase is added to the water phase by stirring continuously until both components reach a temperature of 40°C to form a vesicle suspension.75 Depending on the type of drug (hydrophilic or lipophilic), it can be dissolved in water or ethanol. Mishra et al.28 performed probe sonication or extrusion of the mixture to acquire the desired size of vesicles.
3.3 Ethanol injection-sonication method
The technique of ethanol injection is utilized to create liposomes within the size range of 30–170 nm, primarily focusing on the production of small unilamellar vesicles. The actual size of the liposomes is determined by the concentration of lipids and the speed at which the injection is administered.76, 77 In this method, phospholipids, SA, and the active ingredient are dissolved in ethanol. This organic phase is injected into an aqueous phase through a syringe system at a predetermined flow rate. Then the resultant mixture is homogenized with an ultrasonic probe sonicator.20, 78-80 Salem et al.81 developed an ultrasound-guided injection method to achieve a smaller particle size than the stirring-guided injection technique. The particle size is affected by ultrasound, injection time, and probe ultrasound. Using the ethanol injection method followed by the hot procedure (probe sonication), Singh et al.82 created TEs loaded with hydrophilic drugs. Compared to non-sonicated vesicles, the particle size of sonicated vesicles is smaller.
3.4 Thin-film hydration technique (TFH)
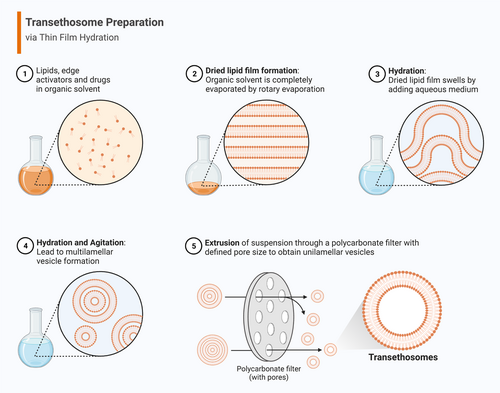
The TFH technique is a popular approach used to prepare TEs. It entails creating a slender lipid layer on the inner surface of a rotary evaporator flask. Subsequently, this lipid film is hydrated by introducing water or a buffer solution. To facilitate the creation of a bilayer, it is crucial to preheat both the lipid film and the water/buffer solution above the lipids' transitional temperature (Tm), if necessary. Additionally, vigorous shaking and potentially using an ultrasonic bath for sonication help the film detach from the flask and form TEs. This method offers the benefit of achieving consistent and reliable results, even when working with the small amounts of compounds. However, it has the drawback of low encapsulation efficiency.77, 83 In this technique, the phospholipids, EA, and drugs are dissolved in the organic phase in a round bottom flask. Then the mixture is kept in a water bath sonicator until a homogenous distribution is obtained. Organic solvents are then slowly removed at lower pressure above the lipid transition temperature by rotary evaporation to form a thin lipid film on the wall of the flask.24, 84 To ensure thorough removal of the organic solvents, the flask is placed in a vacuum oven overnight. The dried lipid film is diluted with an aqueous solution of ethanol or a solution of saline phosphate buffer-ethanol by rotating the lipid film. The TE vesicles are then left to swell at room temperature and stored at a refrigerated temperature of 4°C–8°C.7, 24 The various steps involved in thin film hydration-mediated synthesis of TEs are presented in Figure 3.
Sonbaty et al.80 investigated the effect of technical methodology on quality attributes using the ethanol injection method and TFH methods. The in vitro characterization showed that the average vesicle size of the produced TEs by using ethanol injection method was 245.3 and 62.85 nm by TFH process. Moreover, zeta potential value and EE were 36.3 mV and 82.35%, respectively, in contrast to 49.4 mV and 93.88% with ethanol injection sonication process and TFH. Additionally, the percent of drug deposited in the skin by the TE formulation created by TFH is 1.5 times higher than the percentage deposited in the skin by the TE formulation designed by ethanol injection and more than 2-fold higher than the percentage deposited in the drug solution in propylene glycol.
3.5 Reverse phase evaporation (REV) method
The REV is a technique for preparing TEs by forming water in oil emulsion, followed by evaporation of the organic solvent to create vesicles. This technique is least frequently used and created exclusively to make unilamellar vesicles.77 In this method, the phospholipid, stabilizer, and organic solvent solution are dried in a round-bottom flask by evaporating under vacuum to form a thin lipid film. A thin lipid film is dried with nitrogen gas to separate the residuary solvent.77 The lipid film is then suspended in a solvent or solvent mixture and stirred at room temperature. The prepared formulation is sonicated for 10 min at 5°C–6°C in a sonication bath to form a stable emulsion.85, 86 A gel is formed by removing the solvent under reduced pressure, leading to colloidal dispersion over mechanical agitation.28 According to the study of Nele et al.,85 the formulation technique significantly affects the lamellarity of vesicle populations. Specifically, they performed film hydration followed by freeze-thaw cycles, film hydration followed by agitation on a shaker, and REV. The freeze-thaw processes and agitation on a shaker method gave multilamellar, unextruded, and mixed unilamellar/bilamellar extruded populations. The REV vesicle approach, however, produced a mostly unilamellar extruded population.
4 CHARACTERIZATION OF TRANSETHOSOMES
4.1 Morphology, vesicular size, and poly-dispersivity index (PDI)
The most significant features in TEs characterization are size and PDI. It has been demonstrated that vesicular size is a crucial factor influencing the inhalation and parental administration of TEs and their circulatory half-life.87 The vesicular carriers are typically spherical, physically soft, flexible, and have an enclosed core. They may be small, unilamellar, or multilamellar based on the formulation.15, 32 As most vesicles are nano-sized to study morphology, transmission electron, and scanning electron microscopy are used.37 The importance of uniform size should be considered; it can be attained depending on the equipment and formulation techniques. TEs should typically be between 50 and 200 nm in size for the administration of drugs.88 Photon correlation spectroscopy,68 dynamic light scattering method,50 and Microtrac nanotrac wave are employed to determine the mean vesicular size and size distribution.6
The PDI value reveals the extent of sample homogeneity, which may be monodispersed or polydispersed as measured by size. PDI can be dimensionless and scalable, with a value between 0 and 1. The PDI value equal to or below 0.3 in drug delivery applications denotes an appropriate and homogenous vesicle population.89 In contrast, a high PDI value indicates multiple diverse vesicular populations or a broad size distribution (heterogeneity) in the sample.90 The PDI was assessed utilizing dynamic light scattering (DLS).91 DLS studies the Brownian motion of the dispersed particles in a solution, which scatters the incident light. The level of liposome diffusion in suspension is connected with the scattering of light. The mean particle size is calculated based on the quantity of light dispersed.92 Kim et al.93 recently introduced a tool known as nanoparticle tracking analysis for determining size by measuring the particle diffusion coefficient from a sample. DLS calculates the particle diffusion coefficient based on the intensity variation of scattered light. In contrast, Nanoparticle tracking analysis determines the individual particle movements in successive optical video images. The size determined by DLS can be verified using Nanoparticle tracking analysis since they measure the same physical property.
4.2 Zeta potential
Zeta potential is a physical property that determines the product's stability and can be measured using a Zeta sizer.11 The charge of the vesicular carriers is an essential factor that depends on the formulation excipients and can affect the vesicular properties. In accordance with Salem et al.,1 the positive charge on the vesicular surface imparts a significant reduction in the agglomeration of vesicles due to electrostatic repulsions, which also increases its stability. It offers information on each ingredient in the formulation, its interactions, and surface chemistry. Additionally, the surface of the nanovesicles is negatively charged due to the presence of ethanol, which increases their colloidal stability.94 The vesicles' negative zeta potential was also influenced by the phosphate groups' negative charge.48
A laser is needed as a light source to illuminate the sample's vesicles to quantify the zeta potential. The laser beam travels from the center of the sample cell being examined at a particular angle.95 The charge of TE is inversely correlated with their rate of mobility.
4.3 Entrapment efficiency
An in-depth analysis of vesicle properties may enable the development of TEs with suitable EE and the regulation of drug release. The TE synthesis process, composition, and phospholipid bilayer stiffness can significantly impact a drug's EE. A combination of drug fractions that have been encapsulated and nonencapsulated are found in the TE final product. The free drug must first be separated to calculate the amount of drug present in TE and, subsequently, the EE. Many techniques have been used, such as size exclusion chromatography based on the size difference, gravitation or centrifugal force, dialysis membrane with a sufficient cut-off, and ultracentrifugation.
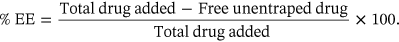 ()
()4.4 Drug content
 ()
()4.5 Measurement and factors influencing deformability index
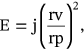 ()
()4.6 Measurement of in vitro skin permeation
In vitro drug release is measured using a Franz diffusion cell.49, 78 The dialysis bag membrane should be chosen in accordance with the drug parameters. The cellophane dialysis membrane with a specific molecular weight is commonly used, and it is hydrated with physiological medium, usually phosphate buffer saline of 7.4 pH. The donor compartment is filled with formulation, and the receptor compartment with 7.4 pH solution and stirred with magnetic beads at 300–400 rpm at 32 ± 1°C temperature to mimic human skin. 1 ml aliquot is collected at periodic intervals and immediately supplanted with an equal quantity of fresh buffer. All drug samples are analyzed by UV spectrophotometry.15, 78
4.7 Stability studies of TE
The two crucial factors affecting TEs' final biological function are their chemical and physical stability. Typically, the main criteria for assessing the physical stability of TEs are their size and appearance. This incident is connected to the propensity for aggregation or agglomeration. Vesicular fusion and rupture during storage can result in drug leakage from TEs.95 Chemical stability is the ability of TEs to maintain the level of EE when changes in the media, such as pH fluctuations, electrolyte composition, oxidizing agents, and the presence of surface-active ingredients, can occur. Lipid membrane permeability changes may be brought on by chemical degradation.97 Moreover, TEs chemical stability may be hampered by interactions between drugs and phospholipids. Hence stability studies are essential during the storage of liposomal vesicles, especially when the hydrophilic compound is incorporated. Freshly developed drug-loaded TE are stored at two different temperatures for 3 months: room temperature at 25 ± 2°C and refrigeration temperature at 4 ± 2°C, with relative humidity at 60% ± 5%. The formulation was kept in a borosilicate container to prevent interaction between the stability study formulation and the glass container. The formulation's physical changes and drug content were evaluated.2, 50
5 THERAPEUTIC APPLICATIONS
Researchers have shown that TEs offer significant benefits in improving the targeted delivery and transport of a wide range of medicinal molecules, irrespective of their diverse physicochemical properties. Their ability to penetrate biological barriers, provide sustained release, and ensure stability positions them as a promising strategy in drug delivery, with potential applications across different medical fields. These ultra-deformable vesicles have recently been administered through various routes for targeted delivery, site-specific action, and avoiding systemic side effects of drugs. Table 2 summarizes the therapeutic application of TEs.
| S. no | Bioactive compound/drug | Method of preparation | Characteristics (Size/EE%/zetapotential/morphology) | Applications | References |
|---|---|---|---|---|---|
| Anti-fungal drugs: | |||||
| 1. | Voriconazole | TFH technique | 191.9 ± 41.5 nm 96.6 ± 2.7% Irregular spherical shapes |
TE displayed enhanced in vivo skin deposition of voriconazole |
[20] |
| 2. | Ketoconazole | TFH technique | 623.06–1532.33 nm 61.45%–85.75% −30 to −40 mV |
Exhibited better in vivo pharmacodynamic activity than conventional liposomes | [18] |
| 3. | Luliconazole | Ethanol injection sonication or TFH methods | Ethanol injection sonication versus TFH 246.3 ± 0.56 versus 62.75 ± 0.16 nm 82.35 ± 3.68% versus 93.85 ± 1.78% 36.3 ± 0.81 mV versus 49.4 ± 0.67 mV |
Results showed that the TFH approach is superior in improving the quality attributes of the nanovesicles. | [80] |
| 4. | Econazole nitrate | Homogenization method | 159.3 ± 4.3 nm 78.3 ± 2.8% −27.13 ± 0.33 mV |
Compared to the marketed econazole nitrate cream, transethosomal gel demonstrated lower ex vivo skin penetration, higher ex vivo skin retention, and higher in vitro antifungal efficacy. | [98] |
| Anti-inflammatory drug: | |||||
| 5. | Flurbiprofen | TFH method | 152.06 ± 5.10 to 215.2 ± 0.53 nm 76.0 ± 0.53% −30.09 ± 0.46 mV Irregular spherical shape |
TE serves as an effective carrier for the management of arthritis | [12] |
| 6. | Apremilast | TFH and probe sonication method | 89–171 nm 40.32% to 82.35% −14.3 mV Spherical vesicles |
TE Showed better skin permeation and sustained release. | [2] |
| 7. | Mangiferin | - | 82 and 411 nm 68 ± 3% Spherical and ovoid multilamellar vesicles |
TE assisted the delivery of mangiferin to keratinocytes, thereby increasing its antioxidant defense status. | [99] |
| 8. | Naproxen sodium | Ethanol injection method | 56.94 ± 0.12 to 291.7 ± 0.09 nm 66.23 ± 1.52 to 93.11 ± 0.96% Spherical shape |
Caused reduction in edema rate related to musculoskeletal pain | [79] |
| Anti-hypertensive drugs: | |||||
| 9. | Olmesartan medoxomil | TFH technique | 222.60 ± 2.50 nm 58.50 ± 1.30% −20.80 ± 0.30 mV Spherical shape |
TE can extensively avoid first-pass metabolism and serve as an excellent transdermal delivery system for OLM. | [8] |
| 10. | Propranolol Hydrochloride | Homogenization method | 182.7 ± 5.4 nm 81.98 ± 2.9% −21.91 ± 0.65 mV Spherical shape |
TE can Avoid the disadvantages associated with oral propranolol, like the first-pass effect, high dosing frequency, and organ toxicity | [52] |
| 11. | Irbesartan | Cold method | 104.63 ± 0.3 to 215.06 ± 0.1 nm 76% −10.7 to −49.3 mV Irregular shape |
TE is a suitable carrier for poorly soluble drugs | [51] |
| 12. | Paeonol | Ethanol injection method | 122.5 ± 7.5 nm 85.5 ± 5.2% 13.08 ± 1.18 mV Spherical morphology |
The paeonol TE demonstrated narrow size distribution, high EE, prolonged residence in the plasma, and a remarkable increase in bioavailability. | [100] |
| Anticancer drugs | |||||
| 13. | Brucine-strychnine | TFH method | 1–100 nm 92.50 ± 0.048% −20 mV Spherical shape |
Transdermal BSTE formulation taken up by the human hepatoma HepG2 cells inhibits their proliferation. | [37] |
| 14. | Rolapitant | TFH technique | <400 nm 90% 42.1–51.6 mV Spherical shape |
Proven promising tool in the treatment modality for lung cancer. | [24] |
| Antiaging drugs: | |||||
| 15. | Cycloastragenol | - | 100–400 nm 31.5 ± 0.02% Spherical morphology |
Aid delivery of cycloastragenol-related antiaging cosmetic products | [7] |
| 16. | Niacinamide | TFH technique | 132 ± 9 to 215 ± 6 nm 5.3 ± 1.2 to 7.6 ± 2.5% −21 ± 6 mV Spherical shape |
Studies on the skin permeation of hydrogels loaded with TEs revealed that nano gels have a more controlled and enhanced penetration. | [5] |
| Miscellaneous bioactive materials | |||||
| 17. | Coenzyme Q10 | TFH method | 146 ± 26 nm 97.63% −55 mV Spherical morphology |
Proved the superiority of CoQ10 TEs as a topical delivery system in the AGA treatment. | [4] |
| 18. | Epigallocatechin-3-gallate | TFH technique | 153.4 ± 1.9 to 650.8 ± 35.85 nm 21.19 ± 1.8 to 58.8 ± 1.08% −2.27 to −5.59 mV |
Proper optimization of TE vesicular properties is essential to maximize the entrapment | [36] |
| 19. | Colchicine | Cold method | 79.8 ± 1.9 to 150.8 ± 4.5 nm 60.04 ± 0.52 to 83.57 ± 0.84% ≥−20 mV Aspherical irregular shape |
TE is proven to be an alternative route to the oral route to overcome bioavailability problems and other side effects | [6] |
| 20. | Cholecalciferol (vitamin D3) | Cold method | 182.17 ± 10.17 nm 100% Spherical and unilamellar vesicles |
TE is proven to be biocompatible and efficient nanocarriers | [32] |
| 21. | Tranexamic Acid | Cold technique | 72 nm 94% −16 mV |
TE would be an excellent alternate carrier for delivering the drug into deeper layers, making it helpful in treating melasma. | [21] |
| 22. | Progesterone | injection sonication method. | 133.3 ± 3.42 to 349.5 ± 1.24 nm 87.93 ± 3.58 to 97.05 ± 2.6% 23.5 ± 3.84 to 74.6 ± 4.97 mV Spherical nanovesicle |
The PRG TE showed a notable increase in serum concentration, echogenicity degree, endometrial thickness, and pregnancy rate. | [1] |
| 23. | 8-methoxy psoralen | - | 265.0 ± 2.9 nm 83.87 ± 4.1% nanospheres |
The TE gel increased the outcome of narrow UVB in treating vitiligo patients with no side effects. | [26] |
| 24. | Tramadol Hydrochloride | Cold method | 149.34 to 278 nm 79.37% −22 mV asymmetrical shape |
TE gel provides an alternative method for drug administration to avoid the drug's poor bioavailability and significant adverse effects due to oral administration. | [74] |
5.1 Transdermal drug delivery
Transethosomes are indeed appealing for the transdermal delivery of bioactive compounds and drugs.101, 102 The TEs of approximately 200 nm size is capable of diffusing through the SC and can reach the upper layer of dermis, allowing their penetration into the skin. Furthermore, these engineered vesicles are composed of lipids with specific charges that help in the transdermal and intradermal delivery of payload into the skin. The penetration of TEs into the systemic circulation is mainly due to the electrostatic forces of the vesicle, such as increased zeta potential and increased net charge.103 These electrostatic forces increase the adhesion of the TEs to the skin.98, 104 Thus, TEs can cross the epidermis and dermis and enter the systemic circulation. Once they reach the dermis, they can be absorbed into the lymphatic system and distributed throughout the body, thus being able to reach the systemic circulation.104 Thus, TEs can cross the epidermis and dermis and enter systemic circulation. Figure 4 depicts the penetration capacity, primary penetration mechanism, and permeation capacity of TEs. Compared with other lipophilic vesicles, clinical applications of TEs focus on treating fungal infections, inflammatory diseases, cardiovascular diseases, cancer, aging, androgenic alopecia, and neurodegenerative diseases.
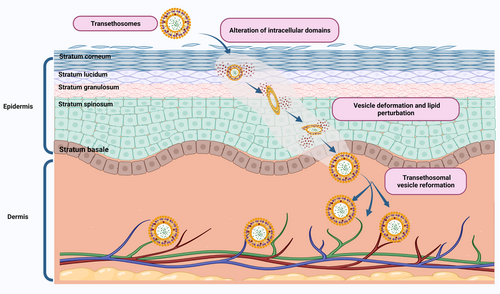
5.1.1 Fungal infections
To ascertain the effectiveness of vesicular carriers for anti-fungal medications, Song et al.20 designed a new carrier based on TEs for the enhanced skin penetration of voriconazole compared with conventional liposomes, deformable liposomes, ethosomes, and controlled polyethylene glycol solutions. According to the study's findings, the presence of ethanol (7%–30%) caused more than 90% of the vesicles to be recovered out of all the vesicles. Therefore, TEs may be used as a potential TDDS for voriconazole.20 The main objective of Verma and Utreja's98 research was to create TEs for the efficient cutaneous administration of econazole nitrate. The results demonstrated better antifungal activity by transethosomal gel than by commercial cream. The TEs could deliver the drug in a controlled pattern to eradicate cutaneous candidiasis. Sonbaty et al.80 recently showed the TFH technique's superiority in enhancing the qualitative attributes of the generated nanovesicles and highlighted its potential for treating cutaneous fungal diseases. Ketoconazole-loaded TEs were created by Nayak et al.18 compared to commercial products and traditional liposomes; they showed improved ex vivo penetration, in vitro diffusion, and greater antifungal effectiveness against Candida albicans.
5.1.2 Inflammatory diseases
The oral administration of flurbiprofen and apremilast results in gastrointestinal damage and short elimination half-life requiring multiple doses. To overcome this, Gadad et al.12 and Rahangdale et al.2 formulated drug-loaded transethosomal gel for transdermal delivery to increase the deposition and skin permeability of the drug, reducing dosing frequency and sustained release. According to the results, the optimized gel formulations with a high concentration of tween 80 exhibited more significant skin deposition and ex-vivo profile than the marketed gel showing that TEs act as an effective carrier for drug release. The effectiveness of ethosomes and TEs for the solubilization and administration of mangiferin was demonstrated by Sguizzato et al.99 Data revealed that both nanosystems could pass through cells intact and distribute Magniferin to the targeted cell, improving antioxidant defense level of the keratinocytes. Kaul et al.79 examined the skin permeation profile of naproxen sodium-loaded TEs incorporated into a hydrogel in vitro and in vivo. The investigation resulted in the formulation that may be the best alternative for treating musculoskeletal pain because it has smaller particle size, enhanced skin deposition, and lower systemic absorption.
5.1.3 Cardiovascular diseases
Paeonol is a phenolic substance primarily found in the Paeonia genus's root bark and cortex. This substance prevents cardiovascular diseases and effectively treats allergies, inflammation, and pain.105 This medicine is constrained in the clinic, nevertheless, due to its poor water solubility and more volatility at the room temperature. Paeonol, which was delivered by TEs, displayed prolonged plasma residence. Furthermore, this formulation significantly increased paeonol's bioavailability.100 The antihypertensive drug olmesartan medoxomil (OLM) has significant first pass metabolism resulted in limited oral bioavailability. Albash et al.8 developed TE to improve OLM's transdermal distribution, utilizing a range of SAs and various phospholipid-to-SA ratios to overcome this. The acquired outcomes proved eminence in the dermato kinetic analysis over a drug suspension and supported the viability of using TEs as a viable carrier for the OLM transdermal distribution.
5.1.4 Cancer
Brucine and strychnine are the major chemical constituents of Nux vomica, traditional Chinese medicine for treating liver cancer, and have limited use because of severe side effects. A transdermal formulation of brucine-strychnine TEs was created by Wang et al.,37 demonstrating in vitro inhibition of the hepatoma cell's proliferation and slow drug release. Therefore, this study offered new perspectives on brucine and strychnine's evolution and clinical use. Rady et al.39 examined the dermal administration of ferrous chlorophyllin, a high molecular weight hydrophilic photosensitizer, via TEs for melanoma treatment by Photodynamic therapy (PDT). The TEs of approximately 500 nm demonstrated deep tissue localization of the medication in the skin and provided effective treatment for resistant melanoma by PDT. To explore dermal delivery and develop a better treatment strategy for skin cancer, Adnan et al.47 created an apigenin-loaded transethosomal gel. The TEs performed sustained release of the medication and improved drug permeability through the skin.
5.1.5 Aging and androgenic alopecia (AGA)
To improve the cutaneous delivery of niacinamide and Cycloastragenol, Basto et al.5 and Wang et al.7 designed encapsulated vesicles. TEs using jojoba oil, dicetylphosphate, span 40, and tween 80 as the penetration enhancer exhibited more controlled and better drug delivery across the skin barrier. These vesicular systems can be utilized to create drugs with antiaging cosmetics to improve skin protection. Patients choose topical therapy over other treatment options for AGA because it is simple to administer topical dosage forms and has fewer adverse effects due to localized application. Therefore, Zaafarany et al.4 attempted to load Coenzyme Q10 (CoQ10) in various phospholipidic vesicular formulations. Results revealed that TEs were the preferred carrier for CoQ10, as they showed the highest deposition percentage in the different skin layers. Furthermore, For maximum effectiveness and minimal adverse effects, Allam et al.106 encapsulated minoxidil in TEs. Oleic acid-containing TEs showed the maximum skin permeability, the most considerable deposition in deeper skin layers, and the minimum in SC.
5.2 Ophthalmic drug delivery
Transethosomes have been shown to improve the bioavailability and efficacy of drugs used to treat various ocular diseases, including glaucoma, macular degeneration, and dry eye syndrome. TEs demonstrated the enhanced drug permeation through the corneal epithelium and improve drug retention within ocular tissues. Some studies have also reported that TEs can reduce the frequency of drug administration, thereby improving patient compliance and reducing potential side effects associated with frequent dosing.107 To improve the drug's ocular permeation, short elimination half-life, and quick eye clearance in the treatment of fungal infections, Ahmed et al.23 developed Ketoconazole transethosomal vesicles, which have the ability to permeate deeply into the posterior eye. Ciprofloxacin-loaded TEs may show prolonged antibacterial activity by improved ocular bioavailability, which may enhance the therapeutic outcomes in Bacterial endophthalmitis treatment.30
5.3 Transvaginal drug delivery
Progesterone (PRG) is a female steroidal hormone that can be used in the treatment of polycystic ovarian syndrome, but it suffers from poor solubility and bioavailability issues due to first-pass metabolism through the oral route. Salem et al.1 formulated PRG-loaded transethosomal mucoadhesive gel for transvaginal drug delivery. The results showed a substantial rise in serum PRG levels, degree of echogenicity, endometrial thickness, and pregnancy rate. Hence the transvaginal gel might be a potential formulation for the delivery of PRG to increase the pregnancy rate in polycystic ovarian syndrome.
5.4 Pulmonary drug delivery
Transethosomes loaded with vinpocetine and piracetam, as well as various vesicular formulations (microemulsion, transfersomes, and composite system), were examined by Nasr et al.3 for their ability to treat scopolamine-induced memory impairment. The TEs showed improved stability and more EE. However, the anti-inflammatory and antiapoptotic properties of the nanocomposite formulation outperformed those of the microemulsion and transethosomal formulations. Due to their small particle size and improved permeation capability over the nasal mucosa, the three nanoformulations (TEs, microemulsion, and nanocomposites) were therapeutically efficacious. Kabil et al.24 created rolapitant-loaded lipid nanovesicles, a novel molecule that could inhibit lung cancer cells from proliferation and deliver a drug nebulization approach. The in vivo studies demonstrated that lipid vesicles are deposited in the lungs through systemic absorption to metastatic lung cancer. Additionally, it showed that TE formulations are safe on lung tissues. Since rolapitant has not yet undergone substantial pharmacological and clinical research, the data obtained offer a chance to examine the potentiality of rolapitant as a new therapy for lung cancer.
5.5 Photodynamic therapy
Photodynamic therapy (PDT) is a localized therapeutic method, since cytotoxicity occurs when light triggers photosensitizer (PS) at the site. It is highly effective in treating dermatological conditions. Accordingly, Rady et al.39 proposed the dermal application of ferrous chlorophyllin, a high molecular weight hydrophilic PS via TEs, for melanoma treatment by PDT. The study demonstrated the effectiveness of nanovesicles in PS delivery for resistant melanoma by providing effective treatment with deep tissue localization of the medicine into the skin. Mahmoud et al.26 examined PDT-mediated 8 methoxy psoralen TEs in the management of vitiligo. The improved TEs displayed maximal skin permeability and high deformability. Samy et al.108 looked into the effectiveness and safety of PDT with Indocyanine-loaded nanocarriers for cutaneous warts. It was proved to be an effective therapeutic modality for wart treatment.
6 CHALLENGES AND STRATEGIES TO OVERCOME
Transethosomes, a cutting-edge drug delivery system, have revolutionized drug delivery by improving the permeation of drugs through biological barrier but the transition of TEs from laboratory-scale production to large-scale manufacturing poses significant challenges that need to be addressed.73 The primary challenge occurs in the selection of raw materials, which plays a critical role in TE manufacturing. Selecting high-quality phospholipids, edge activators, and ethanol is crucial to ensure the efficacy, stability, and reproducibility of the product. To conquer this challenge, quality control measures that can ensure the consistent availability and quality of raw materials can be implemented.109 In addition to that, the large-scale production of TEs can be complex and costly, making it difficult to translate laboratory-scale formulations to commercial manufacturing. Ensuring reproducibility and cost-effectiveness is crucial to facilitate their widespread use in pharmaceutical applications. Developing robust manufacturing processes that maintain the integrity and quality of TEs at larger scales is essential.110 Efficient mixing, temperature control, and homogenization techniques are crucial to ensure the reproducibility and uniformity of TE vesicles. Optimization of TE manufacturing processes through process analytical techniques (PAT) and automation can improve scalability, reduce production costs, and enhance reproducibility.111
Furthermore, TEs are susceptible to degradation and instability during storage. Factors such as temperature, light exposure, and pH can affect their physicochemical properties, leading to changes in drug encapsulation efficiency and vesicle integrity.109 Long-term stability studies are necessary to evaluate the shelf-life of TE formulations and optimize storage conditions. Strategies such as the addition of antioxidants, the use of lyophilization techniques, and protective packaging can be employed to enhance stability and extend shelf-life.83 Moreover, manufacturing TEs for commercial use requires compliance with regulatory standards and quality control guidelines. Developing appropriate quality control methods and implementing good manufacturing practices (GMP) are essential for ensuring batch-to-batch consistency, product safety, and efficacy.112 By employing these strategies, the manufacturing of TEs can be optimized, leading to improved efficiency, product quality, and regulatory compliance.
7 CONCLUSION
The transethosomes have drawn much attention as delivery systems for a variety of bioactive compounds. In addition to the transdermal route, researchers have explored ocular, transvaginal, and intranasal approaches to deliver drugs for a variety of therapeutic applications and proved TEs as potential carriers for drug delivery. A TE consists of a phospholipid, a high content of ethanol, an edge activator, and an encapsulated drug molecule, allowing it to penetrate deeper layers of the skin. Profound investigations were performed to achieve site-specific action and reduce drug toxicity, and it was reported that surface functionalization and photodynamic therapy of TEs were successful. Simultaneously optimizing various parameters can control the complexity associated with the formulation and development of TEs to achieve a safe and effective final product. Although most drug-loaded TEs exhibit deeper skin penetration, sustained drug release, and targeted delivery, more research is needed to address the stability issues. To conclude, through multiple routes, TEs can contribute to various therapeutic applications with site-specific action. Hence TEs shall exhibit a better clinical outcome with fewer side effects by lowering toxicity levels.
AUTHOR CONTRIBUTIONS
Pavani Chowdary: Conceptualization (equal); formal analysis (equal); investigation (equal); resources (equal); writing—original draft (equal); writing—review & editing (equal). Ananya Padmakumar: Conceptualization (supporting); formal analysis (supporting); resources (equal); writing—review & editing (supporting). Aravind Kumar Rengan: Conceptualization (equal); formal analysis (equal); resources (equal); supervision (equal); writing—review & editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge funding from the Department of Science & Technology (DST-SERB CRG/2020/005069; SPR/2022/230), Department of Biotechnology (DBT) (ICMR/35/1/2020-IA/NANO/BMS, ICMR -CoE), and Ministry of Education (MoE), Govt. of India (IITH-SOCH3, MoE/STARS/2023-0640). Author PC wishes to extend her gratitude to IITH and B V Raju Institute of Technology Narsapur, Telangana, for supplementary support. Author AP appreciates and thanks IITH for the financial support of her fellowship. All the graphical illustrations were prepared using https://biorender.com (BioRender).
CONFLICT OF INTEREST STATEMENT
All the authors declare that there is no conflict of interest.
ETHICS STATEMENT
Animal experiments are not involved in this article.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




