Melatonin: Avenues in cancer therapy and its nanotechnological advancements
Abstract
Cancer is one of the most prevailing causes of mortality worldwide. Standard therapies for cancer patients include surgical intervention, chemotherapy, and radiotherapy. Nevertheless, chemoresistance and toxicity are clinical implications of advanced disease. New treatments are obliged to overcome these challenges. According to clinical investigations, melatonin (MLT) has the potential to prevent and cure cancer. It is nontoxic and has a plethora of anticancer properties including apoptosis, antiangiogenic, antiproliferative, and metastasis-inhibitory mechanisms. It enhances the therapeutic sensitivity of malignancies when coupled with conventional medications. The investigations that demonstrate how MLT possesses antitumor characteristics are reviewed in this manuscript. We provided an overview of recent research on the etiology, factors associated, therapeutic property of MLT on various cancer types. We discussed the clinical implications of MLT alone or in conjunction with chemotherapeutic drugs or radiation therapy. Furthermore, we have addressed the mechanisms of its anticancer activities against several types of cancers along with research findings of various investigations done by researchers and advancements in the field of nanotechnology for efficient delivery of MLT. The recent clinical investigations of MLT in various cancer types are reported in this review.
1 INTRODUCTION
Nearly 10 million people die from cancer globally in 2020, making it the major cause of mortality worldwide.1 Breast cancer (2.26 million cases), colon and rectal cancer (1.93 million cases), gastric cancer (1.09 million cases), lung cancer (2.21 million cases), nonmelanoma skin cancer (1.20 million cases), and prostate cancer (1.41 million cases) were the most prevalent types of cancer in 2020 global statistics. Whereas, breast cancer (685,000), colon and rectal cancer (916,000), liver cancer (830,000), lung cancer (1.80 million), and gastric cancer (769,000) deaths were observed worldwide.2 However, the mortality rate is influenced by the level of economic growth along with the interlinked lifestyle and social conditions in the respective countries.3 The incidence of cancer has risen by 0.6% annually on aggregate since 1975, becoming the second most predominant cause of mortality in children between the ages of 1–14 years.
Cancer treatments vary depending on the type of cancer, through clinical modalities, such as surgery, radiotherapy, stem cell therapy, chemotherapy, immunotherapy, hormonal, and targeted medications. Additionally, several products have demonstrated promising results in cancer prevention and treatment.4 Since chemotherapy is noninvasive, it is often preferred over surgery. As a result, a wide variety of chemotherapeutic drugs have been designed for cancer chemotherapy, including cisplatin,5 paclitaxel, docetaxel, and doxorubicin. However, clinical investigations using the aforementioned chemotherapeutic drugs have not always been successful. Several crucial considerations about treatment failure arise. More crucially, the use of chemotherapeutic medications frequently causes the establishment of the “drug-resistance” phenomena. For instance, Doxorubicin (DOX) is a well-known chemotherapy drug that, by blocking topoisomerase II activation and causing damage to DNA, can suppress cancer cell proliferation and cause apoptosis. DOX's activity inhibits the development of the cell cycle and mitosis. However, DOX resistance develops in cancer cells due to repeated application. Cancer cells may have DOX resistance due to chromosomal and genetic motives. Via the stimulation of pathways like NF-B, PI3K/Akt, Wnt, and FOXC2, long noncoding RNAs can cause DOX sensitivity. In accordance with the stress imposed by DOX, which mediates resistance, lncRNAs can trigger the immune system's defense mechanism, autophagy.6 Another study reported that due to the emergence of drug resistance, the clinical application of paclitaxel (PTX) in cancer therapy is constrained. Notably, PTX treatment induces epithelial-to-mesenchymal transition, which causes cancer metastasis and resistance to chemotherapy. Many methods can be used to reduce PTX resistance, including the development of nanomaterials like nab-PTX, antitumor substances like luteolin, and genetic tools. Resistance to docetaxel (DTX) experiences a similar phenomenon.7 The widespread evolution of drug resistance is a key driver toward developing nanomaterials and nanoformulations in cancer treatment. A study reported that Hyaluronic acid (HA) has been widely used in the synthesis of nanoparticles as it interacts with CD44, which is expressed on the surface of cancer cells helps in the uptake of nanoparticles by receptor-mediated endocytosis. To improve the delivery of chemo drugs to tumor sites, HA has been added to a variety of nanostructures, including lipid nanocarriers, carbon nanoparticles, and polymeric nanomaterials.8
The following are the seven distinctive characteristics of cancer cells: independence of growth factors, lack of response to aging signals, escaping the apoptotic cycle, the indefinite potential for replication, maintained angiogenic persistency, tissue infiltration and metastasis, and genome destabilization.9 Malignant tumors can be differentiated from benign (nonmalignant) tumors by their rapid development, increased metabolic activity, highly invasive growth, metastatic property, and penetration of arterial or lymphatic systems. Chromosome abnormalities can be seen in benign tumors.10 The best approach to comprehending the biological importance of tumorigenesis appears to be the categorization of tumors according to the origin of carcinoma cells. It acquires awareness of new opportunities for early detection, prevention, and management, and to develop novel medications for cancer therapy.11
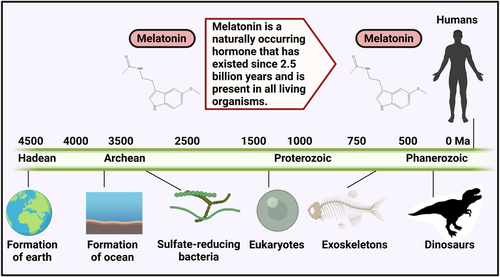
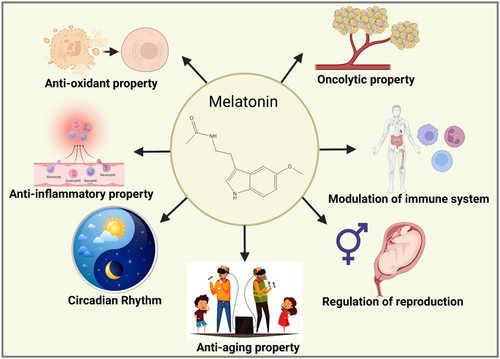
The pineal gland synthesizes and secretes melatonin (MLT). It is a neuroendocrine active molecule that is chemically constituted of N-acetyl-5-methoxytryptamine which is remarkably preserved in prokaryotes and eukaryotes (Figure 1).12, 13 It modulates the incidence, progression, and therapy of cancer by regulating several physiological processes, especially circadian rhythm (sleep-wake cycle), and exerting immunomodulatory, anti-inflammatory, anticarcinogenic, antiaging, and endocrine-regulatory properties (Figure 2).14 MLT concentration is high at dawn and low at noon which can be influenced by environmental factors.15, 16 The absence of daylight merely allows serum concentrations of MLT to increase; it does not directly promote the synthesis of this neurohormone.17 Cancer patients having irregular circadian rhythms gradually develop MLT abnormalities. It functions as a cell regulator in immunomodulatory, antioxidant activities, and hematopoiesis which is essential for human metabolism and pathophysiology.18
Epidemiological investigations revealed that MLT has been associated with significant oncostatic activities on cancers of various origins and levels. Additionally, research using both in vitro and in vivo studies has suggested that MLT could be able to prevent the growth of tumors.19 It also shows oncolytic characteristics via receptor-dependent and receptor-independent pathways. By receptor-independent pathway, it modulates antioxidant properties, cancer cell apoptosis, tumor metabolic activity, preventing angiogenesis and tumor cell migration, and controls circadian rhythm disturbances.20 According to findings, MLT is crucial for tumor development. Low levels of MLT accelerate tumor development. Intriguingly, the individuals with pulmonary and colorectal malignancies who received MLT treatment demonstrated cancer remission and better lifestyles.21 It may be effective as an anticancer supplement by minimizing the adverse effects and bolstering the therapeutic efficacy of cancer treatment, furthermore by possibly inhibiting tumorigenesis. Many cancers, namely lung, prostate, breast, gastric, and colorectal cancers, have been associated with the application of MLT as a cancer therapy.19
Due to its chemical characteristics, MLT has a restricted ability to penetrate mucosal and dermal barriers and has a shorter half-life, and is rapidly eliminated from blood circulation.22 Moreover, some unintended reactions may be noticed following the administration of MLT since it can influence receptors on biological membranes, in the nucleus of cells, and can also behave as an antioxidant molecule. To enhance the effectiveness of therapeutic agents on the targeted tissues and to reduce possible adverse reactions in peripheral tissues, these agents must be administered to the designated area.23 Additionally, this delivery mode must be simple to manufacture on an industrial scale, affordable to purchase, safe and biodegradable, and should be devoid of unintended reactions with the substance it is designed to transport.24 To overcome the drawbacks of MLT for clinical applications in various domains, researchers have been able to experiment with it, especially with the advancement of novel biomaterials and drug delivery systems. The use of nanoparticles to deliver diverse medications is one class of novel delivery strategies that have received much attention.25 Nanoparticles mainly consist of biodegradable substances, polymeric compounds, lipids, metallic elements, and other substances and are typically under 100 nanometers in size. Additionally, nanoparticles possess the majority of the abovementioned features that are required for new delivery strategies.26 Due to this, MLT delivery using nanoparticles has been employed in several investigations.
This review aims to provide an overview of recent research on the etiology, factors associated, therapeutic property, clinical implications, and anticarcinogenic mechanisms of MLT in cancer types. It also discusses how it interacts with chemotherapeutic drugs and radiation therapy. The present article focuses on cumulatively demonstrating the research findings of various investigations done by researchers in the field of novel nanoformulations developed for the delivery of MLT and its role in cancer therapy.
2 BROAD ATTRIBUTES OF MLT HORMONE
MLT is a biologically active amine that is produced by both animals and plants. It is synthesized sequentially. L-tryptophan undergoes hydroxylation to produce hydroxyl-tryptophan, which is subsequently decarboxylated to yield serotonin, which is then metabolized by N-acetyl transferase and hydroxyindole O-methyl transferase to form MLT.27 It is being investigated for its potential use as a medication for a variety of ailments. Because of this, it is essential to understand how this substance's pharmacokinetics takes place. The mentioned aspects of MLT were proposed by a comprehensive and detailed systemic assessment by Groth Harpsøe et al.: a dosage variability between 0.3 and 100 mg can be administered through oral or by an intravenous route, a C max varying between 72.1 and 101.163 pg/mL, a T max vary between 28 and 126 min and a systemic bioavailability of 9%–33%.28 Even though it offers a wide range of applications, its utilization is now confined due to its unfavorable pharmacokinetic properties. Developing innovative delivery systems is one way to increase the future usage of MLT in modern medicine.29
3 ANTICARCINOGENIC PROPERTIES OF MLT
Probably the significant application for MLT administration is to use it as an adjuvant molecule for cancer therapy. There is strong proof that it reduces adverse effects while improving the therapeutic benefits of chemotherapy and radiation, as we will discuss in this section. MLT is regarded as an incredible agent for research and could be employed to prevent and treat a variety of cancers, notably breast, prostate, gastric, and colorectal cancers.19
At normal dose levels ranging from 10 to 50 mg/day, it can treat a variety of cancers in vivo. Breast cancer, metastatic renal melanoma, non-small-cell lung carcinoma (NSCLC), hepatocellular malignancies, and brain tumors are just a few of them. For instance, an investigation of 14 individuals with metastatic, tamoxifen-resistant carcinoma revealed that 20 mg/day of MLT could be therapeutic. Where the malignancy was predicted to advance rapidly, a partial reaction was observed in 28% of those individuals, and those who reacted had considerably reduced serum levels of the tumor growth factor IGF-1 (p < 0.001).30 The responsiveness to NSCLC, renowned for poorly responding to standard treatment, is of particular interest. Individuals who had previously failed the initial course of cisplatin treatment in a prospective study with 63 patients in Stage 4, were treated with either MLT or supportive care alone. The patients treated with MLT had a mean survival time of 7.9 months which is substantially longer than the control groups (4.1 months) (p < 0.05).31 There are quite a few treatments and a short median survival time of 6 months available for patients whose cancer has migrated to the brain. Fifty cancer patients who had documented brain metastasis and had all undergone preliminary treatments were included in a randomized study. They were categorized into two groups for medications that were either provided alone or in combination with MLT. The reported metrics (period without brain tumor development, mean survival rate, and 1-year survival) were all markedly high in the MLT group in comparison to the control.32 Antiangiogenic properties of MLT are believed to be primarily responsible for its anticarcinogenic effects. Antiangiogenic medications are occasionally prescribed simultaneously with chemotherapy to increase the effectiveness of the medication. The properties of MLT in combination with chemotherapeutic agents like docetaxel and vinorelbine were investigated by González et al. They observed that it increased the effects of the medications on the angiogenesis-related mechanisms (cell multiplication, migration capability, and blood vessel development).33
The size of the tumors in the animals was diminished by improved pineal gland activity or exogenous MLT administration. It also revealed that rodents that received MLT had better tumor suppression in the induced studies.34 Researchers have thoroughly investigated the possible anticancer principle of MLT, particularly in hormone-dependent malignancies. Breast cancer is one of the most prevalent malignancies in females depending on hormones. Approximately 70% of occurrences of breast cancer in its early stages are caused by hormones as reported by the American Cancer Society. Its influence on chemo-sensitization is also remarkable. According to Pariente et al., MLT has been found to potentiate the effects of 5-fluorouracil in individuals with colorectal cancer.35 It exhibits antitumoral effects in the prevention of colon cancer in individuals suffering from chronic irritable bowel disease.36 Medical professionals are considering MLT as a possible supplementary medicament for cancer therapy, either alone or in combination with interleukin-2. Its supplementation in this way has been correlated with enhanced outcomes for patients with advanced tumors. Furthermore, it has been demonstrated that MLT can improve the tolerability of chemotherapy and minimize its fatal side effects.18
4 MECHANISM OF ACTION OF MLT AGAINST CANCER
MLT can work by numerous molecular mechanisms among which the most effective mechanism is the stimulation of G protein-coupled receptors which are membrane specific in nature. MLT receptors ML1 is having high-affinity and ML2 is having low-affinity properties which are currently referred to as MT1 and MT2 respectively.37, 38 These receptors have distinctive molecular assemblies, pharmacological properties, and chromosomal arrangements.39 They are of 39–40 kDa molecular weight having amino acids of 350 and 362, respectively. These receptors couple to α, β, and γ subunits of the Gi protein and involves in the transmission of the signals to cells.40 The adenylate cyclase pathway was inhibited by the stimulation of MT1 receptors whereas MT2 receptors activation causes phospho-inositides hydrolysis in the target tissues.41 MT1 receptors are extensively present in the anterior pituitary specifically pars tuberalis and in the hypothalamic suprachiasmatic nuclei. They are also found in the cortex region, substantia nigra, thalamus, hippocampus, cerebellum of the brain, accumbens nucleus, amygdala, cornea, and retina of the eye.42 MT2 receptors are extensively found in the retina followed by the paraventricular nucleus, hippocampus, cortical region of the brain, and cerebellum of the brain.43 MLT receptors are endowed in the retina, brain, pars tuberalis, suprachiasmatic nucleus of the hypothalamus, ovaries, blood vessels, kidneys, adipocytes, pancreas, and immune cells 44 (Figure 3). Furthermore, T and B lymphocytes were also traced for the presence of these receptors.45 According to Liu et al., there are significant species variations in pharmacological aspects of MLT receptors based on affinity assessments that are exhibiting species-dependent modulations in in vivo receptor specificity.46 MLT in combination with chemotherapy treatments allows the use of therapeutic doses of those medications at low concentrations thereby preventing the unwanted harmful effects of both chemotherapy and radiation treatment. It effectively interrupts the signaling pathways of tumors and also operates as a metastasis suppressor by preventing the replication and irregular growth of cancer cells.47

Anticancer activity of MLT is regulated by several mechanisms of which membrane receptor-independent and -dependent pathways are also included. The mechanisms are represented in the following Figure 4. Typically, angiogenic parameters are triggered whereas antiangiogenic parameters are suppressed by tumor cells. One of the essential components of angiogenesis is the vascular endothelial growth factor (VEGF). In practice, VEGF blood levels and tumor vasculature development was disrupted in MLT-treated mice.48 Patients with cancer metastasis who received MLT reported decreased serum VEGF levels, and also pancreatic cancers showed reduced VEGF protein and mRNA concentration.49 Through its direct anticancer property, it can enhance cellular turnover and it helps in the substitution of cancer cells with normal cells by activating caspase-dependent apoptosis. It also prevents healthy cells from converting into neoplastic cells.50 The activation of p21 and p53 suppressor genes showed an oncostatic pathway that limits tumor cell proliferation. Physiological levels of MLT decreased the viability of cancer cells by proapoptotic mechanisms.51 The pro-oxidant condition correlated to the cytotoxic activity or an antioxidant environment that stimulates an antiproliferative activity is required for tumor demise. It showed complex antineoplastic activities, which depend on the therapeutic levels of MLT and the oxidant and antioxidant state, in addition to receptor-linked cytotoxic activity. These mechanisms include the initiation of apoptosis and the downregulation of cell differentiation and proliferation.52
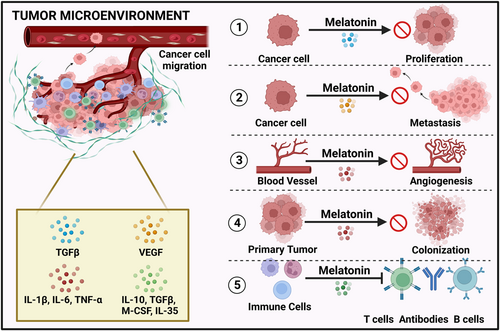
In human breast carcinoma, the MLT in combination with arsenic trioxide selectively destroyed tumor cells by inducing apoptosis, enhancing intracellular ROS production, upregulating Redd1 transcription, and inducing the c-JUN-N-terminal kinase (p38/JNK) signaling pathway.53 Similar to the above study, in the breast cancer treatment, MLT in combination with puromycin promoted cell apoptosis in MDA-MB 231 (epithelial cell line) by suppressing procaspase 3, Bcl-xL, XPO1, and IPO7 and 45S pre-rRNA genes.54 The activation of the MT1 and MT2 receptors by signal transduction pathways, antioxidant properties, suppressing carcinogenic enzymes, reversing Warburg effect, telomerase-suppressing response, and initiation of epigenetic modifications are responsible for the anticarcinogenic activity of MLT.14 Table 1 displays the effects of MLT on various types of malignancies in in vitro and in vivo studies.
| Cancer type | Study performed | Objective | Significance | Reference |
|---|---|---|---|---|
| Breast | In vitro | 1 mM of MLT was exposed to breast cancer cell lines | Metastasis inhibition | Gonçalves et al.55 |
| Breast | In vitro | 10–9 M of MLT was exposed to MCF-7 cells | Inhibition of cancer cell invasion | Mao et al.56 |
| Breast | In vitro | Doxorubicin and MLT were given in combination to MCF-7 cells | Synergic action on mitochondrial oxidative stress and apoptosis | Koşar et al.57 |
| Breast | In vitro | 1 nM of MLT was exposed to estrogen-positive cancer cell lines | Inhibitory activity | Cos et al.58 |
| Breast | In vitro | 1 nM of MLT was given to MCF-7 cells | Reduction in the amount of ERα | Molis et al.59 |
| Breast | In vitro | Troglitazone and MLT (1 mM) | Induces apoptosis | Korkmaz et al.60 |
| Breast | In vivo | 10 mg/kg of MLT and doxorubicin were administered to animals for 15 days | Increases survival rate by causing tumor cell apoptosis | Mao et al.56 |
| Breast | In vivo | 500 mg/day of MLT in combination with DMBA and resveratrol | Reduces tumor incidence rate | Kisková et al.61 |
| Breast | In vivo | 40 mg/kg of MLT was administered to athymic nude mice | Inhibition of angiogenesis, cell division, and reduction of tumor size | Jardim-Perassi et al.62 |
| Breast | In vivo | 33 mg/L of MLT was administered to BALB/C mice | Tumor size reduction | Schwimmer et al.63 |
| Cervical | In vivo | 500 pM of MLT was administered to nude rats | Suppression of tumor growth, metabolism, and proliferation | Dauchy et al.64 |
| Lung | Advanced NSCLC | 20 mg of MLT/day + 20 mg/m2 of cisplatin/day + 100 mg/m2 of etoposide/day | Inhibition of MLT and chemotherapy | Lissoni et al.65 |
| Lung | Metastatic NSCLC | 20 mg of MLT/day + 20 mg/m2 of cisplatin/day + 100 mg/m2 of etoposide/day | Inhibition of MLT and chemotherapy | Lissoni et al.66 |
| Lung | Metastatic NSCLC | 20 mg of MLT/day + cisplatin + etoposide | Tumor regression and improved survival rate | Lissoni67 |
| Lung | Untreatable metastatic NSCLC | 20 mg of MLT/day + (3 million IU/day) for 5 days/4 week, of IL-2 + intensive care | Tumor regression | Lissoni et al.68 |
| Lung | Advanced lung adenocarcinoma | 20 mg of MLT/day + 1–3 mg of somatostatin/day + 5 mL of retinoids + 0.3 mg of vitamin D/day + 2.5 mg of bromocriptine/day + 150 mg of cyclophosphamide/day | Increased survival rate | Norsa and Martino69 |
| Lung | A549 and H1299 cells | 1 mM of MLT + 20–200 μM of berberine | Induces apoptosis | Lu et al.70 |
| Lung | Lewis lung carcinoma | 1 mg of MLT/kg + 40, 160 mg of cyclophosphamide/kg + 20, 40 mg of etoposide/kg | Rescues progenitor cells from apoptosis | Maestroni et al.71 |
| Lung | A549 cells | 1 mM of MLT + 1 µg/mL of doxorubicin | Specific to cancer cells | Fic et al.72 |
| Lung | A549 cells and lymphocytes | 50 μM of MLT + 7.5, 15, 30, and 60 μM of irinotecan | Induced apoptosis | Kontek and Nowicka73 |
| Lung | SK-LU-1 cell line | 1, 2 mM of MLT + 10–200 μM of cisplatin (48 h in culture) | Reduces the IC50 concentration | Plaimee et al.74 |
| Lung | 1.25 mg of MLT/kg/night treatment was given after pinealectomy (2 months) | Subcutaneous administration into mice | Metastasis inhibition | Mocchegiani et al.75 |
| Ovarian | In vitro | 400, 600, and 800 μM of MLT were exposed to ovarian cancer cell lines | Downregulated CDK2 and CDK4, angiogenesis inhibition | Shen et al.76 |
| Ovarian | In vivo | 200 μg/100 g of MLT was administered to rats | Tumor size reduction, regulation of apoptosis, and enhancement of DNA fragmentation | Chuffa et al.77 |
| Prostate | In vitro | 1 nM of MLT was exposed to prostate cancer cells | Prostatic tumor cell line (LNCaP) growth was attenuated | Lupowitz and Zisapel78 |
| Prostate | In vivo | 150 mg of MLT/100 g was given to rats for 4 weeks | Ventral prostate in castrated-testosterone-administered rats showed loss of weight | Debeljuk et al.79 |
| Prostate | In vivo | 4 nM of MLT was administered to mice | Angiogenesis and cell proliferation inhibition | Paroni et al.80 |
| Prostate | In vivo | 10 and 20 mg/L of MLT were administered to mice | Inhibition of tumorigenesis by hampering SIRT1 activity | Jung-Hynes et al.81 |
5 FUNCTION AND THERAPEUTIC APPLICATIONS OF MLT
It has been proved that MLT is involved in the circadian rhythm (sleep-wake cycle) and biological regulations. The regulatory functions of the anterior and posterior pituitary glands are influenced by this hormone.82 Premenopausal women on contraceptives and postmenopausal women have shown increased MLT secretion at night times. It helps in the regulation of jet lag, correction of circadian rhythm, prevents sleep disorders, exhibits potent antioxidant properties, modulation of the immune system, treatment of rheumatoid arthritis, inhibition of neurodegenerative disorders, and in cancer therapy. It also helps in the preservation of DNA veracity.83 MLT can be used as a potential biomarker tool in various fields of research including circadian dysregulation for epidemiological investigations on the detrimental effects of human circadian disturbances,84 cancer theranostics especially in breast,85 ovarian,86 oral squamous cell carcinoma,87 prostate and hepatocellular carcinoma (HCC).88 MLT deficiency could be adopted as a biomarker in healthcare assessment to assist in finding individuals who might favor prolonged monitoring and/or behavioral or pharmacological approaches to disease prevention. MLT production diminishes with age; therefore, a constitutive or untimely deficit of MLT production could indicate a biological aging phenomenon that further influences cancer incidence.89 DNA oxidation is a known mechanism for the development of mutations that play an integral part in carcinogenesis90 and MLT has been demonstrated to be a potent antioxidant (both in vitro and in vivo).91 Reduced MLT levels could facilitate a constitutive rise in radical-driven mutagenesis or render cells more vulnerable to naturally occurring or induced damage from oxidative stress. Inadequate sleep, midnight shift, or excessive exposure to light at night92 may reduce the production of MLT, and various cancers develop, more prevalently in such populations.93 Other findings demonstrated that the hormone acts as a biological oncostatic component, preventing the emergence of malignant neoplasms. It has been exploited extensively in cancer biology and the prevention of the side effects caused by combination therapies. It may eventually find its applications in clinical investigations.
5.1 Significance of MLT in breast cancer
Compared with other malignancies, breast cancer is typically diagnosed malignancy in women worldwide, in both developing as well as developed nations.3 According to Tamarkin et al.,94 women with breast cancers expressing the estrogen receptor (ER) had considerably lower levels of elevated nocturnal MLT plasma levels, confirming that the relationship between ER concentrations and maximum MLT levels is inverse. In addition to altering the structure of estrogen-responsive MCF-7 tumorigenesis, physiological quantities of MLT also suppress the proliferation of tumor cells compared with subphysiological/supraphysiological concentrations of MLT, which are inefficient at reducing the proliferation of MCF-7 cells. It is also interesting to note that the development of MCF-7 cells is not inhibited by precursors and by-products of MLT like N-acetyl serotonin, serotonin, and 6-hydroxy MLT.95 By prolonging the passage of MCF-7 cells into the mitotic cell cycle and ultimately inducing differentiation, it revealed a cell-cycle-specific approach to anticancer properties in MCF-7 cells.96 The ER-positive MCF-7 cell lines responded to consecutive administration of retinoic acid and MLT by completely blocking cellular proliferation and reducing the percentage of cells by induction of apoptosis, which causes cell death by reducing Bcl-2 signaling, enhancing Bax expression, and modifying TGF-β1 interpretation. In ER-negative BT-20 and MDA-MB-231 carcinoma cells, successive therapy exhibited no apoptotic action.97 It lowers MCF-7 cells' ability to invade, which results in a decline in cell adhesion and mobility. In vivo, tests were carried out in athymic naked mice (ovariectomized) inserted with 17 β-estradiol pellets and seeded with 5 × 106 MCF-7 cells in the posterior mammary muscle belly. The results revealed that MLT lowered tumorigenicity.98 A substantial raise in p53/MDM2 ratio and the release of apoptosis-inducing factor (AIF), instead of changes in caspase activation or cleaved-PARP concentrations, are correlated to apoptotic cell death in MLT-treated MCF-7 cells. This effect is observed initially in the incubation period in a TGF-β1-dependent fashion. The Bcl-2/Bax ratio is downregulated, while caspase-7 and caspase-9 are activated and cleaved-PARP is stimulated in the latter phase due to the prolonged incubation period of MLT. According to this study, it causes two distinct apoptotic processes in MCF-7 cells: (i) an initial response; TGF-β1 and caspase-independence, and (ii) a delayed apoptotic phase; TGF-β1 and caspase-1-dependent and likely to have activated-caspase-7 as the ultimate activator.99 In co-cultures of human umbilical vascular endothelial cells (HUVECs) and human breast carcinoma cells (MCF-7), it suppresses angiogenic pathways by reducing VEGF production. Through a downregulating effect on VEGF activity in human breast carcinoma lines, research implicated that MLT is involved in the paracrine connections between carcinogenic epithelium and proximal endothelium.100 By reducing the production of HIF-1α and VEGF-α, MLT decreased the cell sustainability of MDA-MB-231 and MCF-7 cell cultures in hypoxia. MLT therapy in hypoxic conditions decreased the stimulation of matrix metalloproteinase 9 (MMP9) enzyme, VEGF receptors, and angiogenin in cell lines (according to protein array statistics). MLT-treated MDA-MB-231 cells, on the other hand, displayed a marked decline in EGFR, VEGFR2, and revascularization. Together, research findings demonstrated that MLT may have antiangiogenic potential in hypoxic environments.101 It prevents the growth of breast carcinoma caused by bisphenol, an estrogen-like substance that contributes to the development of hormone-mediated malignancies. MLT dramatically reduced bisphenol A (BPA)-induced cell growth by inhibiting ERK and AKT phosphorylation. Additionally, it blocks the upsurge in estrogen response functionality and steroid receptor co-activator production induced by BPA.102 In both healthy cells (MCF-10A) and low-malignant mammary tumor cells (MCF-7), MLT reduced ERK phosphorylation carried out by nicotine, which in turn prevents metastasis and tumorigenesis. Additionally, it drastically suppresses fascin and calpain expression, reorganizing the cytoskeleton's overall architecture and eliminating intrusive membrane projection.103 A recent review described the MLTs' impact on miRNA regulation in both malignant and nonmalignant conditions, among other diseases. This novel and innovative targeted treatment for carcinoma were focused on miRNAs. The development of chemotherapeutic drug resistance is a hindrance to the effective treatment and prevention of resistant malignancies. By causing apoptosis, repressing angiogenesis, and decreasing expression resistance genes, ketogenic nutrition coupled with MLT prevented cisplatin- and vincristine-resistant carcinoma.104 This review examined that irregular circadian rhythms might affect many endocrine activities as well as equilibrium. Thereby, it promotes the emergence of endocrine-related malignancies such as breast cancers, as well as an immunosuppressive and pro-inflammatory phenotypes in the cancer microenvironment.
5.2 Significance of MLT in cervical cancer
In terms of prevalence and fatalities, cervical cancer stands at number four. Approximately 42 countries have cervical cancer as the leading cause of cancer-related death.3 However, because of early screening and prevention, the rate of mortality has dramatically lowered in developed nations. The human papillomavirus (HPV) is the primary cause of cervical cancer, although other extrinsic variables like different biochemical modifications, gene mutations, and epigenetic alterations can also lead to or accelerate the progression of cervical cancer.105 Radiotherapy, surgical removal, and chemotherapy are currently available treatments for cervical cancer. One hundred and thirty-eight women in an Austrian multicenter investigation of endometrial cancer had their anamnestic, histologic, and cytological risk variables analyzed. Regardless of age or menopausal history, 70 of the 138 patients had an intermittent hemorrhage, and 68 had endometrial cancer upon diagnosis. Additional research revealed a connection between endometrial cancer and MLT levels. The mean plasma MLT levels showed a six-fold variation between the two groups of healthy individuals. According to the study, declining MLT plasma concentrations could be a signal of endometrial cancer.106 In a different study, 46 women were split into three categories to examine the relationship between women with genital tract malignancies and blood MLT levels. According to the research findings, none of the three groups had significantly varied circadian MLT patterns. However, there were negligible variations in MLT production between healthy controls and advanced ovarian cancer and squamous cervical cancerous patients, and there were significantly fewer individuals with endometrial cancer of the reproductive tract than healthy control subjects. However, there were noticeable variations between invasive ovarian cancer and endometrial cancer.107 The same investigators revealed blood MLT circadian patterns in women with cervical cancer 5 years later. The first group included 31 cervical cancer patients with the condition at varied stages. The control group, which made up the second category, was composed of 14 healthy volunteers. Its concentrations were noticeably lower in cancer individuals than in healthy people. Additionally, patients with advanced-stage carcinoma had considerably lower nocturnal MLT levels than those with preinvasive cancer. The results of this study indicated that women's MLT levels are impacted by the development of cervical cancer. Additionally, the MLT level is also influenced by cancer stage.108 The vaginal and cervical regions of mice (in vitro studies) were the targets of a study to ascertain the restraining potential of MLT on 7, 12-dimethylbenz[a]anthracene (DMBA)-mediated malignancy. For 2 months, twice a week, 40 CBA mice (female) were given polyurethane sponges containing a 0.1% concentration of DMBA. A portion of the mice was subjected to fresh water containing MLT, five times per week at night, beginning on the day of the initial DMBA treatment and continuing for 4 months. The findings showed that two mice had developed benign cervical cancers while the DMBA-treated mice established malignancies in cervical and vaginal regions. Mice subjected to DMBA and MLT did not develop any cancers in their cervix or vagina. This study led to the conclusion that MLT prevents the carcinogenesis of the cervical and vaginal tissues in mice induced by DMBA.109 Additionally, in vitro research was conducted to investigate how MLT affected ME-180 human cervical carcinoma cells. Cell growth and proliferation were assessed following the cells receiving varied doses of MLT. After 48 h of therapy, MLT at a concentration of 2 mM reduced glutathione levels by 95% while having no effects at doses of 2 µM or 0.1 mM.110 Another research detailed the interaction between MLT and several chemotherapeutic drugs such as cisplatin, 5-fluorouracil, and Docetaxel. Chemotherapeutic drugs investigated, strongly caused lethal impacts in cervical cancer cells using MLT. Additionally, cisplatin- and 5-Flurouracil-challenged cells were notably affected by MLT's enhanced caspase-3 activation. Similarly, co-treatment of MLT with cisplatin greatly increased DNA fragmentation, mitochondrial apoptosis, and ROS production when compared with cisplatin treatment alone.111 HeLa cells were treated with TNF-α to determine the apoptosis in the vicinity of MLT, and cell lysis was then assessed. By activating caspase-9, decreasing mitochondrial viability, increasing ROS, decreasing ATP synthesis, and increasing cyt-c transcription in the nucleus of the cells, it caused cancer cells to perish in the presence of TNF-α. Additionally, by suppressing mitochondrial function, it improved HeLa cells' responsiveness to TNF-α-mediated cancer mortality.112 According to Wang et al., SGK-1 is a protein that supports the ability of cervical cancer cells to survive. When SGK1 is inhibited, MLT becomes more liable to act as a pro-oxidant, increasing the production of ROS and ultimately the lethality of cells.113 Chen et al. investigated the impact of MLT on the apoptosis of HeLa cells treated with cisplatin. In comparison to cisplatin treatment alone, co-treatment with MLT promotes apoptosis through caspase-9-mediated apoptosis, a decrease in mitochondrial potential, an increase in mitochondrial ROS production, and a higher expression of proapoptotic proteins.114
5.3 Significance of MLT in colorectal cancer
Good practices in cancer prevention and treatment are the rationale for the reduced mortality rates noticed in developed nations. Inappropriate food habits, being overweight, a shortage of physical exercise, and routine choices are the leading causes of colorectal cancer.115 Prominent risk factors for colon malignancy include obesity, and intake of manufactured meat and alcohol. Colorectal cancer starts as a cyst in the gastric epithelium, and predominant nodules, which are small lesions that develop without any symptoms.116 Surgery, medication, radiation, immunotherapy, and targeted therapeutics are typical colorectal cancer effective treatments. Recent research has discovered that MLT's significant anticarcinogenic, anti-inflammatory, and antioxidant characteristics can minimize the intensity of colorectal cancer. The elevated risk of colorectal cancer may be significantly attributed to changes in MLT levels, showing that MLT is essential in preventing the growth and advancement of colorectal cancer. People who work both day and night hours experience disruptions in MLT levels.117 It has been documented that MLT has a growth-inhibitory effect on CT-26 cells, a cell line originating from murine colon cancer. This research demonstrated that it slows cell cycle progression in a dose-controlled fashion, but the effect was insignificant at levels below 1 mM. The incidences of cell-cycle suppression at 1, 2, and 3 mM doses of MLT were 22%, 25%, and 47%, respectively.118 Twenty cancer subjects underwent MLT-induced anticancer treatments. For at least 2 months, MLT was given via oral route at a dosage of 20 mg/day in the evening. According to the findings, VEGF average levels dropped during treatment, significantly deviating from pretreatment levels.49 In the vicinity of luzindole, the function of MLT on viable cells was examined in 38 murine tumor cell lines. The outcomes demonstrated that it dramatically reduced cancer cell survivability in the proximity of a selective MT2 receptor antagonist. Colon 38 cell development was unaffected by the antagonist on its own. The gathered information suggests that MLT receptors are not obligatory for MLT's oncostatic effect.119 Human t-cells, A549 pulmonary cancer cells, and HT29 colorectal carcinoma cells were used to study the interaction of MLT with the mutagenic inducer irinotecan. It was proven that irinotecan causes genetic mutations in all tested cultures. Although it was ineffective in causing damage to DNA in normal healthy lymphocytes, the incorporation of MLT at doses of 50 μM with cumulative concentrations of irinotecan (7.5, 15, 30, and 60 M) enhanced DNA damage in the cancerous cells (A549 and HT29).73 In SW480 and LoVo cell lines, the synergistic actions of MLT and ursolic acid (UA) were assessed. The findings demonstrated that combination therapy improved inhibition of cell suitability and relocation as well as facilitated alterations in cellular morphological features and expanded via attenuation of cytochrome c release, initiation of caspase, and remobilization of p300/NF-κB from the nucleus to the cytosol. These results suggested that UA's antimutagenic and proapoptotic properties on colon cancer cells were enhanced by MLT.120 Elevated levels of proteins associated with cell death and the cell cycle were detected in HCT116 adenocarcinoma cells after treatment with 10 μm of MLT. MLT at therapeutic doses dramatically induced apoptosis while suppressing cell progression in a dose-dependent approach. By the Na+/Ca+2 transporter and IP3 receptor, MLT may promote apoptosis in two distinct cancer cell lines, A2780 and DLD1 originating from colorectal adenocarcinoma. The findings revealed that a fundamental mechanism behind the anticarcinogenic action of MLT is the unique selectivity of Ca+2 channels in cancer and normal cell lines.121 A study was conducted to understand the amyloid protein-Oct4 axis in colonic CSCs from a molecular perspective. The study focused on PrPC and Oct4 expression in samples from colorectal adenocarcinoma patients and discovered a strong interlink between Oct4 and PrPC expression and metastasis. Surprisingly, the stem cell biomarkers were suppressed by inactivating PrPC upon treatment with 5-FU and MLT combined.122
5.4 Significance of MLT in liver cancer
In most geographical zones, males are up to three times more susceptible than women to developing liver cancer, and the estimated incidence is twice as high amongst men in affluent nations.3 Chronic hepatitis B or C infection, aflatoxin contamination, diet, excessive alcohol consumption, and overweight are the primary risk factors for HCC. Hepatitis B vaccination is one of the major liver cancer hindrance strategies. HEPA 1-6 murine cell lines were dose-dependently suppressed by co-incubating MLT at various concentrations using tamoxifen and ethanol, indicating a higher level of inhibitory activity than ethanol alone.123 In an investigation, MLT was exposed to HepG2 hepatocarcinoma cell lines at specific doses over 2, 4, 6, 8, and 10 days. In regards, it upregulated the activity of p38, resulting in apoptosis, which was accompanied by enhanced caspase-3 effect, poly(ADP-ribose) protein denaturation, the release of cytochrome c, enhanced caspase-9 effect, and concurrent stimulation of JNK 1, 2 and 3. The proteins p53 and p21 expression considerably improved concurrently with the lowered cell growth and modifications in the cell cycle.124 HepG2 was subjected to MLT under hypoxia to examine the antiangiogenic activity of MLT. A decline in HIF-1α protein production, nuclear distribution, and transcriptional activation are linked at the pharmacological level, which also lowers intra- and extracellular VEGF and inhibits HUVEC tube development under hypoxic conditions. HIF-1α and STAT3 interfere with the gene transcription of VEGF, thereby causing MLT to inhibit angiogenesis in HepG2 cells.125 In a recent paper, the sorafenib responsiveness of HuH7, HepG2, and Hep3B colonies was examined. HepG2 or HuH7 cell viability was inhibited by sorafenib at 1 μmol/L, and Hep3B cell viability was suppressed by sorafenib at 2.5 μmol/L. But when MLT and sorafenib were administered concurrently, HepG2 and HuH7 cells experienced a synergistic lethal impact, and Hep3B displayed susceptibility that had negligible impact when given individually. ROS generation and mitochondrial depolarization increased as a consequence of the additive interactions of MLT and sorafenib, which is a key element in the activation of mitophagy.126 Another investigation eventually revealed that, in comparison to a monotherapy, MLT and sorafenib in combination, dramatically reduced HuH-7 cell lines clonogenicity. Additionally, sorafenib-induced cell death, which is connected to the stimulation of caspase-3 as well as the JNK/c-Jun pathways, was enhanced synergistically by MLT.127 HuH7 and HepG2 cells' abilities to proliferate, migrate, and invade were severely hindered by MLT, while the cellular transcription of let7i-3p miRNA was significantly increased. These results demonstrated that MLT modulates let7i-3p-regulated RAF1 repression to prevent the progression of HCC.128 According to a recent analysis, MLT consumption improved mitochondrial and hepatic activities in the nonalcoholic fatty liver by blocking mitochondrial fission and activating autophagy by inhibiting the p53 pathway. This in turn reduced the disruption of the liver's functionality and structure of the mitochondria.129 It can either be administered alone or in association with other drugs to treat the premalignant cancers cholangiocarcinoma (CCA) and HCC to avoid the progression of the tumor.130 According to several investigations, MLT is crucial for a variety of operations, including endocrine, neurological, immunological, and antioxidant activities in both receptor-dependent and receptor-independent pathways.131 It reduces the activity of glucose transporter 3 (GLUT3) to prevent the absorption of glucose and the generation of ATP.132 The interruption of circadian rhythm or gene expression, which is associated with several hepatic maladies, may promote the development of cancer, inflammation, or steatosis in the liver. Due to MLT's antioxidative characteristics, oxidative stress-related hepatic damage is avoided, the integrity of the liver is restored, and circadian rhythms are also maintained. It may therefore provide a potential therapeutic approach for hepatic abnormalities.133
5.5 Significance of MLT in lung cancer
There has been evidence of MLT's role in lung cancer across several investigations. The findings from a study showed that 10/28 and 11/28 patients, respectively, had exceptionally high MLT concentrations and a low CD4/CD8 percentage.134 Researchers designed subcutaneous low-dosage IL-2 medication plus MLT for solid malignancies, which are typically unaffected by IL-2 monotherapy, to determine the effects of IL-2 immunomodulation in cancers. The findings revealed that 17/82 (21%) of the subjects reported quantifiable tumor cell death. Thirty patients had their disease stabilized, whereas 35 patients experienced their condition worsening. The average lymphocyte and eosinophil numbers increased significantly and the average neopterin levels exponentially reduced when there was minimal progression.135 Eighty individuals with metastatic cancers participated in an investigation to determine the impact of MLT on chemotherapeutic tolerance. Patients with gastrointestinal tract malignancies received 5-fluorouracil with folates, whereas those with lung carcinoma received cisplatin with etoposide, and individuals with breast cancer received mitoxantrone. The thrombocytopenia, lethargy, and fatigue manifestations in subjects assigned to undergo chemotherapy with/without MLT were considerably reduced.136 An investigation was carried out on individuals with advanced lung cancer who were administered a combination of cisplatin and etoposide therapeutic methods. Twenty patients received this treatment combination. According to the research findings, hemoglobin levels are drastically dropped in patient groups. However, the drop in patients receiving only chemotherapy was noticeably greater than in people receiving 5-MTT concurrently. These preliminary findings suggested that concurrent 5-MTT therapy may ameliorate cisplatin-mediated thrombocytopenia in cancer individuals.137 Subjects with advanced NSCLC were offered a cisplatin and etoposide-based chemotherapy regimen with or without the concurrent administration of MLT to determine the impact of MLT on their 5-year mortality. The findings demonstrated that individuals receiving concurrent MLT had considerably higher rates of complete tumor regression and 5-year longevity.66 In a different study, the effectiveness of 5-MTT in combination with chemotherapeutics was investigated. One hundred patients who underwent chemotherapy with MLT showed an improved response and a significant decrease in chemotherapy-related systemic toxicity, specifically thrombocytopenia and neurotoxic effects.67 It not only enhances antineoplastic effectiveness but also safeguards cells from harmful side effects induced by anticancer medications. For instance, MLT inhibited the G2/M phase cell cycle arrest caused by doxorubicin and reduced the expression of cdc2 and cyclin B in IMR90 and A549 cells, inhibiting doxorubicin-mediated aging in a dose-dependent approach. Additionally, it lowered ROS levels caused by doxorubicin, mitochondrial metabolism, and loss of membrane integrity in a manner that was independent of MLT receptors.138 With tissue microarrays, the impact of MLT receptors was examined in noncancerous tissue. It had stronger intensity expression of both receptors than nonmalignant lung parenchyma. Squamous cell tumors showed greater levels of receptors activity than adenocarcinomas.139 To determine histone deacetylase contributions to tumor attenuation and induced apoptosis in NSCLC following MLT treatment, a study was carried out. Three hundred and thirty-seven NSCLC surgical patients were enrolled in this research to evaluate this hypothesis. The results demonstrated that NSCLC patients with elevated HDAC9 expression had lower prognosis and a decreased overall life expectancy. It is fascinating to observe that MLT treatment significantly reduced NSCLC cell invasion and proliferation. It also accelerated cell death and lowered the expression of HDAC9 in NSCLC cells. Treatment with MLT has more anticancer properties when HDAC9 is knocked down. HDAC9 suppression boosted anticancer activity in xenograft cancers, according to an in vivo investigation.140 In B16-F10 animal models, the antineoplastic properties of MLT were examined. B16-F10 cell growth and mobility were inhibited by MLT, which also hindered the G2/M stage of the cell cycle and impaired cytoskeletal morphology. The in vivo results are consistent with the in vitro analysis.141 Pourhanifeh et al. investigated the effects of MLT on alveolar tumors and found its multifunctional properties like pro-oxidant, oncostatic, and anti-inflammatory characteristics, restrict tumor metastasis by triggering apoptosis and limiting autonomous cell proliferation.142
5.6 Significance of MLT in ovarian cancer
Ovarian cancer develops as an aberrant, uncontrolled cell proliferation that causes a tumor to emerge. One of the most frequent gynecologic malignancies-related morbidities is ovarian cancer. Hereditary ovarian carcinoma, being overweight, diabetes mellitus, alcohol addiction, aging, and smoking are possible risk factors. Surgery, chemotherapy, and combinatorial treatment are currently available therapies for ovarian cancer.143 Clinical studies speculated that incorporating MLT into chemotherapy reduced 1-year lethality and chemotherapy-related side effects like asthenia, leukocytosis, nausea, and hypotension, but a recursive study reported no direct correlation between MLT levels and the possibility of developing ovarian cancer.144 An effective medication for the management of advanced ovarian carcinoma that recurrence frequently is IL-2 immunotherapy. The effectiveness of therapy with IL-2 and MLT in individuals with metastatic ovarian melanoma was examined in pilot Phase-II trials. Subcutaneous administration of IL-2 to patients and MLT was ingested orally. This protocol provided only an incomplete response. According to this study, immunotherapy using IL-2 and MLT could be an effective and favorable treatment for metastatic ovarian tumors that are tolerating conventional medical therapies.145 The effectiveness of the treatment of tamoxifen with MLT in patients with advanced tumors was examined in another phase-II research by the same investigator. The outcomes demonstrated that oral intake of these two medications improved patient efficiency and mortality. According to this study, the combination of tamoxifen and MLT is effective in treating metastatic tumors that are resistant to chemotherapy.146 In a different investigation, YC05R pineal extract and anticancer medications were cultured with primary cells obtained from 7 ovarian and 6 mammary gland tumors. All tumors had a dose-dependent inhibition of growth when treated with YC05R pineal extract. Based on the cell types obtained from the individual patient, MLT, and pineal extract both restricted cell proliferation. Neither of the primary cells reacted similarly. According to the findings of the study, the pineal gland possesses potent anticancer components that prevent both mammary gland and ovarian malignancies.147 According to a clinical investigation, MLT production was lower in endometrial cancer patients than it was in tumor-free healthy controls, but there were significant differences between endometrial carcinoma and metastatic ovarian cancer.107 By subjecting mice vitrified ovarian follicles to 10 pmol MLT in an in vitro culture, ovarian follicle proliferation and oocyte maturation were examined. In comparison to the control group, the data revealed a considerable increase in follicular survival and diameter.148 By examining various proapoptotic and antiapoptotic molecules in a rat prototype, no discerpensible difference was observed in body weight, alcohol consumption, or food consumption in ovarian cancer-bearing rats. In contrast to sole administration, co-delivery of ghrelin and MLT prevents cisplatin-mediated follicle breakdown by increasing the count of oocytes in cisplatin-mediated ovaries. Co-treatment of ghrelin and MLT prevented the cytoplasmic redistribution of FOXO3a caused by cisplatin's induction of PTEN and FOXO3a phosphorylation.149 In the development of chemotherapeutic resistance, cancer stem cells (CSCs) are essential. As a result, it was determined how MLT affected CSCs that were separated from SKOV3 ovarian tumor cells. It significantly reduced the protein expression of the epithelial-to-mesenchymal transition (EMT)-related gene and lowered the activation of EMT-related genes. It also considerably lowered the growth of CSCs by 23% compared with SKOV3 cells.150 A study using a mouse paradigm showed that chronic restraint stress (CRS) increased epithelial ovarian cancer (EOC) cell metastasis to the abdomen and the activation of markers relevant to the EMT. The abdominal tumor incidence of ovarian cancer carried on by CRS was significantly decreased by MLT.151
5.7 Significance of MLT in prostate cancer
The second most common cancer and the fifth most prevalent cause of cancer-related mortality in men is prostate cancer.3, 152 Obesity is one of the major risk factors for prostate cancer.153 However, because of earlier stage detection and treatment, the death rates have decreased. According to a case-cohort data analysis, men having low 6-sulphatoxymelatonin (aMT6s) levels in their first-morning urine had a greater risk of developing prostate cancer.154 On the other hand, prostate cancer liability was diminished in patients exhibiting a significant MLT-sulfate/cortisol ratio.155 An in vitro experiment demonstrated that MLT deteriorates cell viability and proliferation, whereas an in vivo investigation reported that testosterone-induced prostatic regrowth was blocked by altering the MLT binding sites in rats when MLT was administered via drinking water.156 It suppressed the proliferation of LNCaP cells of prostate cancer (hormone-independent) by activating the MT1 receptors both in vitro and in xenograft models. According to a preclinical investigation comprising subjects suffering from prostate cancer, prostate-related antigen concentrations can be regulated by the oncostatic characteristic of MLT.157 MLT is renowned for having antitumor activity on a variety of malignancies. The mechanism and influence of dosage, however, are still unresolved. To ascertain the impact of MLT at nanomolar doses, Paroni et al.80 conducted studies in a mouse prototype of prostate carcinoma. To examine this theory, LNCaP cells of prostate cancer were xenografted onto Foxn1nu/nu male mice models that were 7 weeks old and gave MLT treatment. The findings showed that MLT concentrations were 4 times and 60 times higher in plasma and xenograft models of mice treated with MLT respectively, compared with control models (saline-treated mice). MLT-treated xenograft model displayed reduced microvessel density compared with the control. By elevating HIF-1α production and Akt phosphorylation and suppressing Ki67 expression, MLT inhibited angiogenesis. Furthermore, via promoting Nrf2 expression, MLT contributes significantly to establish redox equilibrium. It is essential in regulating mitochondria-mediated apoptotic cell death in prostate cancer by triggering ROS, which in turn activates a number of kinases including p38, SAPK, and JNK. At pharmacological and physiological doses, it may prevent LNCaP cell growth mediated by 17β-estradiol (E2) or 5α-dihydrotestosterone. High MLT concentrations, on the other hand, restrict LNCaP cells from synthesizing prostate-specific antigens. According to an investigation, it reduces the Ca+2 influx mediated by steroids and inhibits the MT1 receptor activation in LNCaP cells.158 The androgen receptor (AR) is excluded from the nucleus by MLT-mediated cGMP, which elevates Ca+2 levels and activates kinase C protein, a potential activation mechanism that controls AR positioning and responsiveness in targeted tissues.159 MLT impeded prostate cancer by preventing NF-κB signaling excitation via suppressing DNA adhesion by MT1 receptor-mediated protein kinase A (PKA) and protein kinase (PKC) activation. MLT demonstrated the antiproliferative activities on hormone-refractory 22Rv1 cancer cells by overexpressing at the MT1 binding site. These investigations demonstrated how the mechanism of MLT involves suppression of NF-κB signaling activity via the MT1 receptor.160 Excessive ROS generation and compromised mitochondrial activity were observed in cells supplemented with DHA, which were probably triggered by AKT downregulation. MLT, on the other hand, suppressed ROS and enhanced OXPHOS, which was correlated to the dephosphorylation of AKT/mTOR. Overall, this investigation has demonstrated that DHA and MLT collaborate to prevent the growth of prostate cancer cells.161 In vitro and in vivo models demonstrated that the tendency of prostate cancer cells to migrate and infiltrate is hypothesized to be inhibited by MLT via the MT1 receptor, phospholipase C, p38, and c-Jun transmission pathways.162 Patients with poor prognosis of prostate cancer have a significantly better survival rate when MLT and radiation are applied in combination.163 MLT restricts pentose phosphate pathway, glycolysis and Krebs cycle in prostatic cancers, indicating that the primary target of indole in this particular tumor type is the lowering of glucose uptake.164
5.8 Significance of MLT in skin cancer
The most complicated, lethal, and diversified cancer is skin carcinoma (melanoma).165 The two types of skin cancers are melanoma and nonmelanoma. The 5th most prevalent malignancy in both genders is the nonmelanoma type of skin cancer.166 Many etiological variables, including skin phototype, hair pigmentation, numerous nodules, genetic factors, and ultraviolet exposure, contribute to the development of this malignancy.167 Radiotherapy and chemotherapy are the standard treatments for skin carcinoma, however, they did not appear to improve patients' likelihood of surviving the disease.168 Additionally, patients may develop cytotoxic drug resistance during chemotherapy and experience unfavorable side effects. Investigating biocompatibility, efficiency, novel treatments or complementary medicines is therefore essential. As an oncostatic and anticarcinogenic drug, MLT, the primary pineal gland secretion, has been demonstrated to be crucial in the treatment of skin carcinoma. It has been demonstrated to have oncostatic and antimutagenic activities on benzo (α) pyrene-mediated oncogenesis in mice. In both the initiation and progression phases of tumorigenesis, the number of mice receiving MLT treatment lowered the average number of papillomas per mouse. It also minimizes lipid peroxides and can block its metabolites from binding to DNA.169 The radio-protective activity of MLT against tissue impairment caused by IR irradiation in rats was investigated. 32 Sprague-Dawley male rats were subjected to IR radiation at varied time intervals, (12 h and 72 h), at a cumulative dose of 800 cGy. Then, before and post-IR, the rats were given saline and MLT at various concentrations. Thereafter, several oxidative and antioxidative characteristics were examined. The findings revealed that tissue concentrations of malondialdehyde (MDA) were markedly increased at both 12 and 72 h after IR, whereas concentrations of glutathione (GSH) were decreased drastically in rats given with saline. On the other hand, rats administered MLT showed much relatively low MDA and elevated GSH levels. Through its antioxidant capabilities, MLT reduced the oxidative damage induced by IR.170 The MLT's radioprotective activities on the Corpora cavernosa of rats subjected to IR were also investigated by scholars. The same factors were examined in rats receiving IR and MLT treatments. Similarly, IR decreased GSH concentrations and increased MDA levels. The oxidative tissue damage was reversed by MLT treatment.171 The objective of another research was to investigate the inhibitory activity of MLT on cancers of epithelial origin. One set of mice received MLT at different concentrations with water during the night, whereas the other set of mice received no MLT treatment and operated as the PB-control. The findings demonstrated that MLT administration enhanced mouse longevity and survival rate, lowered the frequency of subcutaneous meningiomas, and prevented binding protein (BP)-regulated carcinogenesis. MDA and catalase levels have increased as an outcome of BP. In comparison to animals receiving BP treatment, mice administered MLT displayed significantly lower levels of catalase and MDA in tumor territory. Particularly, a small dose of MLT is more favorable than a high dosage in terms of effectiveness.172 MLT, metformin, and a combined treatment of these two dramatically decreased lipid peroxide levels and the frequency and size of skin tumors in mice.173 In another investigation, the endogenous concentration of MLT metabolites such as N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (AFMK), 6-hydroxyMLT (6(OH)M) and 5-methoxytryptamine (5MT) were predicted in a variety of racial groups including Caucasians and African-Americans. African Americans, specifically the younger generation, had the maximum levels of MLT and AFMK, however, 5MT concentrations were comparable across all races. In healthy human melanocytes, the activities of AFMK and 5MT were studied. The outcomes demonstrated that tyrosinase functionality, cell growth, and biogenesis were all suppressed by MLT and its metabolites (10−5 M) in a dose-dependent pattern.174 A further investigation revealed that it inhibits Hsp70 in human endothelial cells, enhancing cellular susceptibility to ultraviolet radiation (UVR)-induced epidermal toxicity, melanoma, inflammatory processes, and DNA depigmentation. Additionally, UVR has pro-inflammatory and proapoptotic properties on healthy human melanocytes, which are inhibited by MLT.175 However, it displayed mutagenic, carcinogenic, apoptotic, and ROS production activities in CCD-1079Sk and A-431cell lines. It considerably outperformed normal cells in terms of its anticancer activities.176 Another research focused on how MLT and vemurafenib synergistically affected the anticancer activity in individuals with V600 BRAF mutated melanomas. The findings demonstrated that MLT dramatically improved the vemurafenib-mediated suppression of angiogenic characteristics and hindered the cell cycle regulation in melanoma cells from mice with metastatic xenografts. Mechanistic investigations showed that MLT increased the anticancer property of vemurafenib by preventing NF-κB p50/p65 from translocating into the nucleus and from adhering to the promoters of iNOS and hTERT, which in turn suppressed the production of iNOS and hTERT.177
6 MLT ADMINISTRATION VIA NANOSTRUCTURED MATERIALS
6.1 Selenium nanoparticles for MLT delivery
The trace element, with enzymatic properties, is available both in organic and inorganic states but is limited by its poor uptake by the gastrointestinal system and potential toxicity when used in higher doses. To overcome the drawbacks associated with selenium, selenium nanoparticles were synthesized by various techniques like microwave-assisted method, chemical reduction method, solvothermal method, and by using microorganisms as well. However, the conventional method used in the synthesis of selenium nanoparticles is the chemical reduction method, wherein chitosan, and ascorbic acid are used in the process of synthesis selenium nanoparticles (SeNPs).178 SeNPs have anticancer effects when used as monotherapy and in combined therapy as well. For instance, increased apoptosis in tumor cells was observed with doxorubicin-selenium nanoparticles-liposomes compared with the individual components used separately.179 However, selenium per se induces DNA damage, ROS synthesis, and disruption of DDR sequences thus causing apoptosis.180 Studies have shown the advantages of using MLT-loaded selenium nanoparticles. When administered to liver cells in mice, MLT-loaded selenium nanoparticles inhibited the initial response to the BCG such as increased levels of NF-⍺, nitric oxide, and IL-1β, thus reducing the damage caused owing to the immunological reactions post-BCG administration.181 Another study by the same team showed the combined therapy enhanced the activity of glutathione-peroxidase thereby reducing the undesirable effects of ROS caused by post-BCG administration.182
6.2 Chitosan nanoparticles for MLT delivery
Chitosan, a naturally available biopolymer is found in crustacean shells, most abundantly in shrimps. Chitosan is derived by the deacetylation of chitin and chitosan nanoparticles are synthesized by emulsification, micellization, and ionic gelation.183 Chitosan nanoparticles have been widely used in cancer imaging, tissue engineering, gene delivery, and even for transdermal, and trans-mucosal drug delivery.184 Herp2 cells undergoing treatment with etoposide, a genotoxic agent, and a topoisomerase II inhibitor were treated with MLT-loaded chitosan nanoparticles. The efficiency of MLT to combat the DNA damage and ROS caused by etoposide was studied and the results showed that the MLT alleviated the oxidative stress, and DNA damage and improved the cells' ability to use glutathione as an antioxidant, in MLT-treated etoposide-treated Herp2 cells. Promising results were obtained when cells were treated with MLT 24 h earlier than treatment with etoposide.185 Chitosan scaffolds loaded with MLT/2-hydroxypropyl-β-cyclodextrin complexes induced cell apoptosis and decreased the G2/M phase cell count in MG-63 osteosarcoma cells.186 Yadhav et al., cultured U87MG cells alone and cocultured them with normal human HEK-293T cells. These cells were treated with chitosan-TPP-loaded MLT NPs and the results showed the better uptake of MLT by malignant cells both in pure and coculture systems treated with NPs when compared with free MLT. It was also reported that MLT delivered by NPs caused cell death in 78% of the cells post-24 h of treatment whereas free MLT caused 58% of cell death. Also, free MLT brought down the cell viability to a minimum whereas NP-delivered MLT caused a decline in cell viability over a period of time, with limited damage to normal cells in pure and coculture systems.187 The studies on lecithin/chitosan nanoparticles coupled with MLT on Caco-2 colorectal cancer cells for transmucosal drug delivery showed better delivery of MLT in the transmucosal spaces.188 Similarly, MLT-loaded chitosan-lecithin nanoparticles enhanced the re-epithelialization of wound models in Human keratinocyte cell lines within 24 h of administration.189 A study by El-Giblay and co-workers synthesized MLT-loaded chitosan microcrystals and used them to treat liver cells of rats sensitized with aflatoxin A1 that induces apoptosis. In vivo, study results showed an imbalance between antioxidant molecules and oxidative products thus showing a reduction in apoptosis.190 An intriguing functionality of MLT is to reduce intraocular pressure in various animals and disease models. In this perspective, chitosan-lecithin and Pluronic® F127-chitosan nanoparticles loaded with MLT for its delivery into HCE-T epithelial cells in vitro developed by Hafner and group. The results showed that Pluronic® F127-chitosan nanoparticles had better uptake within the cells, better adherence to mucosal surfaces, and sustained drug delivery. However, both nanoparticle systems showed no cytotoxicity on the cells and exhibited promising characteristics to be precorneal delivery agents.191
6.3 Solid lipid nanoparticles (SLNPs) for MLT delivery
Lipid emulsions of solid lipids give rise to SLNPs. SLNPs are endowed with the ability to target regions that are guarded by hydrophobic barriers, for instance, CNS. SLNPs have the potential to act as a ferry agent to many lipophilic and hydrophobic drugs and aid in the sustained release of these drugs at the target site.192 Pharmacokinetic studies of MLT loaded in SNLPs administered orally and trans-dermally resulted in an increased serum concentration of MLT following the SLNPs administration, every day. When 3 mg of MLT was administered via SLNPs the serum concentrations were maintained at more than 50 pg/mL for 24 h. The half-life absorption and half-life elimination of MLT-loaded SLNPs were 5.3 ± 1.3 h and 24.6 ± 12.0 h respectively, thus ensuring the sustained release of MLT, like the condition in the body.193 Another study investigated the effect of MLT administered via two different routes against Cyclosporin A-induced cytotoxicity in cardiac tissues in Wistar rats. 1 mg/kg/day of MLT was administered with 15 mg/kg/day of Cyclosporin A intra-peritoneally, and the same dosage of MLT encapsulated in SLNPs was administered for 21 days. The administered MLT antagonized the proapoptotic effect posed on the cardiac cells by Cyclosporin A. This effect could be attributed to the delivery of MLT via SLNPs, the SLNPs were endocytosed by the cells where MLT delivered its antioxidant. MLT functions as a scavenging molecule rather than as the activator of MT1 and MT2 for further downstream signaling pathways.192 The effects of MLT-loaded SLNPs against MCF-7 breast cancer cells treated with tamoxifen showed that the tamoxifen and MLT synergistically enhanced the apoptosis with a reduction in the expression of survivin.194 Studies, where different types of lipids were tested for their efficiency to deliver several agents, revealed that Cy5 (1-Di-((Z)-octadec-9-en-1-yl) pyrrolidin-1-ium iodide) enhanced the MLT's delivery via transdermal route, whereas Cy5 ethanol-MLT NPs showed increased permeability and sustained drug release profile.195
6.4 Liposomes for MLT delivery
Liposomes are phospholipid bilayers of natural or synthetic origin formed spontaneously in the aqueous environment. Drugs can be loaded into liposomes by the pH gradient method, synthesis of liposomes in environments already saturated with drugs, solvent exchange method, supercritical solution technology, and using encapsulated polyanions. Other than drugs liposomes can also be loaded with micro-RNAs, anticancer agents, and chemotherapy drugs.196 Liposomes have been used widely for the transdermal delivery of MLT with reduced lag time, enhanced dermal lipid mobility, improved transdermal flux, and escalated MLT penetration.197 A study by Sana et al., showed the efficiency of bulk MLT and liposomal-loaded MLT against sodium fluoride, chemical toxin-treated rats. The results showed that both the form of MLT reversed the effects of sodium fluoride that include enhanced ROS synthesis, increased expression of TNF-α, and TGF-β, and reduced antioxidant enzymes in the cells.198 The main advantages related to liposomes are their ability to deliver drugs to highly fragile and hydrophobic areas of the host. Glaucoma is one instance where intraocular pressure increases, and it requires continuous application of topical agents to keep the pressure under check. These topical agents though have therapeutic values and have their downsides like prevention of aqueous humor synthesis, the presence of allergens, and toxic preservatives.199 In this regard, liposomes loaded with MLT analog 5-methoxycarbonylamino-N-acetyltryptamine along with mucoadhesives like carboxymethylcellulose and sodium hyaluronate were used for high ocular pressure treatment in rabbits. It was observed that there was a 39.1 ± 2.2% decrease in intraocular pressure compared with liposomes loaded with other formulations, the reduction was a sustained one lasting up to 8 h.200
6.5 PLA, PLGA, and PEG nanoparticles for MLT delivery
Poly (D, l-lactide-co-glycolide) (PLGA) is FDA-approved, an extremely biocompatible and biodegradable polymer used for the parenteral delivery of drugs with sustained drug-release properties.201 PLGA micro- and nanoparticles loaded with MLT prepared by an emulsion-diffusion-evaporation method when administered for 40 days to in vitro MG63 osteosarcoma cell cultures, there was a 70% release of MLT. Furthermore, rapid uptake of the nanoparticles was observed within the first 5 h of treatment and up to 60% of total nanoparticle uptake by cells was observed in the first 24 h. MLT dosage of 1.7 µg inhibited the cancer cells' proliferation but failed to show any induction of apoptosis.201 MLT when loaded onto PLGA nanoparticles showed enhanced antioxidant properties by reducing the oxidative stress on erythrocytes and preventing their hemolysis.202 The ability of MLT to reduce intraocular pressure is well studied and MLT-loaded PLGA and PEG (Poly (ethylene glycol)) nanoparticles showed a significant decrease in intraocular pressure when compared with MLT aqueous solution within 8 h. A maximum reduction of 5 mmHg was observed with the nanoparticle intervention.203 MLT being a well-known antioxidant, could have a positive effect on the cells during ischemia and the reperfusion following it, during which the cells are exposed to higher levels of ROS. In this regard, Ma et al., reported the MLT-loaded PLGA-mPEG nanoparticles' effect on mesenchymal stem cells relocated to the infarcted heart model. In vitro, studies showed that MLT-loaded nanoparticles stabilized the mitochondria and saved the stem cells from injury by decreasing the p53-cyclophilin complex formation. In vivo, studies also exhibited similar results wherein nanoparticle-delivered MLT exhibited better results when compared with MLT injection in rats transplanted with stem cells undergoing cardiac ischemia.204 MLT can also be used in the treatment of sepsis, a disease condition that causes increased ROS levels in the cells of the liver causing morbidity and mortality.205 MLT-loaded PEG and PPS [poly (propylene sulfide)] nanoformulations were studied for their efficiency in treating sepsis in in vivo models. The observations showed that the MLT delivered by nanoformulations decreased the levels of inflammatory cytokines and lipid peroxidation in liver cells when compared with MLT administered per se. Also, reduced hepatic enzymes like ALT and AST indicating reduced liver damage were observed in in vivo mice models when treated MLT loaded nanoparticles. Reduced phosphorylation of p65 of NF-κB was observed in nanoparticle-treated groups compared with MLT alone treated groups.206
6.6 Other nanoformulations for MLT delivery
The shell-like structures made of polymers and mainly composed of a liquid or polymer-based core with an outer polymer membrane are nano-capsules.207 Polymers used in the synthesis of nano-capsules are much less when compared with other formulations and ensure excellent drug stability owing to the protective core surrounded by polymer layers and are resistant to extreme environmental conditions. A study by Komninou and co-researchers reported the effects of MLT loaded into two types of nano-cage systems on a bovine embryo culture model. One formulation had a lipid core loaded with MLT and the other with a polymer core encapsulating MLT. An increase in hatching rates, upregulation of CAT and SOD-2, reduction in apoptosis, and downregulation in the expression of BAX, CASP3, and SHC1 genes were observed in embryos treated with MLT-loaded lipid core nano-cages. These led to increased survival and reduced ROS in embryo.208 MLT-encapsulated nano-capsules loaded onto the hydrogel matrix showed sustained release profiles. Lipid core nano-cages loaded with MLT were effective in targeting the brain and liver to prevent lipid peroxidation. Polysorbate 80 coated nano-cages loaded with MLT when injected intra-peritoneally enhanced the antioxidant reactivity in the hippocampus and reduced the lipid peroxidation in several sites. But the aqueous solution of MLT when injected into the peritoneum showed no above-mentioned effects in mice.209 Metallic nanoparticle systems have also been used in the delivery of MLT. In this regard, Iron oxide magnetic noncomposite particles loaded with MLT served the dual purpose of delivering MLT to the cells and imaging agent for imaging techniques like magnetic resonance imaging. When these nanoparticles were treated on MCF-7 cells in vitro, they showed an enhanced uptake of the nanoparticles and when the magnetic field was added to the culture, it induced hyperthermia and caused cytotoxicity as well as the sustained release of MLT.210 Massella et al. developed a cotton fabric with MLT-loaded polycaprolactone nanoparticles for the transdermal delivery of MLT. Also, these nanoparticles added to dermal patches showed continuous and controlled release profiles.211
Exosomes have been exploited to deliver MLT. Exosomes are promising novel medication systems originating from bodily fluids such as saliva, blood, urine, and saliva, with diameters ranging from 50 to 150 nm. Kumar et al. summarized various methods for isolating exosomes from biological solutions and distinguished drug loading strategies and their use as drug delivery vehicles and diagnostic devices in treating various cancers.212 Kim et al. have used MLT loaded exosomes to treat atopic dermatitis. The HEK293 cells were loaded with exosomes and were observed to have anti-inflammatory properties with their ability to reduce TNF-α and β-hexosaminidase release. Treatment with MLT-loaded liposomes reduced COX-2, TNF-α, and PAR-2 expression levels and restored IFN-γ and IL-4 levels in AD-like mouse.213 Zhang et al. have showed MLT-loaded exosomes potential in repairing myocardial infraction. The as prepared MLT exosomes reduced ROS production, helped in microvessel formation, decreased mitochondrial dysfunction and cardiac fibrosis thus helping in myocardial repair.214 The summarized delivery of MLT via various nanosystems is represented in Figure 5.
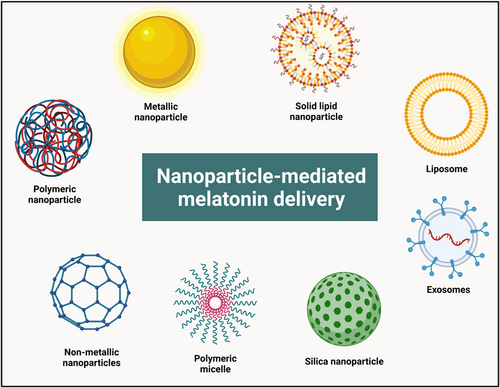
7 STATUS OF CLINICAL TRIALS OF MELATONIN IN VARIOUS TYPES OF CANCERS
According to experimental and clinical research, MLT has substantial preventive capabilities against the hazardous adverse effect characteristics of both radiation and chemotherapy.215, 216 MLT as an adjuvant to various cancer therapies reported are enlisted in Table 2 below. Due to its antioxidant property, it was additionally investigated as an added benefit to chemotherapy. MLT continues to be investigated in this regard, and it is not officially used in conventional clinical practice.225 It has been demonstrated to possess an array of therapeutic properties in several investigations. These include anti-inflammatory, cytostatic, antiproliferative, and proapoptotic implications, along with various functions associated with its potential to manipulate epigenetic modifications.226, 227 Although current investigations have demonstrated that these effects can only be observed during specific stages of tumor development and progression,14 there is evidence that suggests that these antimalignancy features are present during numerous phases.
| Therapy | MLT effect | Action mechanism | Reference |
|---|---|---|---|
| MLT + Chemotherapy | Inhibited cell proliferation | Regulates EZH2 expression | Zhang et al.217 |
| Enhanced cytotoxic effects | HER2 protein destruction | Liu et al.218 | |
| Suppressed autophagy | Inhibits NR4A1, CTSL, and Atg12 | Zhao et al.219 | |
| Induced apoptosis | Decrease AMPK α1 expression | Tran et al.220 | |
| MLT + Radiotherapy | Enhanced preadipocyte differentiation | Decrease TNF-α expression | González-González et al.221 |
| Improved life expectancy of patients | Reduce toxicity | Lissoni et al.222 | |
| MLT + Immunotherapy | Improved IL-2 immune response | Induction of cytolysis | Lissoni et al.223 |
| Enhanced anticancer effect | Inflammatory reaction | Tassoni224 |
Numerous clinical investigations have suggested that using MLT along with chemotherapy may increase the effectiveness of the latter and lessen its negative effects. Data also show that longevity and life expectancy have improved in such cases.228, 229 Improvements in outcomes are believed to be the effect of MLT's ability to eliminate free radicals and its antioxidant properties.230 Further research on the characteristics and therapeutic use of MLT employing in vivo animal models and clinical trials is required when used as an adjuvant to chemotherapy. It has been suggested that double-blind placebo-controlled trials be used to investigate the role of melatonin in reducing the harmful effects of chemotherapy and its relationship to abnormal mitochondrial function. Over the next 10 years, a wealth of knowledge about how melatonin interacts favorably with anticancer medications is anticipated to become available.231 The current clinical research on the use of melatonin to treat various cancer malignancies is summarized in Table 3.
| Cancer type | Sample size | Daily dose | Treatment | Duration of the study | Outcome of the study | Reference |
|---|---|---|---|---|---|---|
| Breast cancer | 95 | 3 mg | Oral | 4 months | Participants in the MLT group noticed considerably significant improvements in their perception of their sleep quality without experiencing any notable side effects. | Chen et al.232 |
| Breast cancer | 95 | 3 mg | Oral | 4 months | IGFBP-3, IGF-1, or circulating estrogen levels were unaffected by MLT. The low baseline estradiol levels may have hindered the detection of any additional estradiol-lowering effects of MLT. | Schernhammer et al.233 |
| Breast cancer | 54 | 6 mg | Oral | 3 months | Melatonin considerably lowered the possibility of experiencing depressive symptoms. | Hansen et al.234 |
| Breast cancer | 20 | 70 mg | Multimodal (10 mg in the morning, mid-day, evening, and 40 mg in the night) | – | 75% of patients resulted in an overall therapeutic benefit. The mean survival percentage for those with metastatic disease was 71%. | Di Bella et al.235 |
| Lung cancer | 151 | 10 mg (51 patients) 20 mg (53 patients) |
Oral | 7 months | Combining melatonin with chemotherapy improved the normalized quality of life and discreetly enhanced the social well-being index. However, it had no impact on individuals with NSCLC's survival or adverse events. | Sookprasert et al.236 |
| Advanced stage of cancer patient receiving palliative care | 72 | 20 mg | Oral | – | Physical exhaustion, additional consequences, or exploratory results did not differ significantly between the melatonin and control groups. | Lund Rasmussen et al.237 |
8 CONCLUSION AND FUTURE PERSPECTIVES
Uncontrolled proliferation and dissemination of abnormal cells describe the collection of diseases known as cancer. The prevalence of cancer has considerably raised and possesses a huge number of fatalities. MLT secreted by the pineal gland controls the sleep-wake cycle in addition to performing a diverse range of other functionalities like antioxidant, immunomodulatory, anti-inflammatory, antiaging, antimetastatic, antiproliferative, antineoplastic, antiestrogenic, and anticarcinogenic effects. There is a significant amount of data from both clinical and laboratory trials revealing that MLT can show anticarcinogenic activity against all types of cancers, encompassing apoptosis, oncostatic, antiproliferative, and antineoplastic properties. Cancer's origin, development, and metastasis are all restricted by MLT. It is a crucial physiological anticancer medication since it can stimulate multiple anticancer mechanisms in a variety of cancer types. We have included pertinent information about the etiology, epidemiology, factors associated, therapeutic effectiveness, medical importance, and cytotoxic mechanism of MLT in this review. We also explored its anticarcinogenic mechanism in connection to malignancies. The primary mode of action of MLT remains to be emphasized, and it is uncertain what molecular basis is governing the anticarcinogenic activities of MLT toward various cancer types. Both receptor-dependent and receptor-independent mechanisms are employed by MLT to regulate cancer. The distinctive quality of MLT is that it blocks cancer cells' entry into the circulatory system and prohibits them from spreading to distant regions. The significance of novel drug delivery techniques was highlighted in this review, and it was recognized that nanoparticles were one of the most intriguing agents in this aspect. Additionally, it emphasized how the fundamental properties of nanoparticles made them suitable for the safe and effective administration of both hydrophilic and hydrophobic compounds, such as MLT. MLT was delivered using different nano-delivery systems, and it was described how these therapeutic approaches make it possible to administer drugs effectively and have other essential properties.
MLT has been proven in numerous studies to have favorable anticancer characteristics, although it does have minor adverse effects, including tiredness, migraine, nausea, and apathy in addition to weight issues. Semen integrity and motility deteriorated after prolonged MLT therapy. Photoreceptors in the retina are damaged by elevated MLT levels. Therefore, deeper investigation is still required to completely understand its anticancer principles and consumption safety assessment. Preapoptotic and antiapoptotic activities are two of its well-known dual functions. For instance, the antiapoptotic activity is detected in both cancer and normal cells, demanding further exploration into the enigmatic function of MLT in the treatment of carcinoma and the differential mechanism between malignant and healthy cells. Studies are indeed essential to get uniform and consistent results as well as to avoid ambiguity. Future research is necessary to address the inconsistent outcomes of epidemiological studies, which is another crucial and critical element. To regulate the dose, safety, and specific function of MLT, particularly in correlation to cancer, research must first discourse the oncostatic function. Further, future research needs to fully explain the anticancer properties of MLT to improve its clinical benefits. Finally, it was observed that the usage of MLT, a substance with multiple benefits and a novel drug delivery mechanism, was restricted to in vitro or in vivo trials and that extensive research was required to determine its effectiveness in humans.
Overall, the MLT is proven to have potential anticancer properties which can be enhanced upon developing into a nanoformulation provides the efficient delivery of the drug at the targeted tumor sites. Additional clinical evaluation is expected to concentrate on improving its capacity to lower the unfavorable consequences of anticancer medications. Clinical trials are further required to ascertain whether MLT can improve the effectiveness of anticancer medications. Even though there is limited evidence regarding human subjects, the majority of studies being conducted today highlight the significance of MLT administration and drug delivery techniques for the medical profession which might improve the drug effectiveness in disease treatment.
AUTHOR CONTRIBUTIONS
Chandra Lekha Putta: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (lead); supervision (equal); validation (lead); visualization (lead); writing—original draft (lead); writing—review and editing (lead). Kalyani Eswar: Methodology (supporting); writing—original draft (supporting); writing—review and editing (supporting). Aravind Kumar Rengan: Conceptualization (supporting); project administration (lead); supervision (equal); validation (equal); visualization (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
All the authors acknowledge the Indian Institute of Technology Hyderabad, Telangana, India, for providing the resources and infrastructure for their work. Schematics were prepared using Biorender.com. The authors would like to thank ICMR (No.35/1/2020-IA/Nano/BMS), DST-AMT (DST/TDT/AMT/2017/227), SERB-CRG (CRG/2020/005069) Grants, ICMR-CoE Grant, SERB-SUPRA (SPR/2022/230) Grant, and IITH/BME/SOCH3 Grant. The authors, Chandra Lekha Putta and Kalyani Eswar would gratefully acknowledge MoE/MHRD for funding their fellowship.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not Applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not Applicable.




