Recent progress in orodispersible films-mediated therapeutic applications: A review
Abstract
Orodispersible films (ODFs) as a relatively novel delivery platform have increasingly attracted enormous attention owing to their various advantages compared to conventional oral dosage forms, including fast disintegration, ease of administration without consumption of water, and rapid absorption of incorporated drug, high patient compliance for pediatrics, geriatrics and patients with swallowing difficulties. This review aims to summarize the developments and possibilities that ODFs as the potential carrier for chemical drugs, vaccine, probiotics, and herbal extracts. Especially, with the outbreak of coronavirus disease 2019 (COVID-19), the advantages of ODFs related to ease of transporation and distribution without cold-chain as well as low-cost manufacturing make ODFs a promising carrier for vaccines against COVID-19. Subsequently, the current therapeutic applications of ODFs delivered either locally or systemically for potential patients suffering from various diseases are discussed, including oral inflammation, cardiovascular diseases, pain disorders, nausea and vomiting, mood or mental disorders, erectile dysfunction and pulmonary diseases. Finally, this review provides overview of the novel inkjet printing and three-dimensional printing techniques, and the possibility of extemporaneous preparation for ODFs in hospital pharmacies via printing techniques are discussed as well.
1 INTRODUCTION
Oral dosage forms are the most preferred and most commonly used drug delivery system due to its ease of self-administration and high patient compliance. Among them, oromucosal films are gaining many interests from researchers owing to its fast drug disintergration. Oromucosal films, as listed in the European Pharmacopoeia (Ph. Eur. 9.0),1 including orodispersible films (ODFs) and mucoadhesive buccal films.
ODFs are single- or multilayer sheets that can disperse rapidly when orally administrated, usually within 30 s. ODFs were directly administrated to the tongue and would rapidly dissolve once ODFs are hydrated with saliva in oral cavity. The dissolved ODFs together with the incorporated drug compounds are swallowed along with saliva to absorbed from gastrointestinal tract into the systemic circulation. Therefore, ODFs can be administrated without the need of water, minimizing the risk of choking and the patient compliance is improved. ODF is a kind of promising dosage form with significant advantages: 1) Administration of ODFs do not require water. The easy administration of ODFs makes it a promising dosage form for pediatric and geriatric patients. Since ODFs are difficult to be spit out once administered, uncooperative patients can benefit from it as well. 2) After contacting with saliva, the dissolvable characteristics of the ODFs lead to the fast disintegration in the oral cavity. 3) The active pharmaceutical ingredients (API) from ODFs can be absorbed via gastrointestinal tract after swallowing. Additionally, ODFs enable rapid onset of action of API by penetrating oral mucosa.
With the advent of first oral film Listerine® Pocketpaks Breath Strips in 2001 by Pfizer,2 there are some ODFs products becoming commercially available now. For example, Chloraseptic® with benzocaine was launched in 2003 for sore throat treatment. In 2013, Setofilm™ with ondansetron was available in the market for the treatment of chemotherapy and radiotherapy-induced nausea and vomiting (shown as Table 1).
| Commercial name | Active ingredients | Dose | Film type | Use | Manufacturer |
|---|---|---|---|---|---|
| Suboxone® | Buprenorphine/naloxone | 12 mg/3 mg, 8 mg/2 mg, 4 mg/1 mg, 2 mg/0.5 mg | Monolayered sublingual film | Opioid dependence | Indivior, Inc. |
| Triaminic thin strips | Dextromethorphan/Phenylephrine | 3.67 mg/2.5 mg | Monolayered ODFs | Children's cold and cough | Novartis, Inc. |
| Listerine POCKETPAKS® | Mints | / | Monolayered ODFs | Fresh breath and killing bad-breath germs | Pfizer, Inc. |
| Chloraseptic® Sore Throat Relief Strips | Benzocaine | 3 mg | Monolayered ODFs | Sore throat | Medtech Products, Inc. |
| Orajel® Toothache Strips | Benzocaine/menthol | 15 mg/2.5 mg | Monolayered ODFs | Relieving pain of the mouth and gums | Church & Dwight Co., Inc. |
| Gas-X thin Strips® | Simethicone | 62.5 mg | Monolayered ODFs | Relieving belching, bloating, and feelings of pressure/discomfort in the stomach/gut | Novartis Consumer Health, Inc. |
| Snoreeze Oral Strips® | Peppermint oil/vitamin E/sodium hyaluronate | / | Monolayered ODFs | Reducing snoring | Passion For Life Healthcare (UK) Ltd. |
| Benadryl® Quick Dissolve Strips | Diphenhydramine HCl | 25 mg | Monolayered ODFs | Relieving allergy, sneezing and runny nose | Johnson & Johnson, Inc. |
| Donepezil RapidFilm™ | Donepezil | 5 mg and 10 mg | Monolayered ODFs | Alzheimer's disease | Tesa Labtec GmbH/APR Applied Pharma Research s.a. |
| Olanzapine RapidFilm™ | Olanzapine | 5 mg, 10 mg and 15 mg | Monolayered ODFs | Schizophrenia | Tesa Labtec GmbH/APR Applied Pharma Research s.a. |
| Zolmitriptan RapidFilm™ | Zolmitriptan | 2.5 mg and 5 mg | Monolayered ODFs | Migraine | Tesa Labtec GmbH/APR Applied Pharma Research s.a. |
| Setofilm™ | Ondansetron | 4 mg and 8 mg | Monolayered ODFs | Treatment for chemotherapy and radiotherapy-induced nausea and vomiting | Tesa Labtec GmbH/APR Applied Pharma Research s.a./Norgine BV |
| Zuplenz® oral soluble film | Ondansetron | 4 mg and 8 mg | Monolayered ODFs | Prevention of nausea and vomiting | Galena Biopharma, Inc. |
| IvyFilm | Ivy leaves (Hedera helix) | 16 mg | Monolayered ODFs | Relief of productive cough | Lamar International (Pty) Ltd. |
| Onsolis®/Breakyl® | Fentanyl | 200 μg, 400 μg, 600 μg, 800 μg and 1200 μg | Bi-layered buccal soluble film | Breakthrough pain in patients with cancer | Meda Pharmaceuticals, Inc./BioDelivery Sciences International, Inc. |
| BELBUCA® | Buprenorphine | 75 μg, 150 μg, 300 μg, 450 μg, 600 μg, 750 μg, and 900 μg | Bi-layered buccal soluble film | Relieving of chronic pain | Endo Pharmaceuticals Inc./BioDelivery Sciences International, Inc. |
| BUNAVAIL® | Buprenorphine/naloxone | 2.1 mg/0.3 mg, 4.2 mg/0.7 mg and 6.3 mg/1 mg | Bi-layered buccal soluble film | Treatment for opioid dependence | BioDelivery Sciences International, Inc. |
- Abbreviation: ODFs, orodispersible films.
However, the main hurdle of ODFs is the low drug-loading capacity that can be formulated mainly due to the limited thickness. Attempting to overcome this limitation, many multilayered or bilayered ODFs were developed nowadays aiming to increase drug-loading.3 Besides, the choice of the incorporated drug compounds is crucial. The highly potent drug with relative low dose is more suitable for embedded in ODFs. For example, mosapride citrate is used for the treatment of gastroesophageal reflux disease with swallowing difficulties at the dose is either 5 mg or 10 mg three times per day. Hence, ODFs can be an ideal carrier for this kind of drug with low-dose requirement.4
In addition to chemical drugs, ODFs were also used as the carrier for delivery of herbal extracts, probiotics, and even vaccines.5-7 With a wide range of suitable drug compounds that are capable to load into ODFs, the therapeutic application of ODFs rapidly increased with more and more potential patient groups can benefit from using ODFs as the delivery platform in recent years. Currently, the production method of ODFs is mainly solvent casting method. Lately, various printing techniques occur and were employed in ODFs, such as inkjet printing and three-dimensional printing.8, 9 These novel production methods of ODFs indicated the possibility of individualized medicine, which means the dose can be tailored based on the different requirements of a specific patient by a community or hospital pharmacist.
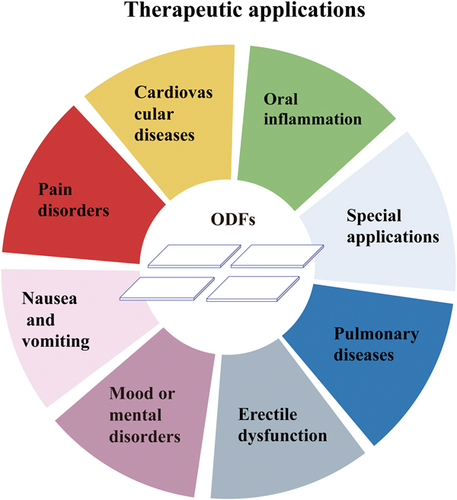
This review is focused on the recent development of ODFs for the delivery of chemical drugs, herbal extracts, probiotics, and vaccines. The further section of the review highlights the current applications and potential patient groups that are capable to utilize ODFs as a drug delivery system (Figure 1). Finally, the personalized medicine based on printing techniques employed for the fabrication of ODFs is also discussed.
2 ODFS AS THE POTENTIAL CARRIER FOR VARIOUS INGREDIENTS
2.1 Chemical drugs
Both water-soluble and poorly water-soluble chemical drugs can be incorporated into ODFs.10, 11 When a drug is absorbed via the buccal route, the absorption of the drug is mainly passively diffuse across the lipid membranes via para-cellular and trans-cellular pathways, therefore, both hydrophilic and lipophilic drugs could be transported.12
Water-soluble drugs can be easily dissolved with saliva in the oral cavity, however, the excessive first-pass metabolism and short half-life of drugs may lead to poor bioavailability after oral administration, for example, propranolol.13 Therefore, frequent administration might be needed for drugs with short biological half-life. As ODFs are an easy administration dosage form, hence, ODFs are capable to be utilized for administration frequently even without of consuming extra water.
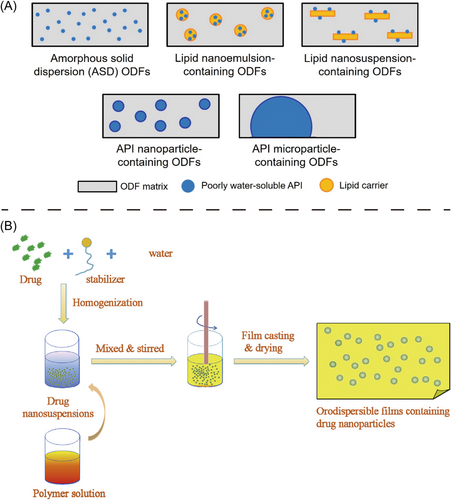
In addition, poorly water-soluble drugs can be embedded in ODFs as well, despite their characteristics of limited aqueous solubility and slow dissolution rate limit the oral bioavailability.14 Different strategies have been made to improve solubility of poorly water-soluble drugs in ODFs formulation. For instance, micronization, nanoparticle technology,15 solid dispersion,14 solubility enhancers,16 all these techniques contributed to enhanced dissolution rate and bioavailability of the drug as well as improving the incorporated amount of drug. Among these methods, particle size reduction technologies are commonly used to increase surface aera of drugs, and hence enhance its bioavailability.10 Recently, Nguyen et al. nanosized the poorly water-soluble loratadine by wet balling milling technique, afterward embedded it on ODFs, which showed enhanced bioavailability in rats compared to the raw loratadine.10 The ODFs based on amorphous solid dispersions enhance dissolution rate of the drug via formation of interaction between the drug and soluble carriers.17, 18 Senta-Loys et al.17 proposed tetrabenazine dispersed in citric acid and polymer to form amorphous solid dispersions in ODFs delivery platform. In their study, the dissolution rate of tetrabenazine was considerably improved, tetrabenazine could even remained in amorphous state during 6 months storage when HPMC or pullulan was used as the polymer.18 In terms of nanotechnology, on the one hand, nanoparticles contributed to protecting the incorporated API against degradation from the digestive enzymes or acidic pH condition. On the other hand, the ingredients of nanoparticles like lipids enhance the absorption of the API via the lipolysis pathway.15, 19 Lately, Steiner et al.15 used five formulation strategies to incorporate different poorly water-soluble APIs in ODFs, including preparation of amorphous solid dispersions, incorporation of APIs in lipid nanosuspension and lipid nanoemulsion, wet-milling APIs into nanoparticles or microparticles, afterward APIs were embedded into ODFs (Figure 2). The results demonstrated that amorphous solid dispersions-based ODFs and lipid nanoemulsion containing ODFs showed the most desirable characteristics with fast disintegration. However, different solubility-enhancing formulation strategy has its pros and cons. The suitable formulation method should be considered according to the lipophilicity of APIs as well.21 Additionally, thiolation of polymeric excipients enables to improve the solubility of less water-soluble drugs as well. The inter- and intrachain disulfide bonds during the swelling process can improve the stability of carrier matrix and achieve sustained or controlled drug release.22, 23 Furthermore, solubility enhancers (such as organic solvent and organic acid) were also employed with poorly soluble drugs. For example, organic acid could affect pH-dependent solubility like weakly acidic or basic molecule. However, the recrystallization of API and the containing traces of solvent residues should be considered.16
2.2 Vaccine
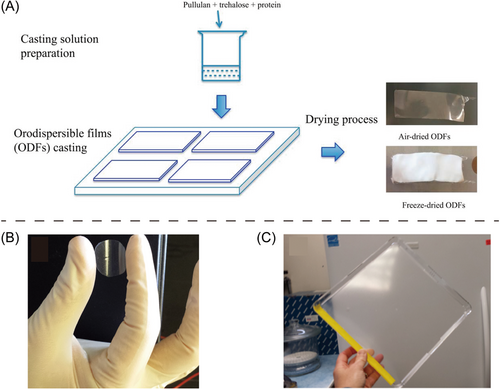
Oral administration of vaccine remains to be a challenging task due to its low inherent stability, degradation, and metabolism, causing poor bioavailability. Many efforts have been applied to preserve vaccine in ODFs while maintaining its stability and activity.24 Tian et al.25 first incorporated protein in the trehalose and pullulan-based ODFs and used it for protein delivery in oral cavity (Figure 3A). Afterward, they developed an ODF containing influenza virus vaccine and achieved the vaccine remaining stable even at high temperature (60°C) or humidity (56% relative humidity) conditions for 1 month.5 Moreover, Bajrovic et al.24 suggested that influenza virus vaccine formulated in an ODF platform and administered via oral route induced similar immune responses or even better than those that were administered via intramuscular route (Figure 3B,C). In sum, ODFs are flexible, portable, and enables to maintain vaccine stability during transportation, as well as ODFs with ease of administration are cost-effective, which allows ODFs a kind of potential alternative carrier for vaccine, especially during the outbreak of epidemic and pandemic.
Remarkably, the current coronavirus disease 2019 (COVID-19) has highlighted the importance of using vaccine for reducing infection and transmission of SARS-CoV-2 and preventing serious illness or death from COVID-19. At present, several vaccines for COVID-19 have been developed, however, most of them are intramuscular injections. Those aqueous vaccines need to be maintained in cold-chain condition during transportation and storage, increasing costs and even limiting the utilization of COVID-19 in some tropical countries. Hence, using an alternative dosage form would be beneficial not only to the government for reducing shipping cost, but also to the patients who have needle phobia. In 2021, oral recombinant vaccines against COVID-19 have been developed by Vaxart, which was the world's first oral pill vaccine for COVID-19. Vaxart announced the this oral vaccine had positive results in a clinical phase II study.26 Based on this, ODFs would also be a promising carrier for vaccine against COVID-19, because ODFs cost even less than the manufacturing of solid tablets, and also some problems of tablets can be obviated, such as the risk of choking.
2.3 Probiotics
Probiotics have shown great potential in the treatment of oral diseases and improving oral health, including caries and caries-associated microbes, periodontal diseases, oral candida, and halitosis.27 Probiotics are living microorganisms, they may easily lose probiotic viability in harsh stomach conditions. Therefore, the alternative delivery route such as delivering to the oral cavity is preferred, which is capable to rapidly disintegrate and release incorporated probiotics in the mouth, thereby bypassing acidic condition of gastrointestinal tract.28 Studies have shown that administration of probiotics using ODFs is an ideal alternative dosage form as the viability of probiotic can be maintained in ODFs.29 For example, an ODF loaded with probiotic Enterococcus faecium CRL 183 was proposed by Lordello et al.7 The probiotic in the ODFs maintained high viability up to 90 days of storage and high antifungal activity to prevent oral candidiasis. Saha et al.30 formulated ODFs with carboxymethyl cellulose (CMC) as film-forming polymer and delivered Lactobacillus fermentum NCIMB 5221 for the treatment of periodontitis. Probiotics maintained their probiotic viability in this CMC-based ODFs within 150 days of storage at room temperature under ambient humidity. Lately, various techniques have been explored to increase the loading of probiotics and prolong its release in the oral cavity with the aim to achieve better antifungal activity of probiotics. For instance, an ODF was prepared with xylitol using inkjet printing for oral delivery of Streptococcus salivarius. This ODFs made of S. salivarius and xylitol caused 2.86-Log reduction of S. mutans numbers after co-incubation and achieved complementary and enhanced antibacterial activity against S. mutans.29, 31
2.4 Herbal extracts
Natural or herbal pharmaceuticals are widely used in Asian countries. These natural pharmaceutics have gained constant interest from many researchers due to their extensive pharmacological effects, including anti-inflammatory and antioxidant activity (curcumin),32 anticancer (Cucurbitacin B),33 anesthetic activity (Jambu).34 However, the poor aqueous solubility, fast degradation at physiological pH, the lack of flexible and high patient-compliance dosage form have hindered the extensive application of natural pharmaceuticals. Nowadays, researchers have investigated the incorporation of natural pharmaceuticals into ODFs. For example, Indonesian medicinal plants were embedded in ODFs allowing them to be more flexible for intraoral delivery.35 Nguyen et al.6 prepared an ODF with the loading of Panax notoginseng showing high stability at acidic pH, fast disintegration, and in vitro release. As the stability of herbal extracts can be maintained in ODFs with the ease of administration for elderly people, ODFs containing herbal extracts could overcome their inborn defects and serve as an efficient carrier for those people in the treatment of chronic diseases.
3 CURRENT THERAPEUTIC APPLICATIONS FOR POTENTIAL PATIENTS
ODFs can be formulated and designed to obtain either local or systemic absorption. The total surface area of the oral cavity is around 170 cm2.36 About one-third of these areas, including buccal mucosa are lined by non-keratinized stratified epithelium, which is more elastic and permeable, hence more suitable for drug delivery.37 The rich capillary network and large surface area of buccal mucosa enable the systemic absorption of drugs for buccal delivery. Also, ODFs can achieve systemic absorption via gastrointestinal tract after swallowing.
On the one hand, ODFs are advantageous dosage forms for topical use in oral cavity. Compared with gels, ointments, and sprays, whose drawbacks are the lack of bioadhesiveness and having difficulties in accurate dose due to the possibility of dilution by the saliva, ODFs have high local drug concentration, nonirritating and flexible to use, adjustable size, and personalized dose, making it an attractive candidate for the treatment of diseases affecting the oral cavity.
On the other hand, systemic delivery of ODFs by swallowing through gastrointestinal tract is commonly preferred route, or through oral mucosa directly to the systemic circulation, which can trigger prompt onset of action. However, there is an absence of available dosage forms targeting for pediatric, geriatric, and dysphagic patients. The ease of administration and no need of water make ODFs are ideal dosage form for those patients.
3.1 Oral inflammation
The most common oral mucosal disorders are oral ulcer, erosive lesions, oral mucositis, and stomatitis.35 Oral ulcer is often recurrent, painful, and slow to heal and is estimated to affect around 20% of the general population.38 When a situation gets worse, bullous, erosive lesions would form. Topical treatment of oral ulcers and erosive lesions using ODFs offers the formation of a layer above the wound surface to protect it against secondary infection and further possible irritation, thus enhancing the therapeutic effect on ulceration. Moreover, oral mucositis and stomatitis were the main adverse effect of cytotoxic chemotherapy and radiation therapy for cancer patients. The chemotherapy drugs and radiation would cause damage on mucosal epithelial cells, increasing the risk of oral infection and inflammation. However, the current market lacks local, long-acting oral infections dosage forms, rather than mouthwash or oral ointments. ODFs seem to be an alternative formulation to mouthwash or oral ointments.7 Pechová et al.39 incorporated a topical anti-inflammatory drug benzydamine hydrochloride into the ODFs. In addition to film-forming polymers and plasticizer, the sweeteners and a superdisintegrant Kollidon® CL-F were also used in this ODF.
3.2 Cardiovascular diseases
Cardiovascular diseases include several heart and blood vessels-related disorders. According to World Health Organization (WHO), cardiovascular diseases cause the highest amount of death globally. It is estimated 17.9 million people die each year from cardiovascular diseases.37 Extensive drugs were used for the treatment of cardiovascular diseases. There are also many commercially available dosage forms for these drugs, such as oral tablets, capsules (Table 2). However, some of these drugs (e.g., propranolol) may suffer from extensive first-pass metabolism after oral administration, thus leading to low bioavailability. Moreover, fixed-dose cardiovascular drugs in form of tablets or capsules are not suitable for pediatric use. Therefore, many research works have been done to incorporate cardiovascular drugs, for example, propranolol, enalapril maleate, metoprolol tartrate in ODFs.11, 40, 41 The ODFs containing enalapril maleate fabricated by inkjet printing were developed. The therapeutic single dose for children 0.5 mg enalapril has been successfully printed onto the film. The hydrochlorothiazide films were used to imprint with enalapril ink for further investigation of the enalapril immigration, and no enalapril immigration was observed.8 Zhao et al. reported the amlodipine besylate-loaded ODFs using an electrospinning technique, showing the enhanced physicochemical properties.42 The angiotensin-converting enzyme inhibitor captopril can also be loaded in ODFs for hypertension.43 Based on the numerous advantages of ODFs as mentioned above, to utilize ODFs in the treatment of cardiovascular diseases would considerably improve patient compliance, especially for hypertensive patients after stroke.
| Types | APIs | Brand name | Dosage forms | Use |
|---|---|---|---|---|
| Anticoagulants | Apixaban | Eliquis | Tablets | Preventing first or recurrent stroke; Reducing blood clots in the blood vessels; Treatment for deep vein thrombosis and pulmonary embolism |
| Dabigatran | Pradaxa | Capsules | ||
| Edoxaban | Savaysa | Tablets | ||
| Heparin | Heparin | Injection | ||
| Rivaroxaban | Xarelto | Tablets, oral suspension | ||
| Warfarin | Coumadin | Tablets, injection | ||
| Antiplatelet agents and dual antiplatelet therapy (DAPT) | Aspirin | Aspirin | Capsules, tablets | Preventing blood platelets clumping together; Reducing blood clots in the blood vessels; Treatment for heart attack, coronary artery disease, and ischemic strokes |
| Clopidogrel | Plavix | Tablets | ||
| Dipyridamole | Persantine | Tablets | ||
| Prasugrel | Effient | Tablets | ||
| Ticagrelor | Brilinta | Tablets | ||
| Angiotensin-converting enzyme (ACE) inhibitors | Benazepril | Lotensin | Tablets | Treatment for high blood pressure and congestive heart failure |
| Captopril | Capoten | Tablets | ||
| Enalapril | Vasotec | Tablets | ||
| Fosinopril | Monopril | Tablets | ||
| Lisinopril | Prinivil, Zestril, Qbrelis | Tablets, oral solution | ||
| Moexipril | Univasc | Tablets | ||
| Perindopril | Aceon | Tablets | ||
| Quinapril | Accupril | Tablets | ||
| Ramipril | Altace | Capsules | ||
| Trandolapril | Mavik | Tablets | ||
| Angiotensin II receptor blockers | Azilsartan | Edarbi | Tablets | Treatment for high blood pressure; Reducing the risk of stroke and heart failure |
| Candesartan | Atacand | Tablets | ||
| Eprosartan | Teveten | Tablets | ||
| Irbesartan | Avapro | Tablets | ||
| Losartan | Cozaar | Tablets | ||
| Olmesartan | Benicar | Tablets | ||
| Telmisartan | Micardis | Tablets | ||
| Valsartan | Diovan | Tablets | ||
| Angiotensin receptor-neprilysin inhibitors | Sacubitril/valsartan | Entresto | Tablets | Treatment for heart failure |
| Beta-adrenergic blocking agents | Acebutolol | Sectral | Capsules | Treatment for high blood pressure, chest pain, and ventricular arrhythmias |
| Atenolol | Tenormin | Tablets | ||
| Betaxolol | Kerlone | Tablets | ||
| Bisoprolol/hydrochlorothiazide | Ziac | Tablets | ||
| Bisoprolol | Zebeta | Tablets | ||
| Metoprolol | Lopressor, Toprol XL | Tablets | ||
| Nadolol | Corgard | Tablets | ||
| Propranolol | Inderal | Tablets | ||
| Sotalol | Betapace | Tablets | ||
| Combined alpha and beta-blockers | Carvedilol | Coreg CR | Capsules | Treatment for congestive heart failure, chest pain, and high blood pressure |
| Labetalol hydrochloride | Trandate | Tablets | ||
| Calcium channel blockers | Amlodipine | Norvasc | Tablets | Treatment for high blood pressure, chest pain, and coronary artery disease |
| Diltiazem | Tiazac | Capsules | ||
| Felodipine | Plendil | Tablets | ||
| Nifedipine | Procardia | Capsules | ||
| Nimodipine | Nimotop | Capsules | ||
| Nisoldipine | Sular | Tablets | ||
| Verapamil | Calan, Verelan | Capsules | ||
| Diuretics | Acetazolamide | Diamox | Tablets | Treatment for congestive heart failure, high blood pressure, and edema |
| Amiloride | Midamor | Tablets | ||
| Bumetanide | Bumex | Tablets | ||
| Chlorothiazide | Diuril | Oral suspension | ||
| Chlorthalidone | Thalitone | Tablets | ||
| Furosemide | Lasix | Tablets | ||
| Hydrochlorothiazide | Esidrix, Hydrodiuril, Microzide | Tablets, Capsules | ||
| Indapamide | Lozol | Tablets | ||
| Metalozone | Zaroxolyn | Tablets | ||
| Spironolactone | Aldactone | Tablets | ||
| Torsemide | Demadex | Tablets | ||
| Vasodilators | Isosorbide dinitrate | Isordil | Tablets | Treatment for angina pectoris |
| Isosorbide mononitrate | Imdur | Tablets | ||
| Hydralazine | Apresoline | Tablets | ||
| Nitroglycerin | Nitrostat | Sublingual tablets | ||
| Minoxidil | Rogaine | Tablets |
3.3 Pain disorders
Pain relief medicines or analgesia are in high demand for many pain disorders needed for systemic delivery, for example, back pain, headaches or migraine, joint pain, and even cancer pain. As the fast onset of action of drugs is highly required for treating pain disorders, the combination of analgesia with ODFs seems to be prominent. The nonsteroidal anti-inflammatory drugs (NSAIDs) are mostly utilized as pain relief medicines. Among these NSAIDs ibuprofen is the most prominent drug and has been widely used for many pain disorders.44 While, opioid analgesics may be needed for some pains failed to be relieved by NSAIDs, but there are underling risks for abuse liability. Buprenorphine is the only opioid agent that can be safely used for pain relief and also used for opioid dependence treatment.45 Pergolizzi Jr et al. designed a buprenorphine buccal film by using BEMA® technology for the treatment of low back pain. This buprenorphine-incorporated film showed higher pain reduction compared with placebo films and good tolerability during 12 weeks clinical studies.46 Another kind of opioid analgesia, fentanyl is 80–100 times stronger than morphine. Fentanyl buccal film was approved by the FDA for the treating of cancer pain with the name of Onsolis®. In Phase III trial, fentanyl buccal film exhibited high clinic efficacy and good tolerability by reducing the common adverse events (e.g., vomiting, nausea, fatigue).47 In addition to pain, associated symptoms could occur (e.g., nausea and vomiting) in the meanwhile. A combination of pain relief (sumatriptan succinate) and antiemetic (prochlorperazine maleate) for treating migraine and associated nausea and vomiting was loaded in ODFs for rapid release of drugs. The obtained ODFs have demonstrated desirable characteristics with fast disintegration time and 100% permeation through ex vivo goat mucosa.48
3.4 Nausea and vomiting
Motion sickness may cause nausea, vomiting, dizziness or vertigo. Besides, patients after treated with chemotherapy, radiotherapy may also vomit. But drugs for the treatment of nausea and vomiting can be easily expelled by vomiting, thus the sufficient dose and intended treatment may not be reached. To avoid this problem, researchers incorporated oral antiemetic to ODFs.49 Meclizine hydrochloride was reported to load in ODFs for the treatment of nausea and vomiting associated with motion sickness.50 Besides, Yildiz Pekoz et al.51 prepared dimenhydrinate loaded films, which can be sustained release. Also, the dimenhydrinate administered by buccal route bypass the first-pass metabolism, resulting in the two-fold higher AUC than oral delivery route, thus bioavailability is enhanced.
3.5 Mood or mental disorders
Mood and mental disorders include depression, anxiety, bipolar disorder, neuroses, psychoses, and schizophrenia. As the administration of ODFs does not need any water and can be easily applied, allowing ODFs more suitable to be used for these disorders. ODFs have more accurate doses and better patient compliance than other dosage forms. In the pediatric population suffering from hyperkinetic movement, adult tablets were usually administered due to the lack of suitable pediatric dosage forms. Senta-Loys et al.17 developed a tetrabenazine incorporated ODFs for the treatment of pediatric hyperkinetic movement, which simplify drug administration and would benefit to children. Aripiprazole is an antipsychotic indicated for the treatment of bipolar disorder and schizophrenia. Lee et al.52 designed a three-dimensional (3D)-printed aripiprazole incorporated ODFs, which showed accelerated water penetration and disintegration compared with films prepared by solvent-casting method. Olanzapine, an antipsychotic, was commercially available as orodispersible tablets (ODTs) Zyprexa®. Olanzapine-embedded ODFs were fabricated to obviate problems happened during manufacturing, storage, and handling of ODTs, such as frangibility.14
3.6 Erectile dysfunction
Sildenafil citrate is the most commonly used drug for the treatment of male erectile dysfunction. The commercial dosage form of sildenafil citrate for clinical use is a tablet, which causes inconvenience during administration especially when water is not nearby. In this case, the fast disintegration ODFs are more suitable to be the deliver platform for sildenafil citrate with its ease of administration and carrying.53 While, the extensive first-pass metabolism and poor aqueous solubility of sildenafil citrate contribute to the poor oral bioavailability. Hosny et al. used hydroxybutyl-β-cyclodextrin to improved solubility of sildenafil citrate, and incorporated it into ODFs. From in vivo studies on human volunteers, sildenafil-loaded films exhibited more than 2.2 folds higher oral bioavailability than commercial tablets.54 A pharmacokinetics of sildenafil-loaded ODFs (at the dose of 100 mg) was reported to show similar results as sildenafil in conventional tablet (Viagra®) after single-dose administration to 53 healthy male volunteers, which satisfied the criterion of bioequivalence.55 Cocci et al.56 concluded that ODFs were an efficient and safe carrier for sildenafil, which serves as a interchangeable formulation for more precise and tailored delivery of sildenafil.
3.7 Pulmonary diseases
Pulmonary infections like tuberculosis remain to be a challenge worldwide. Nowadays, the main treatment for tuberculosis now is injection. However, long-term drug administration, low patient compliance, high dose, and frequent dosing intervals all contribute to the rising risk of drug resistance. To avoid this issue, many new delivery systems have been developed, including ODFs.57, 58 Matawo et al.59 reported an ODF containing pyrazinamide as a potential alternative with flexible dosing for pediatrics. In addition to tuberculosis, employing ODFs as carrier for the treatment of some chronic obstructive pulmonary disease has gained researchers' interests as well. The ease of administration, high patient compliance, and avoiding the risk of choking make ODFs a desirable alternative dosage form to asthma, bronchiolitis, and prophylaxis of apnea. Speer et al. developed the theophylline-loaded matrix particles incorporated ODFs for obstructive pulmonary diseases, which had prolonged release till 1000 min depend on the size of incorporated particles.60 Khan et al.61 reported an ODF with the loading of antibacterial agent cefixime trihydrate for infections of the respiratory tract. According to their research, β-cyclodextrins were used to improve poor water solubility and bad taste of cefixime trihydrate.
3.8 Special applications
The mucoadhesive buccal films (MBFs) is another kind of oromucosal films, which mainly placed to the buccal mucosa.59 As drugs enable to be absorbed rapidly via buccal delivery and circumvent high hepatic first-pass metabolism as well as prevent degradation in the harsh gastrointestinal environment, the bioavailability of drugs can be improved. MBFs were mainly used for the treatment of local disorders, such as oral mucositis and stomatitis, oral cancer, and topical anesthetics in the oral cavity.62
Due to the above-mentioned advantages, ODFs are gaining popularity, especially for pediatric and geriatric patients who are suffering from dysphagia or an increased the risk of choking. With the growing interest of ODFs, researchers have investigated ODFs delivery system for some new applications. For instance, a high drug-loading ODFs has been developed, which contained 30 mg racecadotril for the treatment of acute diarrhea in pediatric patients.63 The anti-histamine drug loratadine has been embedded into ODFs. As ODFs provides the possibility of adjusting dose by dividing the films into appropriate parts, it could be a suitable alternative for a traditional antiallergic agent for pediatrics.64 The nutritional supplements could also be delivered by ODFs. The ODFs containing vitamin D3 for daily supplementation were reported by IBSA.65 Later, the bioavailability of the vitamin D3-loaded ODFs was compared with the marketed oral vitamin D3 preparation in healthy volunteers. The ODFs with the loading of vitamin D3 showed a slightly higher in rate (Cmax) and extent of absorption (AUC 0−t and AUC 0–∞) than vitamin D3 oral solution. Therefore, these results proved that ODFs could be a promising alternative to the commercial vitamin D3 oral solution.66
An acceptable taste is important for ensuring patient acceptability and compliance. The taste should be taken into consideration when preparing formulations, especially for pediatrics, who are more sensitive to bitterness. Flavor and sweetening agents were usually used to improve palatability of drugs. Taste-masking polymers can be employed as a popular method for masking the bitter tastes of incorporated drugs, for example, ethylcellulose.67 When sweeteners are insufficient to overwhelm the bitterness, forming the barrier between drugs and taste buds by using microparticles or forming complexes with cyclodextrins were also efficient taste-masking technologies.68, 69
4 PRINTING TECHNOLOGIES FOR PERSONALIZED MEDICINE
The lack of suitable age-appropriate dosage form for pediatrics frequently occurs in many diseases. The common solution of tailoring the dose is splitting the tablets in half or smaller parts. However, it always causes uneven splitting, which changes the correct dose and leads to the risk of under- or overdosing. Besides, the release behavior of the tablet also can be changed because of the splitting. Therefore, a tailored dosage form, like ODFs, is urgently needed. Recently, there has been growing interest in developing ODFs by using printing technologies. Printing technology makes it easier to achieve a personalized or tailored dosage of ODFs in hospital pharmacy by controlling the amount of link (drug suspension) to be printed.67 As shown in Figure 4, there are two kinds of printing methods for the manufacture of ODFs: 2D printing and 3D printing. In 2D printing, the liquid drug or formulation (as inks) would be deposited onto ODF as substrate in a designed pattern. For 3D printing, ODFs were printed layer by layer by adding materials or excipients.
4.1 Inkjet printing
Inkjet printing is used for the fabrication of ODFs which provides flexible dosing to meet different requirements of individuals. There are three main steps using inkjet printing to manufacture ODFs: (i) the preparation of drug suspension as the ink, (ii) the deposition of the ink onto the ODFs in a defined pattern and (iii) the drying.67 Due to the high precision and accuracy of printing technology, even very small volumes of drugs could be dispensed onto film substrate allowing to deliver the precise therapeutic dose for pediatric patients. Also, inkjet printing provides a great possibility for personalized medicine owing to its high printing accuracy and flexibility. Therefore, inkjet printing technique could be employed in a hospital setting for the preparation of ODFs with tailored dose in the future.
To obtain desirable drug embedded ODFs by inkjet printing, both ink formulation and substrate properties should be taken into consideration. To avoid blockage of nozzle, viscosity and surface tension of printable ink formulation are the most important parameters. The viscosity of print inks must not exceed 20 mPa.s to assure the flowability of the ink. The viscosity cannot be too low, because otherwise ink would be ejected too early and form the fluid tail, which produces satellite droplets. Hence, the manufacturer recommended having a viscosity in the range between 8 mPa.s and 20 mPa.s, which provides the surface tension in the range of 24–36 mN/m.8 As viscosity considerably effects the printing process, ink formulation usually contains viscosity modifiers and surfactants. Glycerol, polyvinyl alcohol, and sodium carboxymethyl cellulose (CMC) are commonly used as viscosity modifiers.71 In addition, the contact angle between printing droplets and substrate is an essential element for inkjet printing. The high contact angle usually indicates poor ink spreading, which leads to insufficient absorption of inks into substrate, hence, crystallization of drugs may easily occur. Various technologies have been used to decrease contact angle, for example, by improving hydrophilicity of ODFs with hydrophilic component (HPMC).72
As ODFs are edible, flexible and uniform, which act as a suitable candidate for printing substrate. In addition to ink formulation, the properties of substrate are of crucial importance to obtain the optimal printing ODFs. Firstly, sufficient mechanical properties of ODFs are the key characteristics for the printability, handling, and transporation of the printing ODFs. For example, Visser et al. defined the limits for mechanical properties of plain ODFs. The tensile strength and elongation at break can be set to higher than 2 N/mm2 and 10%, respectively. The Young's modulus of optimal plain ODFs should be lower than 430 N/mm2.73 The fast disintegration of ODFs is considered essential as well. The disintegration time had to be 30 s or less.74 The good absorption capacity of the substrate ensures the ink drug penetrates inside the film matrix rather than sliding away from the substrate surface, thus improving drug loading and preventing drug crystallization. Edinger et al. used mesoporous fumed silica as porosity enhancing agent to enhance the absorptivity of the substrate.75 Additionally, the thickness, palatability, and taste of the printing substrate greatly affects patient acceptability and need to be considered as well.
4.2 3D printing
The 3D printing, also known as additive manufacturing, is able to build up 3D geometries in a layer-by-layer fashion with the help of computer-aided design (CAD). In inkjet printing, the inks need to be formulated individually for different active ingredients to achieve suitable properties before printing, while the production process of 3D printing is relatively simple for operation in the hospital setting. Plus, 3D printing technique is cost-effective and considerably improves dose accuracy, the application of 3D printing in ODFs provides the opportunity for producing tailor-made and individualized medicines in hospital pharmacies.
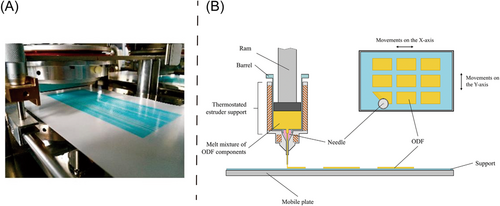
One of the typical 3D printing techniques that is frequently used for the production of ODFs is fused deposition modeling (FDM) 3D printing. FDM is a continuous process and usually involves three main steps for the manufacturing of ODFs: (i) mixing and melting drug and excipients into liquid state, (ii) using hot-melt extrusion (HME) to obtain drug-loaded printing filaments, and (iii) printing and deposition of the filaments to form the 3D ODFs. The multi-step 3D printing was able to simplified, for example, Cho et al.14 proposed holt-melt pneumatic extrusion-based 3D printing. The main limitation of FDM is the high processing temperature, leading to the degradation of thermolabile drugs. Thermoplastic polymers are commonly used in FDM to avoid the exposure of high temperature during extrusion, polylactic acid (PLA), polyvinylpyrrolidone (PVP), and polyvinyl alcohol (PVA)76 are most commonly used thermo-resistant polymers. In additional to high temperatures, disintegration time of 3D-printed ODFs may be prolonged due to the formation of filaments. Musazzi et al.70 proposed the hot melt ram extrusion 3D printing for the manufacture of ODFs. In their research, the drug (paracetamol) maltodextrins, excipients and plasticizer were mixed and heated, followed by printing directly on the packaging foil and sealing the ODFs.70 During printing process, the critical parameters that must be controlled are the infill density, speed of extruder, layer height, and the temperature of both nozzle and building platform.9
Semi-solid extrusion (SSE) 3D printing, also called pressure-assisted microsyringe, is another typical 3D printing technique that is commonly used for the fabrication of ODFs. SSE 3D printing is the one-step process to fabricate ODFs at room temperature without the preparation of drug-loaded filaments. The semisolid materials are extruded continuously layer-by-layer through a syringe-based nozzle onto the build plate, followed by in-process drying. The semi-solids formulation contains drug, excipients, and solvent. Therefore, the optimal mixture of these components is of utmost importance for the successful printing of ODFs by SSE. For example, the semi-solids formulation needs to have appropriate rheological characteristics and extrudability for desirable flowability to pass through nozzle before printing. Panraksa et al.77 investigated various hydrophilic polymers as printing material that are suitable for ODFs fabrication by SSE 3D printing. In this study, ODFs prepared by 5% sodium carboxymethylcellulose showed fast disintegration time with desirable printing resolution as well as accurate dimensions.77 Drying condition needs to be considered as well, otherwise shrinking or deformation may easily occur during the drying process.9 Chemical drugs such as levocetirizine hydrochloride, warfarin, and mirtazapine have been used in SSE 3D printing for ODFs fabrication.78-80
5 FUTURE PERSPECTIVE AND CONCLUSIONS
ODFs have shown great promise as a novel alternative to conventional dosage forms, not only due to easy administration for pediatric, geriatric, and non-cooperative patients, but also for cost-effectiveness in manufacturing as well as convenience in transportation, storage, and handling. With the possibility of incorporating various drug compounds, including chemical drugs, vaccine, probiotics, and herbal extracts, the ODF act as a versatile platform for drug delivery. Especially during the outbreak of pandemic and epidemic (e.g., COVID-19), vaccines delivered by ODFs would greatly reduce cost and benefit more people as ODFs makes it easier to transport and distribute in tropical developing countries without the need of cold-chain. Compared with solid dosage form, vaccine embedded in ODFs disintegrate rapidly in oral cavity without the consumption of water, thus decreasing the risk of choking and are more suitable for patients with swallow difficulties and dysphagia. Additionally, ODF also gain popularity as the deliver platform for the treatment of oral inflammation, cardiovascular diseases, pain disorders, nausea and vomiting, mood or mental disorders, erectile dysfunction, pulmonary diseases, and and so forth. Finally, the latest developed printing techniques considerably improve efficiency and dose accuracy in fabrication of ODFs. The combination of printing techniques and ODFs allow to have the on-demand preparation of tailored or personalized dosage of ODFs for a specific requirement in hospital or community pharmacies.
Nonetheless, how to achieve extemporaneous preparation of ODFs for individualized dosage in local pharmacy just before patient consumption requires further investigation and research. One the one hand, the fabrication (printing) process time should not last too long. On the other hand, the printing process need to be standardized and simplified for pharmacist compounding. Further, more efforts have to be made to achieve large-scale industrialization and commercialization of ODFs.
AUTHOR CONTRIBUTIONS
Conceptualization: Yu Tian and Yourong Duan. Methodology: Yu Tian and Jiangtao Lin. Formal analysis: Yu Tian and Hongshu Jing. Investigation: Yu Tian and Quan Wang. Writing—original draft: Yu Tian. Writing—review and editing: Jiangtao Lin, Hongshu Jing, Quan Wang, Zhihua Wu, and Yourong Duan. Supervision: Yourong Duan. Funding acquisition: Yu Tian. Project administration: Yu Tian and Yourong Duan. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This research was financially supported by the National Natural Science Foundation of China (82102906) and Shanghai Sailing Program (21YF1444800, China).
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All the authors hereby declared that the research and study related to the manuscript has been done under proper ethical code.
Open Research
DATA AVAILABILITY STATEMENT
No data were used for this article.




