Recent advances in 3D printing sacrificial templates for fabricating engineered vasculature
Shuai Li, Hangyu Li, and Xiushuai Shang contributed equally to this work.
Abstract
Fabricating engineered vasculature within biological scaffolds is one of the most common strategies to maintain high cell viability before implantation. Many studies have been conducted from the aspects of the manufacturing process, materials science, and cell biology to fabricate engineered vasculature with the aim of enhancing the integration between scaffold and host. Among them, the method of combining three-dimensional (3D) printing and sacrifice-based technique has attracted extensive attention. Taking advantage of 3D printing, the method of separating the printed sacrificial template from the biological scaffold to form a 3D channel has become a widely used approach to advance the engineered vasculature. With the development of 3D printing techniques and material science, numerous sacrificial materials have shown their potential in fabricating engineered vasculature. However, several issues remain in this multimethod design, including, but not limited to, the printing process, removal method of sacrificial material, and cell seeding method. This review aims to summarize recent strategies for 3D printing sacrificial templates for fabricating engineered vasculature. The pros and cons of sacrificial materials used in these studies are analyzed. Future perspectives are proposed to fabricate biomimetic-engineered vasculature. Flexible fabrication processes and materials should be advanced to support the 3D printing of sacrificial templates.
1 INTRODUCTION
Significant progress has been made in tissue engineering and biomanufacturing for fabricating in vitro tissues to regenerate the in vivo defect sites. Numerous studies have revealed that the fabrication of engineered vasculature within in vitro tissues plays a vital role in promoting tissue regeneration.1, 2 The vasculature system distributes throughout the entire human body to deliver oxygen, nutrients, and metabolites. Thus, vasculature is essential and crucial in maintaining organ/tissue functions and physiological stability.1 The diffusion limitation for maintaining cell viability is 150–200 μm.3 Therefore, building dense vasculature in engineered tissue is necessary, especially in thick tissues. A dynamically perfused vasculature within engineered tissue could provide a model to better understand the interactions between surrounding cells and the vascular niche.4 Several studies have demonstrated that in vitro tissue with engineered vasculature can connect with the host tissue more rapidly than in vitro tissue without engineered vasculature.5-7 In vitro tissues with highly complex and mature vasculature could be rapidly invaded by host vessels, which promotes perfusion and anastomosis efficiency.8 Hence, building an engineered vasculature in artificial tissue is imperative.
Various approaches have been proposed to fabricate engineered vasculature. The most common methods include mold-based techniques,9 three-dimensional (3D) printing techniques,10, 11 sacrifice-based techniques,12, 13 and multimethod techniques.14, 15 In mold-based techniques, the shape of engineered vasculature highly depends on the structure of molds. Thus, the geometries of engineered vasculature prepared by mold-based techniques mainly focus on the two-dimensional (2D) plane, which is distinct from the vasculature in the human body. 3D printing techniques allow 3D digital models to be produced as physical objects with the use of a 3D printer.16 In the last two decades, 3D printing techniques have revolutionized the biomanufacturing field and found various applications in the biomedical area. Generally, 3D printing techniques for fabricating engineered vasculature can be classified into three main categories: extrusion-based 3D printing, inkjet-based 3D printing, and light-assisted 3D printing. Extrusion-based 3D printing uses a printing nozzle to consistently extrude ink for fabricating target constructs. In this method, 3D printing of hollow hydrogel fibers extruded by a coaxial nozzle is the most common strategy to build engineered vasculature.17, 18 However, vasculature with complex geometries and branched features is hard to be created with this approach due to the continuous extrusion process. Suspension bath-based printing emerged as an effective solution for fabricating biomimetic vasculature in recent years.19, 20 One strategy of suspension bath-based printing is extruding sacrificial ink into a bulk of biomaterials to generate biological constructs with perfusable vasculature. The other design is printing biomaterials in a mass of sacrificial materials. After removing the sacrificial materials, the printed vascular-like structures can be retrieved. Suspension bath-based printing can be a potential method to fabricate vasculature that has the same structure as its' native counterpart. However, the suspension bath follows the flow characteristics of a Bingham plastic, which means the suspension bath flows as a viscous fluid at high stresses but behaves as a rigid object at low stresses.21 Thus, the applications of suspension bath-based printing are limited by the specific suspension bath. Inkjet-based 3D printing can extrude the printed ink at intervals.22 Thus, it can fabricate branched vasculature by using inkjet-based 3D printing with the support of a suspension bath. CaCl2 and gelatin have been widely used as suspension baths to support inkjet-based printing. However, inkjet-based printing is generally limited to low-viscosity materials.23, 24 With the development of materials science, various photo-sensitive materials have been utilized in light-assisted 3D printing. Digital light processing (DLP) is the main technique used in light-assisted 3D printing.25 For the DLP method, a 3D object can be fabricated layer-by-layer by exposing the prehydrogel solution to the light. A remarkable study of fabricating engineered vasculature was conducted using food dyes as photo absorbents in DLP.26 A lung model with an air sac and surrounding blood vessels was generated through this method. The most significant advantage of light-assisted 3D printing is the high resolution.27 Moreover, support materials are not required in the fabrication process. Stereolithography printing has also been widely explored for fabricating vascular structures.28-30 However, light-assisted 3D printing requires specific light-sensitive materials and printing apparatus, which may be hard to be widely used due to the high cost and the material limitations.
Sacrifice-based techniques are mainly used to fabricate biological scaffolds with perfusable channels. A sacrificial template is first shaped to the designed structure and cast by the scaffold materials, as shown in Figure 1. After the gelation of the scaffold materials, the sacrificial template can be removed under the specific condition to form the perfusable channels.31 In the case of sacrifice-based techniques, the sacrificial template is usually generated by 3D printing, electrospinning, and mold-based method. Therefore, sacrifice-based techniques are generally combined with other methods to manufacture engineered vasculature, which is known as a multimethod technique. The multimethod technique is formed by combining two or more technologies. Three-dimensional printing can be used to create a sacrificial template that has the same structures as the engineered vasculature, which shows great potential to fabricate vasculature with complicated features. Thus, the combination of 3D printing and sacrifice-based techniques is the most widely used approach for fabricating engineered vasculature. Despite the significant processes in 3D printing sacrificial templates for fabricating engineered vasculature, drawbacks of manufacturing techniques, sacrificial materials, and material interfaces remain.
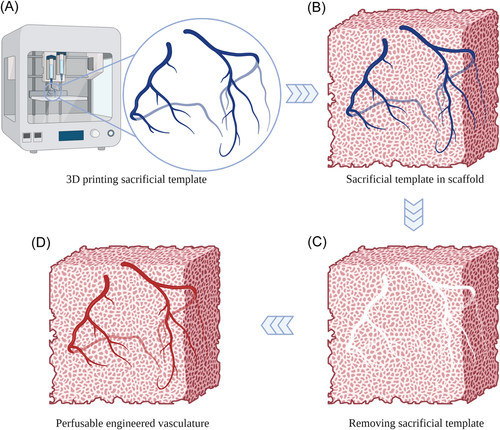
This review focuses on various sacrificial templates fabricated by 3D printing for producing engineered vasculature in recent years. We briefly introduce the sacrificial materials and the 3D printing methods used for manufacturing sacrificial templates. The sacrificial materials used for 3D printing sacrificial templates are categorized into hydrogel and polymer in this work. The printing and fabrication methods are briefly introduced and the pros and cons of each sacrificial material are discussed. Finally, future perspectives are proposed to leverage applications of 3D printing sacrificial templates in fabricating engineered vasculature. 3D printing sacrificial templates have shown great promising for fabricating engineered vasculature with biomimetic structures. The advancement of engineered vasculature structure, cell seeding method, and 3D printing processes can further improve the quality of engineered vasculature and fabrication efficiency.
2 3D PRINTING SACRIFICIAL HYDROGEL
Hydrogels have been widely used in tissue engineering and biomanufacturing due to their similar microenvironment to soft tissues.32 For fabricating biological scaffolds, native hydrogels including collagen, gelatin, and alginate remain prevalent choices for regenerative treatments because of their capacities of imitating native extracellular matrices and high biocompatibility.33 With the in-depth investigation of these native hydrogels, the properties of being used as sacrificial materials have gradually been explored. Among them, alginate, gelatin, and agarose are the most attractive sacrificial materials.
2.1 3D printing alginate as sacrifice template
For fabricating engineered scaffolds, alginate can be covalently crosslinked by divalent cations, such as Ca2+ and Ba2+.34 This crosslinking method has been widely used for fabricating hydrogel constructs due to the mild reaction process. However, the crosslinked networks can be broken by solvents including ethylene diamine tetraacetic acid (EDTA) and sodium citrate.35-37 Thus, alginate can be used as sacrificial material based on the above principle.
To maintain the stable release of the laden drugs from the polydopamine (PDA) coated Gel/polycaprolactone (PCL) core/shell scaffolds, Liu et al.38 used thermosensitive gel containing sodium alginate and gelatin as the sacrificial material to 3D print sacrificial template, as shown in Figure 2A. The crosslinked alginate/gelatin scaffolds were utilized to load drugs and control the releasing ratio of drugs by a NIR irradiation. The release of drugs could be maintained for a long time to match the regeneration ratio. Moreover, the dissolving of alginate/gelatin released the engineered vasculature, as shown in Figure 2B. In their latter study, the self-sacrificial gelatin and alginate-based bio-ink were designed to construct scaffolds with interconnected microchannel networks.39 The sacrificial templates were established by 3D printing the gelatin/alginate. Then, the sacrificial templates were immersed in the CaCl2, CuCl2, or FeCl3 solution for a certain time to partially crosslink the sacrificial templates. The inner noncrosslinked hydrogels were dissolved by water at a temperature of 37°C to form hollow channels. Then the hollow scaffolds were treated with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS) and CaCl2 to form fully interconnected microchannel (FIM) networks with macropores, as shown in Figure 2C,D. In another study, Dong et al.40 used alginate and gelatin-based hydrogel as the sacrificial material to fabricate scaffolds with hollow channels transformed from core/shell fibers coated with PCL/SrCuSi4O10 (wesselsite, CS). The solution of alginate and gelatin with stromal cell-derived factor-1α (SDF-1α) was 3D printed to the designed template, followed by immersion in CaCl2 to crosslink the alginate. Through incubation into PCL/CS solution, the scaffolds with core/shell structure were constructed completely. The hollow channel networks appeared by removing the hydrogel core with phosphate-buffered-saline (PBS) at 37°C in vitro, as shown in Figure 2E. In the above works, the extra solvent was not used to dissolve the core alginate/gelatin template. The dissolving of alginate/gelatin depends on the temperature at around 37°C to liquefy gelatin and break the crosslinked alginate networks. In this process, drug or factor delivery can be realized at the same time. Moreover, the alginate/gelatin blend can be successfully 3D printed. Thus, the combination of different sacrificial materials to reach various functions may be a potential trend for using sacrificial materials to fabricate engineered vasculature.
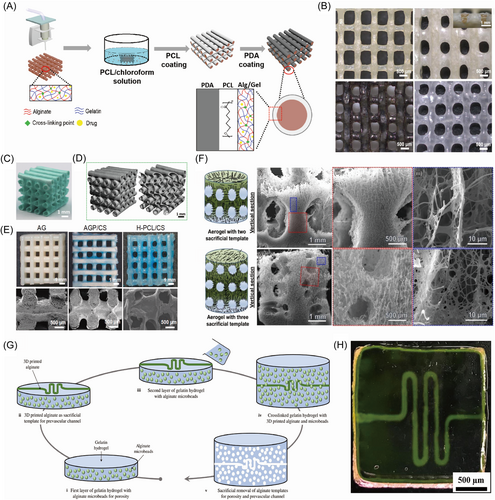
To construct the anisotropic microchannels and patterned macrochannels in the novel nanofiber aerogels, John et al.41 demonstrated the fabrication of anisotropic microchannels and patterned macrochannels within aerogels by the method of partially anisotropic freezing configurations. PCL/gelatin (1:1) short nanofibers and 3D-printed alginate sacrificial templates were used to generate the microchannels and macrochannels, respectively. After the crosslinking of aerogels by glutaraldehyde (GA) vapor, the alginate-based templates were dissolved by a solution of EDTA, leaving patterned macro channels and aligned anisotropic microchannels in the aerogel scaffold, as shown in Figure 2F. Negrini et al.42 utilized alginate with the shape of microbeads and 3D printed filaments as the sacrificial template to fabricate gelatin construct for mimicking adipose tissue structure and promoting angiogenesis, as shown in Figure 2G. The alginate microbead and filament were crosslinked by CaCl2 after printing, exhibiting adequate mechanical properties, ease of removal, and no cytotoxicity. The alginate-based templates were washed away by trisodium citrate owing to the Ca2+ ions chelator effect, leading to the formation of pores and hollow channels in gelatin constructs, as shown in Figure 2H. The fabricated alginate-based microbeads and filaments played a significant role in the construction of gelatin scaffolds to mimic the more virtual adipose tissue, which provided more ideas for designing relevant models.
With the development of 3D printing techniques, suspension bath-based printing has emerged as a powerful tool to print designed structures with the support of a suspension bath. For fabricating engineered vasculature, the sacrificial material can be printed in the scaffold material. After the gelation of the scaffold, the printed sacrificial template can be removed to form perfusable channels. Wang et al.43 used calcium alginate hydrogel (Ca–Alg) as the sacrificial template to fabricate tailored channels in the hydrogel scaffold. The alginate ink was injected into the prepolymer such as agarose and gelatin (AG), gelatin, and gelatin methacryloyl (GelMA) prepolymer according to the defined geometry and crosslinked immediately with calcium ions in the prepolymer to prevent the suspending channel templates from deformation, followed by the full crosslinking of the prepolymer. Then the Ca–Alg templates were dissolved by sodium citrate and pulled out, remaining the customized microchannels. Besides, the vascularized hydrogel could be successfully fabricated with the seeding of human umbilical vein endothelial cells (HUVECs). With the utilization of a sacrificial Ca–Alg hydrogel template, the direct printing approach satisfied the demand for quick construction of scaffolds and provided a novel platform for various applications of engineering vascularized models. In a novel mold-sacrificial approach proposed by Ching et al.,44 the tunable alginate-based inks were utilized to fabricate versatile multilayer vasculature. Based on the 3D printed porous poly-(ethylene glycol)-diacrylate (PEGDA) mold mixed with CaCl2, the alginate-based inks were perfused into the mold with PEGDA added in the outer layer (Alg-PEGDA) and GelMA added in the inner layer (Alg-GM) for adequate mechanical properties. By controlling the crosslinking duration and concentration of CaCl2, the tube wall with a suitable thickness was obtained after the removal of the uncrosslinked inner part of alginate-based ink by flushing with HEPES. Based on the multilayer alginate-based ink, various kinds of vascular structures could be fabricated freely.
2.2 3D printing gelatin as sacrificial template
Gelatin has been widely used as a scaffold material to attract the attachment of cells due to the abundant Arg-Gly-Asp (RGD) sequence for cell adhesion.45 Gelatin physically crosslinks at 4°C and liquefy at above 22°C. Thus, this property can be used to assist with the 3D printing and the removal of sacrificial templates.
In the study of Hong et al.,46 gelatin was utilized in a coaxial 3D-bioprinting method to fabricate cell-laden vascular constructs. Gelatin and gelatin–PEG–tyramine (GPT) were used as core and sheath materials, respectively, as shown in Figure 3A. The gelatin core could also be used as a carrier for loading HUVECs. After removing the gelatin core under physiologic temperature, HUVECs could be easily released in the hollow channel. The hollow GPT channel can be seen in Figure 3B. The sacrificial gelatin-based template within H2O2 results in no significant harm to cell culture, which provides extremely possible designation, printing, and organization of 3D tissue architecture vascularization. Liu et al.47 utilized gelatin as a sacrificial material for the formation of multiscale perfusable vasculature within centimeter-scale liver-like tissues by multimaterials 3D printing. The gelatin-based template hydrogels were easily removed away at physiologic temperature through gel–sol transition to form channels for endothelialization by HUVECs. The tridimensional “one-to-two” channeled tissues were as shown in Figure 3C. Ouyang et al.48 proposed a novel void-free 3D printing (VF-3DP) approach to fabricate perfusable constructs. Sacrificial gelatin-based ink that could carry endothelial cells and photo-crosslinkable GelMA could be printed in the multi-ink bioprinting system. After crosslinking, the entire void-free hydrogel was incubated at 37°C to remove the gelatin template for producing a porous hydrogel scaffold. In addition, the microfluidic devices with customized in situ vascularized interconnected channels could be fabricated with the additive step of polydimethylsiloxane (PDMS) casting before incubation, as shown in Figure 3D. In another study by Ouyang et al.,49 CaCl2 was added to gelatin to partially crosslink alginate to fabricate multilayer tubular hydrogels. First, the thermosensitive gelatin containing CaCl2 was filled in the customized polylactic acid (PLA) mold, and gelled at 4°C, followed by immersion in alginate solution again and again at intervals. After that, the gelatin was removed at 37°C to generate the multilayer ionic hydrogel, as shown in Figure 3E. The constructed hollow hydrogel structure with branched geometries can be seen in Figure 3F. The sacrificial gelatin templates were fabricated by custom molds instead of 3D printing. However, the partially crosslinking method provides a method for fabricating complex and multilayer vasculature.

Gelatin can also be applied as a sacrificial material in suspension bath-based printing. In the study by Scott et al.,50 gelatin ink was designed as a sacrificial template for sacrificial writing into a functional tissue (SWIFT) biomanufacturing method. The gelled gelatin ink with a suitable rigidity at about 20°C was extruded into the cold organ building block (OBB)-based matrix in silicone molds with a designed extrusion rate, print speed, and pathway to obtain the vascular template with desired diameter and geometry, as shown in Figure 3G. The gelatin template was removed at 37°C, leaving behind the fully gelled OBB-ECM matrix with hierarchical vascular networks to mimic the cardiac tissue matrix. Based on the sacrificial gelatin template, the SWIFT manufacturing method could provide therapy with the quick assembly of patient- and organ-specific vascularized tissues in the future.
Eltaher et al.51 utilized gelatin-sucrose matrix (GSM) as the sacrificial material to fabricate scaffolds with hollow channels at the human scale. The printed sacrificial GSM-based template with appropriate density and flowability could be dissolved after the crosslinking of the casted matrix. Results demonstrated that the vasculature fabricated by GSM could support cell metabolism and protein secretion, which highlights the importance of the engineered vasculature again. In a sustainable reversible multilayered patterning approach proposed by Wen et al.,52 the thermosensitive gelatin (Gel) and pH-sensitive chitosan (Chit) were utilized to print Gel/Chit-H+ hydrogel as sacrificial templates. The printed Gel/Chit-H+ hydrogel could be used to form the nonsacrificial Gel/Chit-H+-Citr hydrogel by electrostatic crosslinking between Chit and citrate (Citr). The uncrosslinked Gel/Chit-H+ portion could be removed facilely by heating to 50°C. Through the 2D, 3D, and 4D patterning, various customized patterns of the interconnected or segregated fluidic network within the Gel/Chit-H+-Citr hydrogel could be obtained. The dynamic sacrificial printing approach depended on the stimuli-sensitive materials not only indicating the development of patterning methods but also providing a novel method to fabricate complex multilayered structures for various aspects of medical applications. To fabricate independent branched vessels and complex vascular networks in distinct porous scaffolds, Su et al.53 designed the sacrificial template based on the above Gel/Chit-H+ hydrogel. The initial labile Gel/Chit-H+ templates were tailored by the 2D formation method or 3D printing with Gel/Chit-H+ solution, as shown in Figure 3H. Then, the templates were immersed into a sulfate solution to form the electrostatic network within the outer layer of the templates (Gel/Chit-H+-SO4). The thickness of Gel/Chit-H+-SO4 depends on the immersing time. The removal of the Gel/Chit-H+ core was achieved by immersing it in warm water. The tubular Gel/Chit-H+-SO4 structure transformed from an electrostatic network to a crystalline network in alkaline conditions to form a more stable Gel/Chit0 hydrogel. Interconnected channels fabricated by the proposed method can be seen in Figure 3I. The Gel/Chit0 hydrogel channels showed excellent physiological stability, mechanical properties, semipermeability, biocompatibility, and weak in vivo inflammatory response, which possessed the potential for wide application in engineered vasculature.
Fitzsimmons et al.54 assessed gelatin and Pluronic F-127 in the aspect of printing features, toxicity, rheological properties, and compressive moduli for being used as sacrificial materials. Hyaluronan (HA) was also added to gelatin to modify the printing properties of gelatin. In the aspect of printability and filament diameter, Pluronic F-127 exhibited the highest homogeneity and resolution. In terms of rheology, Pluronic F-127 and gelatin-HA showed shear thinning behavior. Besides, Pluronic F-127 showed acceptable cytotoxicity when cultured with endothelial cells (ECs) and adipose-derived stromal cells (ASCs). Pluronic F-127 and gelatin-HA could construct interconnected channels successfully within the hydrogel scaffolds, as shown in Figure 3J. Results demonstrated that Pluronic F-127 is better than gelatin as a sacrificial material. However, the addition of HA to gelatin could narrow the gap, which indicated that it might be beneficial to use multihydrogels as sacrificial materials.
2.3 3D printing agarose as sacrificial template
Agarose is a natural polysaccharide that can form a densely packed gel with low porosity. The lack of crosslinking sites at the interface of agarose and other hydrogels makes it easy to isolate the sacrificial agarose and scaffold materials.55 Thus, in this study, GelMA could be used to encapsulate 3D printed agarose. After manually removing the agarose template, perfusable microchannels formed. Based on this removal principle, Duchamp et al.56 explored the fabrication of a sacrificial template composed of agarose by proposing a strategy of rapid cooling gelation in the glass capillary. The GelMA layer was injected with customed agarose microfiber, which could be tailored in diverse curvature, shapes, and diameters, as shown in Figure 3A. Then, the agarose-based template was extracted easily and quickly from the hydrogel matrix by physical methods, which reduced the possibility of harmful effects on the seeded cells later. The microchannels were absolutely visible, as shown in Figure 4A. With flexibility, operational ease, and negligible effects on cell viability, the agarose-based template exhibited a possible extension to engineer more duct-related cancer models. In their later study, agarose further served as the sacrificial material to fabricate hollow microchannels in GelMA hydrogel to mimic the in vivo microenvironment of breast tumors.57 Owing to the capillary effect, the heated agarose solution was sucked into a glass capillary, followed by cooling to be gelled. Then, the agarose microfibers were printed onto a layer of partly crosslinked GelMA in the designed geometrical shape. After fully cast with breast cancer cells loaded-GelMA and photo-crosslinking, the agarose templates were extracted manually to release the hollow microchannels that supported the survival of seeded lymphatic endothelial cells (LECs), as shown in Figure 4B. The lymphatic vessel-breast cancer (LV-BC) model was successfully constructed, as shown in Figure 4C. The sacrificial agarose template protected cells in the GelMA matrix from osmotic damage, which assisted the construction of multi-size capillary-like microchannels in models such as in vitro LV-BC model.
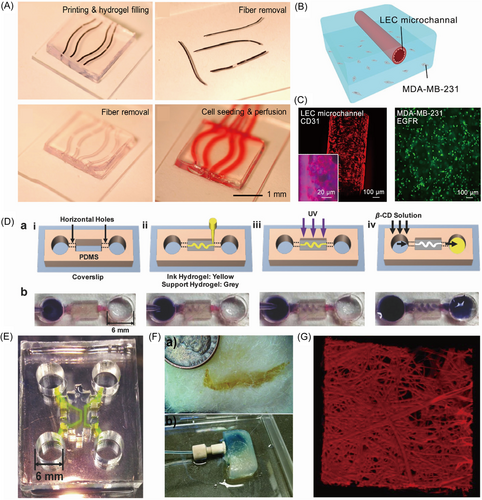
2.4 3D printing other hydrogel as sacrificial template
Due to the flexibility of using sacrificial hydrogels to fabricate engineered vasculatures, several customized hydrogels have been developed. Song et al.58 designed a modified hyaluronic acid (HA)-based template consisting of the guest–host pairs of adamantane (Ad) HA (Ad-HA) and β-cyclodextrin (β-CD) HA (CD-HA), which owned the properties of shear-thinning and self-healing. The modified HA-based template could maintain a tailored shape with different curvatures, lines, and pockets after printing in the supporting matrix, as shown in Figure 4D. The modified HA-based template was washed out by β-CD to break the guest–host bonds after the support hydrogels were cross-linked. The interconnected microchannel could be seen obviously in Figure 4E. The modification of HA had lots of options to adjust the properties of the sacrificial template, which meant the potential for wide application in vascularized structure engineering. Štumberger et al.59 utilized xanthan gum as the sacrificial material to fabricate hollow channels in hydrogel matrices. The xanthan gum-based filament was injected into the hydrogel matrices by 3D printing according to the designed routes and geometries, and removed by flushing with water. An earlobe-shaped channel could be fabricated through the proposed method, which provided a novel method for the construction of engineered vasculature. In the study of Bellan et al.,60 the pH-sensitive water-soluble shellac was utilized to print the sacrificial microfiber network in the hydrogel. Obtained from a cotton candy machine, the melt-spun shellac microfibers with a wide range of diameters were placed into a silicone mold, which decided the final shape of the hydrogel, followed by exposure to ethanol at physiological temperature. Then, the gelatin solution containing microbial transglutaminase (mTG) overlaid the shellac microfiber networks and crosslinked at 37°C. The shellac fibers were dissolved by ammonium hydroxide to generate the hollow channels, as shown in Figure 4F. The gelatin hydrogel with a microchannel network can be seen in Figure 4G. The utilization of a sacrificial shellac template in a novel approach to fabricate microfluidic devices successfully indicated that other new materials also had the potential to be involved in the construction of engineered vasculature.
2.5 Summary of sacrificial hydrogel
Hydrogels have attracted tremendous attention in the field of biomanufacturing and tissue engineering.61 3D printing sacrificial hydrogels, then removing the printed templates are considered to be an effective method to fabricate engineered vasculature. In the removal process, the hydrogels can be dissolved by solvent or liquified at specific conditions.
Alginate can be crosslinked to fabricate engineered scaffolds. The key to using alginate as sacrificial material is dissolving the crosslinked alginate by EDTA or sodium citrate. A significant advantage of alginate as sacrificial material is its printability, especially when alginate is used with CaCl2. Thus, 3D printing alginate in the suspension bath with Ca2+ is one of the methods to create sacrificial templates. In the other approach, the printing/fabrication of hollow alginate fibers can be a principle of using sacrificial alginate. Hollow alginate fibers are hard to be used to fabricate engineered vasculature with complicated geometries, especially branched features. This is mainly caused by the coaxial extrusion process of using alginate and Ca2+. This shortcoming can be overcome by using suspension bath-based printing. With the support of a suspension bath, sacrificial alginate templates with arbitrary geometries can be achieved. Thus, sacrificial alginate shows great potential in the technique of suspension bath-based printing to fabricate engineered vasculature. However, the removal of crosslinked alginate requires chelators. Studies have revealed that EDTA and sodium citrate have a negative effect on cell viability.62, 63 This may hinder the application of alginate as sacrificial material. The concentration and exposure time of chelators should be strictly controlled.
Gelatin can be easily removed at the cell culture temperature when being used as sacrificial material. Moreover, no additional solvent is required to assist in the removal process. Cells can be loaded in sacrificial gelatin to aid cell seeding. In addition to this, gelatin can promote cell adhesion.64 Based on these advantages, gelatin can be an ideal sacrificial material for fabricating engineered vasculature. However, viscous gelatin solution shows low printability.65 Thus, gelatin often cooperates with other hydrogels to improve its printability. For instance, alginate can be blended with gelatin to promote printability.66, 67 In this case, gelatin can be utilized to enhance cell adhesion.45 Therefore, the blended sacrificial hydrogel can potentially be widely utilized for fabricating engineered vasculature.
The use of agarose as sacrificial material avoids the interfacial issue between sacrificial material and scaffold matrix, which can be a constructive method to maintain the structural integrity of the fabricated scaffold. Studies mentioned above used the same method of manual operating to remove the printed sacrificial agarose. This removal method may present difficulties in the fabrication of engineered vasculature with complex geometries, especially for perfusable structures.
With the development of materials science, more and more native and customized hydrogel materials have been explored as sacrificial materials for fabricating engineered vasculature. Among them, several materials require the corresponding solvent to aid the removal process. Moreover, the printing of sacrificial templates may involve specific support materials or apparatus, which reduces the applicability and scalability. Despite the numerous studies and remarkable advances of 3D printing sacrificial hydrogel to fabricate engineered vasculature, the easy and mild removal process and cytocompatibility can be the crucial characteristics of a sacrificial hydrogel.
3 3D PRINTING SACRIFICIAL POLYMER
With the advancement of sacrifice-based techniques, several polymers and synthetic materials have also been explored to fabricate engineered vasculature. Among them, polyvinyl alcohol (PVA), PLA, Pluronic F-127, Poly(N-isopropylacrylamide) (PNIPAM), saccharide, and PCL are the most common sacrificial materials.
3.1 3D printing PVA as sacrifice template
PVA has excellent biocompatibility, mechanical stability, and low toxicity.68 Besides, PVA can be 3D printed at the temperature around 180°C. As a hydrophilic polymer, PVA can be dissolved in water easily.69 Thus, PVA can be an excellent sacrificial material for fabricating engineered vasculature.
Based on the mild water-soluble process, Zou et al.70 utilized PVA as the sacrificial material to fabricate a prevascularized face-like construct based on a 3D tai chi pattern. The PVA sacrificial mold was printed with one nozzle of a fused deposition modeling (FDM) printer, while the hydrogel composites of nanocellulose, agarose, and sodium alginate with HUVECs and human fibroblasts (hFBs) were printed by the other nozzle to fill the PVA scaffold layer by layer. After crosslinking with CaCl2, the sacrificial PVA scaffold was removed by PBS solution. The bioprinted vessel-like networks with nutrient networks could be seen in Figure 5A. To fabricate customized ultrathin tubes with adequate mechanical flexibility to mimic bile ducts, Park et al.71 demonstrated the fabrication of thin-wall tubular scaffolds based on 3D printed sacrificial PVA-based templates. The PVA-based templates were 3D printed at a high temperature according to the 3D geometry obtained from medical imaging technologies. Ultrasonic treatment was conducted in 50°C water transiently to increase the surface smoothness of PVA templates. Then, the PVA templates were coated with a PCL layer by immersing the PVA templates in the PCL solution. Finally, the PVA templates were removed by water and ultrasonic treatment, leaving behind the hollow ultrathin tubular structures, as shown in Figure 5B. Besides, the surgery of bile duct regeneration in rabbits demonstrated the feasibility and usefulness of tubular structures in practical surgery. PCL and thermoplastic polyurethane (TPU) coating were used in the study of Lee et al.72 Commercial PVA filaments were 3D printed to fabricate the sacrificial templates. Ultrasonic treatment in 50°C water was used to smooth the surface of the printed PVA sacrificial templates. Salt-included PVA or TPU solution was utilized to endow the wall of the tube structures with a porous morphology. The PVA templates were finally removed by ultrasonic treatment in 50°C water for a long time, leaving behind the tailored porous TPU vessel structure with matched flexibility with native soft tissues, as shown in Figure 5C.
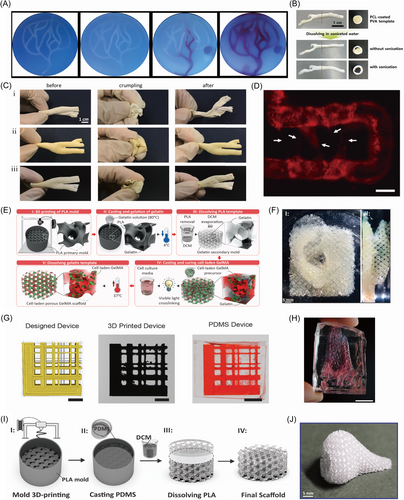
Except for the FDM of PVA sacrificial templates, electrospinning was used to fabricate sacrificial PVA templates with small sizes. Hsu et al.73 utilized PVA as the sacrificial material to fabricate hollow channels in poly (glycerol sebacate) (PGS). The aligned PVA fibrous membranes were constructed by the electrospinning approach, followed by immersing PVA fibrous membranes in partially crosslinked PGS prepolymer (pPGS) to form the PVA-PGS laminated membrane with PVA fibers. After curing, the PVA sacrificial fibers were removed according to the successive steps of sterilization, ultrasonic treatment, and stir in water, leaving behind the porous anisotropic PGS membrane with microchannels. The sacrificial electrospun PVA fibrous membrane provided an affordable and biocompatible method to construct anisotropic microchannels for vascularization. The fabrication of engineered vasculatures with different sizes can also be achieved by using sacrificial PVA templates. In the study of Hu et al.,74 PVA was utilized as the sacrificial material for the formation of microchannel networks in engineering tissues. The deposited PVA structure was printed by the 3D printer Ultimaker. After that, the PVA sacrificial template was placed in a silicone holder, followed by embedding with matrices consisting of matrigel, fibrin, and gelatin. Then, the sacrificial PVA template was removed by perfusing warm media, leaving behind the hollow channels. To fabricate dense vascular beds in the constructs, commercial PVA-based thread intertwines the 3D-printed PVA template to form a sacrificial template of various sizes. The fabricated interconnected channels can be seen in Figure 5D.
3.2 3D printing PLA as sacrificial template
The key point in the process of fabricating engineered vasculature by using sacrificial materials is dissolving the 3D-printed sacrificial parts. Thus, a few polymers that can be dissolved by organic solvent were also introduced in the sacrifice-based techniques to fabricate engineered vasculature. In the rigid, facile, and low-cost double-templating approach proposed by Davoodi et al.,75 PLA and gelatin templates were used to fabricate GelMA scaffolds with microchannel networks, as shown in Figure 5E. The PLA sacrificial template was 3D printed, followed by immersion in the gelatin solution. After gelation at 4°C, the PLA template was dissolved by dichloromethane to form the gelatin template. The GelMA solution with cells was then used to cast the gelatin template. Finally, the gelatin template was removed by incubating the construct at 37°C to generate the microchannel networks after crosslinking the cell-laden GelMA by visible light. A porous GelMA human tissue model with a microchannel network fabricated by the approach can be seen in Figure 5F. With the porous microchannel network suitable for cell culture and various cellular activities, the sacrificial PLA and gelatin-based double-templates in such a method provided the potential for wide application of artificial thick engineering tissues. In the study of Chen et al.,76 PLA was utilized to 3D print the sacrificial template for fabricating vessel networks according to the 3D microscopy images of the vessels in vivo. The printed PLA template was immersed in PDMS first, and removed by dichloromethane after the solidification of PDMS, as shown in Figure 5G. Then, the printed vessel networks were carpeted with endothelial cells and formed perfusable microfluidic devices in vitro through the proposed Clarity-to-Reality (C2R) method. The C2R device that replicates the vessels in healthy tissues can be seen in Figure 5H. Similarly, Montazerian et al.77 fabricated porous PDMS scaffold with channel networks by sacrificing 3D printed PLA shell mold, as shown in Figure 5I. According to the mathematically tailored printing path, the unsupported and robust PLA shell mold was printed by an affordable FDM 3D printer with high resolution. The printed PLA shell mold maintains superior structural integrity. A dichloromethane solution was used to remove the PLA mold. The porous nose-like PDMS scaffolds could be seen in Figure 5J.
3.3 3D printing PNIPAM as sacrificial template
PNIPAM is a widely used material for the nondestructive release of cells.78, 79 Thus, PNIPAM shows great promise in the biomanufacturing field. PNIPAM is soluble when the temperature is low due to the hydrophilic amide group. When the temperature is elevated, a volume phase transition and volume shrinkage happen to release water due to the hydrophobic propyl group.80 Therefore, PNIPAM is commonly used in drug delivery and soft robotics. Several studies have also explored the possibility of using PNIPAM as a sacrificial material to fabricate engineered vasculature.
In the study of Lee et al.,81 a strategy of sacrificial template, which was thermosensitive, was proposed to fabricate microvascular networks within the gelatin scaffold, as shown in Figure 6A. This study used PNIPAM with the properties of biocompatibility, simplicity of processing, and a suitable lower critical solution temperature (LCST) as the sacrificial template by the technique of solvent-spinning. With the easy solubility at the physiological temperature by water, the microvascular networks were exposed obviously, as shown in Figure 6B. The authors deduced that it would be widely utilized if a sacrificial PNIPAM-based template could yield microchannel in the gelatin scaffold with scales, complexity, and thicknesses based on various demands in the future, without significant harm to the cultured cells. Lee et al.82 further compared the effects of microchannel and macrochannel both fabricated by PNIPAM on the formation of normal functional vessels, as shown in Figure 6C,D. The study verified that the capillary-like microchannel with a range of diameters from 10 to 25 μm roughly owned elegant promotion of polarization of M2 macrophages and angiogenesis. With the limitation of the resolution for processing microfibers of traditional 3D bioprinting, though, the suitable accurate sacrificial PNIPAM-based template may be produced by the cotton candy machine as a novel idea. A new liver model with a similar structure and function to those in vivo was proposed by Lee et al.83 to mimic a virtual microvascular network in the liver by sacrificial PNIPAM-based template covered with enzyme-crosslinking gelatin hydrogels, loaded with dense hepatocyte spheroids, as shown in Figure 6E. The PNIPAM fibers were produced by the cotton candy machine and removed away from the crosslinked gelatin hydrogels below its LCST, organizing microchannel networks that were important for the maintenance of cell viability. Then, the model could be used to mimic the process of inflammation and fibrosis in the early and late stages of nonalcoholic fatty liver disease, respectively, showing the hepatic lobe by liver buds. Briefly, the liver tissue-laden channel chip with the vital perfusable microchannel network portion could integrate the adjacent host vessels and exchange the nutrients and signals, which provided the possibility for the chip system to improve the organ-replacing technique.
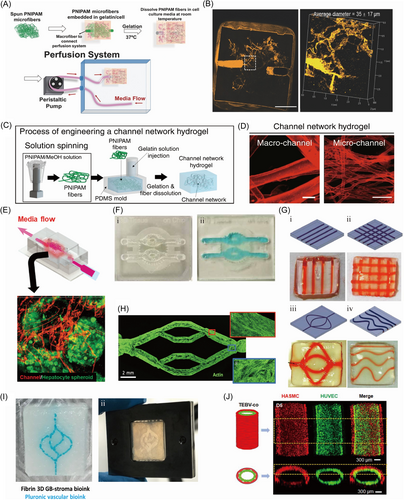
3.4 3D printing Pluronic F-127 as sacrificial template
Pluronic F-127 shows superior printability and shear thinning feature at room temperature (25°C) and liquefies at 4°C.84 Thus, Pluronic F-127 has been widely used as a sacrificial material to fabricate engineered vasculature. 3D printing sacrificial Pluronic F-127 on a crosslinked layer of hydrogel is the most common method for fabricating engineered vasculature. Nothdurfter et al.85 utilized thermo-responsive Pluronic F-127 as the sacrificial material to fabricate hollow channels in a microjetted cell-laden hydrogel chip with polymethylmethacrylate as a rigid shell to mimic neuroblastoma tumor-environment model. Pluronic F-127 was printed on a layer of crosslinked cell-laded hydrogel. After that, multiple layers of cell-laden hydrogel were printed to fill the remaining space in between and above the strands of Pluronic F-127. The Pluronic F-127 templates liquefied and left the hollow channels at a temperature lower than 15°C. The final constructed chip can be seen in Figure 6F. With the channels fabricated by printing sacrificial Pluronic F-127, the artificial tissue in fluidic chips owned afferent and efferent supply vessels, which provided a new artificial platform for precise tumor-related research. Ji et al.86 utilized sacrificial Pluronic F-127 and traditional 3D printing in a cost-effective and highly operational approach to fabricate the scaffolds with customized channels. After printing the photocured matrix to the desired height, the construct was exposed to light to partially crosslink the printed matrix. Then, the additional layer was printed to embed the printed sacrificial Pluronic F-127 without exposing it to light. This printing process could be repeated several times before the entire hydrogel was fully photocured. With the removal of Pluronic F-127 by immersion in PBS, the remaining hollow channels within the hydrogel scaffold were interconnected, as shown in Figure 6G. Hynes et al.87 utilized Pluronic F-127 as the sacrificial material to construct vessel-like microchannels to mimic in vivo branched or straight vessels. With the support of a basal gel layer consisting of gelatin, fibrinogen, transglutaminase (TG), and thrombin, the sacrificial Pluronic F-127 ink with thrombin was extruded to construct tailored channel geometry. Next, the Pluronic F-127 channel template was cast with gelatin-fibrin hydrogel, and the whole template was dealt with 37°C to absolutely crosslink the fibrin. The Pluronic F-127 channel template was removed by liquefication at 4°C and gentle irrigation of cooled EndoGRO-MV medium, leaving behind the vessel-like channels. The vascularized channels with human cerebral microvascular endothelial cells could be seen in Figure 6H. The sacrificial Pluronic F-127 template represented an efficient material to fabricate a vascular network. Neufeld et al.88 designed a Pluronic F-127-based template in the form of the vasculature structure by the method of 3D printing to construct vessel-like scaffolds that mimic the tumor-stroma interacting model. With the formation of a printable platform by glioblastoma (GB)-bioink and PDMS, the tailored Pluronic F-127-based template was printed with the bioink containing Pluronic F-127 and thrombin, followed by full immersion in the fibrin 3D-bioink with patient-derived glioblastoma (PD-GB4) and microenvironment cells. The construct was overlaid with a coverslip glass, sealed in a metal frame, and dealt at 37°C until absolute crosslinking was completed and the rigidity can match the brain tissues. Finally, the sacrificial Pluronic F-127 was liquefied at 4°C and washed away, remaining the luminal channels for vascularization as shown in Figure 6I.
In the other method of using sacrificial materials, the Pluronic F-127 can be employed to fabricate hollow hydrogel fibers for fabricating engineered vasculature. Rué et al.89 utilized Pluronic F-127 as a sacrificial material to fabricate dual-layer tissue-engineered blood vessels (TEBV) to replace the damaged or pathological vessels. Pluronic F-127 was extruded from the inner syringe of a triple coaxial nozzle, while the HUVECs-laden collagen type I and the human aortic smooth muscle cells-laden alginate were printed from the middle and outer syringe, respectively. The tip of the nozzle was immersed in CaCl2 solution at 37°C. After the crosslinking of outer-layer alginate with Ca2+ and gelation of the inner-layer collagen type I at 37°C, the sample was incubated in H2O miliQ to remove the Pluronic F-127 core, leaving behind the hollow TEBV structure, as shown in Figure 6J. Based on the sacrificial Pluronic F-127 template, the triple coaxial printing approach promoted the fast construction of TEBV-like structures.
Furthermore, the sacrificial Pluronic F-127 can also be used to fabricate microfluidic chips with channels. Zhou et al.90 mixed Pluronic F-127 and nanoclay (Laponite RD) as the sacrificial material to construct PDMS microfluidic devices. The Pluronic-nanoclay ink showed adequate mechanical capacity and higher fidelity than pure Pluronic F-127 as the concentration of laponite increased. The printed Pluronic-nanoclay template was encapsulated by PDMS. After curing at 65°C, the sacrificial Pluronic-nanoclay template was dissolved in water at 4°C.
3.5 3D printing saccharide as sacrificial template
Monosaccharides and disaccharides are soluble in water due to the presence of numerous hydroxyl groups that have a strong affinity to water molecules.91 Thus, several saccharides have been explored as sacrificial materials to fabricate engineered vasculature.
Lei et al.92 utilized caramel transformed from commercial sucrose via aldol condensation and polymerization to print a caramel-based template by a simple one-pot 3D bioprinting method, as shown in Figure 7A. The customized sacrificial caramel-based template with manageable, tunable, and various parameters could be removed easily by water to fabricate perfusable and permeable hierarchical microchannel networks (PHMs). The fabricated PHMs could be seen in Figure 7B. The fabricated caramel-based template showed a rigid mechanical property and long storable time, which provided a novel technology to customize transient devices with gentle microstructures in different biomedical applications. Fang et al.93 designed a carbohydrate-based template that consists of glucose, sucrose, and maltose to fabricate a scaffold with branched channel networks and oriented micropores. The branched channel networks were formed by dissolving the 3D-printed carbohydrate-based template in water. The oriented micropores were generated using oriented thermally induced phase separation. Ha et al.94 explored the fabrication of bone scaffolds based on 3D printing sacrificial template composed of caramel transformed from sucrose, as shown in Figure 7C. An insoluble PCL layer was used to cover the caramel template. The sacrificial could be removed by water easily to form hollow microchannels owning a consistent size with the caramel fibers, which were beneficial to the formation of vessels and transportation of nutrients and bio-signals. Besides, the micropores on the PCL layer were caused by the phase separation of organic solvents. The dual-drug-laden nanofibrous scaffolds with interconnected microchannel networks were absolutely visible. Depending on the sacrificial caramel-based template, the formed microchannels played a vital role in angiogenesis and even ossification.
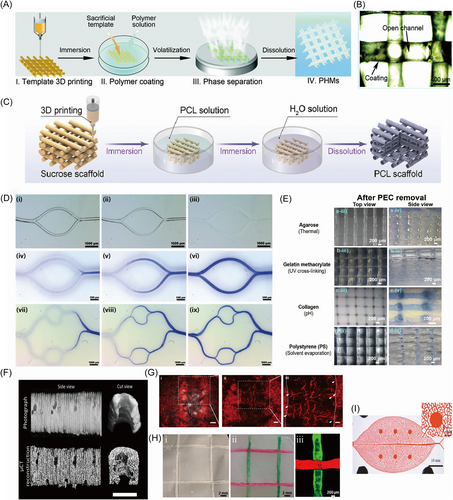
Water-soluble sugar substitute has been investigated as sacrificial materials. Kinstlinger et al.31 designed an isomalt-based sacrificial template based on isomalt and cornstarch, which possessed laser sintering ability and fluidity, respectively. Through the selective laser-sintering (SLS) system with a multimode powder distributor and a shaking sieve reservoir, the isomalt-based sacrificial material was printed into customized overhanging robust geometry with multilayered branches, smooth curvature, and high resolution. Then, the isomalt solution was used to increase the surface smoothness of the printed sacrificial template. After casting with a series of elastomers, rigid plastics, and hydrogels, the isomalt-based template was dissolved by water or PBS, leaving behind the interconnected microchannel networks. Accompanying the ability to fabricate tunable multiscale vascular networks (MSVTs), the sacrificial sintered isomalt-based template exhibited the potential to construct thick engineered tissues with suitable and functional vessels carrying oxygen and nutrients to the inner part.
3.6 3D printing PCL as sacrificial template
PCL is one of the common melting extrusion materials in 3D printing.95 Organic solvents including chloroform, dichloromethane, and dioxane can be used to dissolve PCL. Therefore, several studies explored the possibility of using PCL as sacrificial material to fabricate engineered vasculature. In the study of Yuan et al.,96 the sacrificial PCL-based template was utilized to fabricate the vascular niches and sweat gland (SG) interacted model. For the fabrication of angiogenic extracellular matrix (ECM) scaffolds (AES), the PCL microfiber templates were fabricated via a customized melt-spinning device and were extruded onto the rotating rod, and then were implanted into the subcutaneous tissue of rats to obtain fiber-cell-ECM constructs. The sacrificial PCL templates were removed by incubation with chloroform after dehydration, leaving behind the porous constructs (cell-ECM). The AES was obtained through rehydration and decellularization, which exhibited higher porosity than nonangiogenic ECM scaffolds (NES). Nie et al.97 designed an ultrafine fiber network as a sacrificial template to construct multiscale channels within hydrogel chips to manufacture a cancer-vessels interacted in vitro model. The tailored ultrafine fiber network was established by FDM printing and electro-hydrodynamic (EHD) printing, followed by incubation in gelatin-GelMA pre-polymer solution. A twice-crosslinking strategy of gelatin gelation at 4°C and GelMA crosslinking under UV light was called a twice-crosslinking strategy was applied to form the hydrogel construct. The sacrificial tailored ultrafine fiber network was removed manually, leaving behind the multiscale hollow channels. An in vitro breast cancer tissue with a functional vascular network was made successfully. With no harm to cell culturing, the sacrificial ultrafine fiber template could be utilized in the fabrication of various in vitro engineering vascularized models. In their later study, soft ultrafine fiber mold (SUFM) was proposed to fabricate ultrafine channels.98 Sacrificial PCL was used in EHD printing to prepare SUFM. After casting, cooling, and curing of the hydrogel matrix, the SUFM could be separated from the cured hydrogel to release the microchannels, which was called damage-free demolding. Compared to the traditional molds and the demolding approach, SUFM and the damage-free demolding method could better guarantee the integrity of hydrogel.
3.7 3D printing other polymers as sacrificial template
Several customized materials have been developed for 3D printing sacrificial templates. Recently, Ryma et al.99 explored the fabrication of vessel-like branched multiscale microchannels based on a novel print-and-fuse approach by using poly (2-cyclopropyl-2-oxazoline) (PcycloPrOx). Through melt electrowriting, thermosensitive PcycloPrOx filaments were deposited together. And then, PcycloPrOx filaments swelled and fused in an aqueous environment at a temperature of 25°C higher than the LCST. After the gelation of hydrogel matrices, the PcycloPrOx template was removed facilely at a temperature below the LCST, leading to the formation of microchannels, as shown in Figure 7D. Owing to the functions such as tuneable channel parameters, facile storage condition, ease of removal, and promotion of endothelialization, the PcycloPrOx polymer represented significant progress in the development of vascularized tissue constructs. Through the embedded 3D printing (EB3DP) approach proposed by Wang et al.,100 a sacrificial polyelectrolyte complex (PEC)-based template was printed into the supporting matrix of Pluronic F-127. The PEC ink was prepared by using Poly (diallyldimethylammonium chloride) (PDADMAC), poly (sodium 4-styrenesulfonate), and sodium chloride. The printed PEC-based templates were cast with natural or synthetic materials such as agarose, collagen, poly (1, 8 octanediol-co-citrate), GelMA, and so on, with no harm to PEC when crosslinked. And then, all the PEC-based templates were removed by potassium bromide aqueous solution, while the PEC coacervate could be collected and recycled. The perfusable and hierarchical scaffolds with porous channel networks could be seen in Figure 7E. As one of the rare materials tunable with various castable materials, PEC exhibited a bright possibility of wide application for the formation of interconnected hierarchical porous channels in the scaffolds.
The integration of engineered vasculatures with host vessel networks can be a challenge in the biomanufacturing field. Therefore, Szklanny et al.101 proposed a method of fabricating engineered hierarchical vascular constructs. Water soluble butanediol vinyl alcohol copolymer (BVOH) was used to print a mold in the form of a pipe for producing poly (l-lactic acid)/poly (lactide coglycolide) (PLLA-PLGA) vessel-like scaffold, as shown in Figure 7F. Endothelial cells were seeded in the lumen of the vessel-like scaffold to organize natural-like endothelia. Then, the cell-laden scaffold was stuck into a vascularized tissue printed by recombinant human collagen methacrylate (rhCollMA), which formed the engineered tissue flaps together. The results demonstrated that the engineered tissue flaps can anastomose with a rat femoral artery to promote the integration of host and implanted tissues, which shows great promise for creating functional tissues. In their later study, templating rods and a PDMS mold were utilized as sacrificial templates for the formation of MSVTs within the implantable tissues.102 BVOH mold was 3D printed to fabricate the PDMS mold. The templating rods were inserted into the PDMS mold directly. Then, the space that remained in the PDMS mold was cast completely with salt-leached PLLA/PLGA to provide porous scaffolds with mechanical properties. After that, a human adipose microvascular endothelial cells—and dental pulp stem cells—laden collagen gel was used to fill the porous scaffolds. After removing the templating rods, endothelial cells were seeded into the channel to form an endothelial lumen within macrovessels. The MSVTs within thick engineered tissues for implantation containing constructed macrovessels and self-assembling microvessels could be seen obviously in Figure 7G.
PDMS has been widely used to fabricate microfluidic chips due to its high optical transparency, low autofluorescence, low toxicity in its cured form, and biocompatibility.103 Owing to the easy formability of PDMS, it has also been explored to be used as sacrificial material. Li et al.104 utilized PDMS as the sacrificial material to construct microfluidic channels in tailored paper chip devices for tridimensional in vitro tissues. The PDMS inks were injected into the bacterial cellulose matrices to form microfiber with the designed shape, followed by rigidification of PDMS, drying, and immersion in sodium borohydride (NaBH4) solution, without destroying the completeness of PDMS microfibers. With lyophilization of the matrices, PDMS microfibers were facilely extracted, remaining interconnected and perfusable microchannels. A vascularized breast tumor model was constructed with HUVECs in channels and MCF-7 breast tumor cells in surrounding matrices. The sacrificial PDMS microfibers exhibited low cost, adequate mechanical properties, and ease of extraction from matrices, which indicated the potential for the development of volumetric human tissue models.
Suspension bath-based printing shows great potential for fabricating 3D structures. Cheng et al.105 printed the hydrophobic petroleum jelly–liquid paraffin in bacterial cellulose hydrogel to fabricate microchannels. The printed petroleum jelly–liquid paraffin was removed by liquidation at 70°C and rinsing with n-hexane, ethanol, and water, leaving behind the paper device with interconnected and perfusable microchannels as shown in Figure 7H. HUVECs in the microchannels and MCF-7 cells in the matrix showed high viability, which demonstrated that the vascularized in vitro breast tumor model was successfully fabricated.
Ozbolat et al.106 designed Carbopol as a novel sacrificial material in an extrusion-based printing method to fabricate microchannels in PDMS microfluidic devices. After constructing and curing the basal layer and framework of PDMS with different ratios, the Carbopol ink with Bingham plastic behavior was printed inside the frame to form a stable channel template according to suitable extrusion parameters, followed by casting and curing with the PDMS layer. The sacrificial Carbopol templates were washed away by water and Dulbecco's phosphate-buffered saline (DPBS).
Celikkin et al.107 utilized reverse thermosensitive PolyIsoCyanide (PIC) hydrogel as the sacrificial material to fabricate hollow channels in GelMA hydrogel. The synthetic PIC water solution was printed by a 3D bioplotter freely with the cartridge temperature at 12–15°C while the temperature of the printing bed was set at 37–40°C. Then, the fugitive PIC template was soaked by GelMA at a temperature above the gelation temperature of PIC. After UV crosslinking of GelMA, the sacrificial PIC template was removed by precooled PBS to generate hollow channels in GelMA. The cytocompatibility of PIC exhibits great potential for fabricating vascularized tissues.
Inspired by the ubiquitous biological systems in leaf venation, Mao et al.9 proposed a method of fabricating a leaf skeleton-like vascular network based on a mold-based method. The flat venation skeleton was first extracted from Osmanthus fragrans leaves. Then, silicon wafers were sculptured into the leaf skeleton-like network by the SU-8 micropatterning method, followed by the treatment with octafluorocyclobutane (C4F8) for convenient removal later. After that, the defined silicon mold was cast with PDMS prepolymer and removed after the solidification of PDMS at 95°C to release the leaf skeleton-like vascular network. The human-on-leaf-chip system with a hierarchically branched vasculature network in chambers could be seen in Figure 7I. The leaf skeleton-like sacrificial silicon mold exhibited great possibility to freely tailor vasculature structure for engineering vascularized tissues.
3.8 Summary of sacrificial polymer
PVA, PLA, and PCL are commonly used as printing filaments in FDM. Thus, sacrificial templates with complicated structures can be easily fabricated by using these polymers. In FDM, PVA is widely used as a support material to assist the fabrication of 3D constructs due to its water-soluble feature.108 In the field of tissue engineering, PVA has been explored as a drug delivery carrier.109, 110 Therefore, the high biocompatibility of PVA provides great potential of being used as a sacrificial material in fabricating in vitro tissues. To produce biological scaffolds with engineered vasculature, sacrificial templates are usually encapsulated by hydrogels. PVA shows high solubility in water.111 Thus, it is possible to dissolve the PVA templates before the gelation of hydrogels, which results in size deformation caused by the swelling of PVA in the water-contained environment.112 Although some studies have explored the coating on PVA templates to alleviate the swelling caused by the rapid dissolving, an extra agent for coating should be introduced in the fabrication process.113, 114 Therefore, the rapid dissolving and swelling of PVA templates should be further explored to reach precise fabrication of engineered vasculature. PLA can be extruded from a nozzle in a 3D printer based on FDM by heat to a temperature of around 200°C.115 The temperature for melting PCL is 55–60°C.116, 117 Therefore, sacrificial templates with intricate structures can be easily fabricated by using PLA and PCL. In the studies introduced above, the fabrication of sacrificial PLA templates is mainly based on FDM. For sacrificial PCL templates, electrospinning and electrohydrodynamic (EHD) jet printing can also be applied to form PCL templates with small sizes, which offers the feasibility of fabricating multi-scale engineered vasculature. However, the removal of PLA and PCL requires organic solvents including chloroform and dichloromethane. This may cause cell damage when using a cell-laden scaffold matrix. Thus, cells are usually seeded into the hydrogel scaffolds and engineered vasculature after removing and sterilizing the constructs.
PNIPAM is an attractive material in the field of biomedical engineering applications. The LCST of 32°C renders PNIPAM with different behaviors at different temperatures.118, 119 The dissolving of PNIPAM at the temperature under LCST can be applied to removed sacrificial PNIPAM templates. In the studies of using PNIPAM as sacrificial material to fabricate engineered vasculatures, PNIPAM is electrospun to produce sacrificial templates with microfibers, which increases the scalability of 3D printed sacrificial templates. However, rare studies have explored the 3D printing of PNIPAM to fabricate macrosize templates. Thus, using PNIPAM as sacrificial material to create multiscale templates can be a potential research topic.
Pluronic F-127 is a thermoreversible polymer and a representative sacrificial material for engineered vasculature creation.120, 121 At the temperature of 25°C, Pluronic F-127 shows shearing-thinning behavior, thixotropic properties, and superior printability,122, 123 which can be used to print sacrificial templates with structural integrity. The printed Pluronic F-127 templates can be liquified and removed after cooling to 4°C.124 Thus, Pluronic F-127 can be a prominent sacrificial material for fabricating engineered vasculature. Moreover, the function of using Pluronic F-127 as a suspension bath has also been examined in the study of Wang et al.100 However, in situ casting of scaffold matrix should be conducted after the printing of sacrificial Pluronic F-127 templates due to the poor mechanical of Pluronic F-127.125 Thus, the printed Pluronic F-127 templates are not stable. Moreover, Pluronic F-127 lacks cell adhesion properties. This can be explained by the fact that Pluronic F-127 does not contain arginine/glycin/aspartate motifs.126 Hence, it may not be suitable to load cells during the 3D printing process.
Saccharide-related sacrificial materials have been widely used in 3D printing sacrificial templates for fabricating engineered vasculature. FDM and SLS are utilized to print saccharide-related sacrificial materials in the study introduced above. It shows that saccharide-related sacrificial materials can be processed by various methods to form designed structures. The printing of saccharide-related sacrificial materials requires heating to be melted.127, 128 Thus, cells cannot be loaded into the printing process.
Various customized sacrificial materials have been proposed to 3D print sacrificial templates, which exhibit considerable potential for fabricating biomimetic-engineered vasculature. A specific solvent may be required to remove the printed sacrificial templates. For instance, the solvent for dissolving the printed PEC-based templates is potassium bromide, which increases the operational complexity. Some customized sacrificial materials, including PcycloPrOx and PIC that can be dissolved in water, show great promise for fabricating engineered vasculature. Several customized sacrificial materials can be used as printed ink in suspension bath-based printing. This offers distinct choices for fabricating engineered vasculature with 3D and intricate geometries. The study of Mao et al. used the mold-based technique to fabricate biomimetic-engineered vasculature. Although 3D printing was not used in this study, we also introduced this work. Interestingly, the flat leaf venation skeleton was used as the sacrificial template to produce engineered vasculature which has the same geometry as its native counterpart. This study provides an advanced approach for duplicating bionic structures.
4 CONCLUDING REMARKS AND FUTURE PERSPECTIVES
3D printing sacrificial templates have shown remarkable potential for fabricating engineered vasculature due to their feasibility and versatility. Numerous engineered vasculatures with intricate structures were fabricated by using the method based on 3D printing and sacrifice-based technique. Different cell seeding methods were proposed to mimic the in vitro tissues or models. However, current strategies of 3D printing sacrificial templates for engineering vasculature still have some drawbacks in producing biomimetic vasculature.
Extrusion-based printing is one of the main approaches to fabricate hydrogel or polymer sacrificial templates. Thus, the size of the sacrificial templates is uniform due to the continuous extrusion of the printing nozzle. The corresponding vasculature lacks the hierarchical structure of the native vessels. The hierarchical structure of native vessels could reduce energy dissipation and reach maximum efficiency of energy use when transporting blood.129, 130 Therefore, it is crucial to build hierarchical vasculature within tissue engineering scaffolds.
The other research topic of engineered vasculature is the formation of endothelial monolayers. Several studies introduced above lack the seeding of endothelial cells and further investigation of cell interactions. Lack of the endothelial monolayer results in blood infiltration and thrombosis after implantation due to direct contact of the platelets with the scaffold matrix.131, 132 The endothelial monolayer acts as a barrier between the blood and interstitial compartments.133 Therefore, the creation of endothelial monolayer within engineered vasculature plays a central role in the successful implantation. Moreover, multiple cell types are distributed at the corresponding position to realize the precise physiological appearance of native vessels.134 The inner surface of the blood vessel contains a monolayer of endothelial cells, the media layer consists of smooth muscle cells that act as a support to maintain intravascular pressure and tissue perfusion, and the outer layer is composed of fibroblast cells to maintain quiescence of blood vessels.135-137 The crosstalk between endothelial cells and smooth muscle cells or fibroblast cells is essential for the formation of blood vessel functions.138 Thus, it is necessary to build engineered vasculature with multiple layers of cells to enhance the integration efficiency between a scaffold and host. In the study of Gao et al.139 printed coaxial hydrogel fiber in a support matrix achieved the fabrication of engineered vasculature with endothelial cells, smooth muscle cells, and fibroblast cells, which offers an innovative method to construct biomimetic vasculature.
Suspension bath-based printing grants a great promise technique for fabricating freeform and 3D structures in a support bath. Hence, printing sacrificial materials in scaffold materials that also act as support matrices has been an attractive method to fabricate engineered vasculature.140 We can observe that the studies of fabricating engineered vasculature by using suspension bath-based printing require specific support material to hold the printed structure, which limits the serviceable range and applications of the method. Therefore, the development of a versatile support matrix to assist the printing of multiple inks can be a research topic in the future, which can also be a focus of scale-up manufacturing.
In summary, the fabrication of engineered vasculature is crucial to maintain the viability and function of cells loaded in the in vitro scaffolds. It is critical to guide the design of biomimetic scaffolds to have a better understanding of the analogous physiological features in vivo. Therefore, the advancement of the manufacturing process, cell loading mode, and versatile material can be the main research interests of 3D printing sacrificial templates for fabricating biomimetic-engineered vasculature.
AUTHOR CONTRIBUTIONS
Shuai Li: Conceptualization (lead); investigation (equal); project administration (lead); writing—original draft (lead); writing—review and editing (lead). Hangyu Li: Investigation (equal); writing—original draft (equal). Xiushuai Shang: Writing—original draft (equal); writing—review and editing (equal). Jiayan He: Investigation (supporting). Yihe Hu: Methodology (equal); supervision (lead); writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank the support from the Research Start-up Fund of the First Affiliated Hospital, Zhejiang University School of Medicine. We thank Dr. Yihao Liu at Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine for providing the support of preparing Figure 1.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




