A pilot study investigating the role of 18F-FDG-PET in the early identification of chemoradiotherapy response in anal cancer
Abstract
Introduction
Anal cancer (AC) is 18F-FDG-PET avid and has been used to evaluate treatment response several months after chemoradiotherapy. This pilot study aimed to assess the utility of semi-automated contouring methods and quantitative measures of treatment response using 18F-FDG-PET imaging at the early time point of 1-month post-chemoradiotherapy.
Methods
Eleven patients with AC referred for chemoradiotherapy were prospectively enrolled into this study, with 10 meeting eligibility requirements. 18F-FDG-PET imaging was obtained pre-chemoradiotherapy (TP1), and then 1-month (TP2), 3–6 months (TP3) and 9–12 months (TP4) post-chemoradiotherapy. Manual and semi-automated (Threshold) contouring methods were used to define the primary tumour on all 18F-FDG-PET images. Resultant contours from each method were interrogated using quantitative measures, including volume, response index (RI), total lesion glycolysis (TLG), SUVmax, SUVmedian and SUVmean. Response was assessed quantitatively as reductions in these measures and also qualitatively against established criteria.
Results
Nine patients were qualitatively classified as complete metabolic responders at TP2 and all 10 at TP3. All quantitative measures demonstrated significant (P < 0.05) reductions at TP2 for both Manual and Threshold methods. All reduced further at TP3 and again at TP4 for Threshold methods. TLG showed the highest reduction at all post-chemoradiotherapy time points and classified the most responders for each method at each time point. All patients are recurrence-free at minimum 4-year follow-up.
Conclusion
Based on our small sample size, semi-automated methods of disease definition using 18F-FDG-PET imaging are feasible and appear to facilitate quantitative response classification of AC as early as 1-month post-chemoradiotherapy. Early identification of treatment response may potentially improve disease management.
Introduction
Anal cancer (AC) is a rare cancer, with squamous cell carcinoma (SCC) the predominant histological subtype, that is routinely managed with a chemoradiotherapy regime.1, 2 Treatment follow-up and response assessment in AC is primarily based on clinical examination assessing for physical changes in the primary tumour.3 In recent years there has been increasing interest and utilisation of imaging to assess response for the surveillance of AC.
Conventional morphological imaging modalities (CT, MRI and ultrasound) evaluate physical characteristics and changes in the gross position, size and structure of a tumour to assess treatment response over time.4, 5 In contrast, functional imaging modalities, including positron emission tomography (PET), can visualise the cellular function of a tumour, both relative to surrounding normal tissue and within the tumour itself. PET, primarily using the 18F-fluorodeoxyglucose (FDG) PET tracer, is the main functional imaging modality employed in cancer disease staging and radiotherapy planning in AC. It defines AC with greater sensitivity than CT, altering staging and treatment intent.6, 7
Changes in the functional state of a tumour can be identified earlier on 18F-FDG-PET than the physical changes in tumour mass observed on conventional imaging or physical examination.8 A reduction or loss of PET signal indicates treatment response, though the timing and magnitude of this change is important. A high negative predictive value highlights the strength of 18F-FDG-PET in AC.9 While measurable response on functional imaging has been observed during treatment, post-treatment imaging is most often utilised to ensure visible changes are not confounded by acute treatment-related effects.10 Several measures of cancer treatment response identification using 18F-FDG-PET imaging are outlined in the literature. Methods include measuring the relative change in tumour volume, SUV, total glycolysis volume (TLG)11, 12 or via a visual response criteria.13 The response index (RI), or percentage reduction in maximum SUV (SUVmax), has been shown to be effective in rectal cancer response assessment.14
Interobserver and intraobserver variation in tumour definition may lead to incorrect measurements of tumours and categorisation of treatment response.15 Semi-automated methods of tumour definition on 18F-FDG-PET utilise the standard uptake volume (SUV), a qualitative measure of PET activity. SUV Gradient and SUV Threshold methods define tumour extent and tumour change with reduced error.16, 17 Semi-automated methods of tumour definition can ensure consistency and equivalence of patient assessment and care, integral to precision radiotherapy. Our previous work18 demonstrated the ability of the 40% Threshold method to define AC in the pre- and post-chemoradiotherapy context. In this method all voxels with an SUV value >40% of the maximum within a region of interest are included within the resultant contour.
The timing of post-treatment PET-based imaging and the benefit of early identification of response/non-response on a patient's long-term outcome has been established in other anatomical sites including head and neck, oesophagus and rectum.11, 19, 20 The timing and correlation of 18F-FDG-PET imaging to long-term outcomes in AC are not yet established. Clinical assessment in AC response may take up to 26 weeks before being identifiable.21 Measurable changes in tumour 18F-FDG-PET activity occur earlier than these physical changes and have shown a strong correlation to response in pathology studies in AC.7 Early identification of persistent disease may lead to earlier intervention, including radiotherapy dose escalation or surgery.
The optimal time point to assess treatment response in AC using 18F-FDG-PET warrants investigation. To address this issue, we conducted a pilot study using qualitative and quantitative measures of response at the ‘earlier’ time point of 1-month post-chemoradiotherapy to investigate the potential of 18F-FDG-PET in the early identification of chemoradiotherapy response in AC.
Methods
All eligible patients with histologically proven squamous cell carcinoma (SCC) AC referred for chemoradiotherapy at our institution between 2015 and 2017 were prospectively and sequentially enrolled into the study following informed consent (Fig. 1 inclusion criteria). This study was approved by, and performed in accordance with the Austin Health Research Ethics Committee requirements, reference number HREC/14/Austin/643.

Intensity modulated radiotherapy (IMRT) was delivered 5 days a week, over 5–6 weeks, on Elekta™ Infinity or Integrity treatment units (Elekta Solutions, Stockholm, Sweden) to a planned primary tumour dose of 54 Gy in 30 fractions. All patients were imaged daily using cone beam CT (CBCT), with a pre-treatment online zero-action threshold for adjustment. Radiotherapy was completed as prescribed in all but one patient, where treatment ceased at 48.6 Gy (fraction 27 of 30) due to acute toxicity. All patients were reviewed by a Radiation Oncologist 1 month after treatment completion, then every 3–6 months for up to a year following the completion of radiotherapy with imaging and clinical examination (Fig. 2). Thereafter follow-up transitioned to 6–12 monthly assessments as per our department's standard practice.

PET imaging
Whole body 18F-FDG-PET/CT scans were performed as per institutional standard anal/colorectal imaging protocols using a Philips Ingenuity® or Gemini® TF64 PET/CT (Philips Healthcare, Cleveland, Ohio). Pre- and post-chemoradiotherapy imaging was performed in the same facility using the same parameters. CT attenuation corrected images were used for viewing and analysis. Images were obtained at four time points (TPx): within 1-month prior to treatment (TP1), 1-month post-chemoradiotherapy completion (TP2), then at standard departmental time points of 3–6 months post-chemoradiotherapy (TP3) and 9–12 months post-chemoradiotherapy (TP4) (Fig. 2). Images obtained within 1 year of completing treatment were included for analysis.
Image fusion and contour definition
Image fusion and contour definition was performed as described previously18 using MIM Maestro® version 6.8.3 (MIM Software Inc., Cleveland, Ohio). Registration of post-chemoradiotherapy imaging to pre-chemoradiotherapy imaging using low dose CT images included an initial rigid assisted alignment, followed by a box-based alignment of the region around the pelvic region of interest. The final fusion was visually inspected by a single experienced colorectal radiation therapy clinician with respect to the anus and distal rectum, with manual adjustment to ensure relative soft tissue motion or filling was accounted for.
Following image fusion, primary AC tumours were contoured according to two methods on each PET study set. Manual (Clinician) and Threshold contours were defined on all pre- and post-treatment images as described previously.18 Manual contours were created where visual 18F-FDG-PET activity was present by a senior nuclear medicine specialist experienced in anal/colorectal PET reporting and contouring. Resultant contours were then viewed and accepted by a senior radiation oncologist experienced in AC. Threshold methods were performed independently by a second experienced senior clinician. A threshold of 40% of pre-treatment (TP1) SUVmax was used to define contours for the Threshold method on all study sets using the MIM Maestro® auto-threshold tool. Threshold method contours were checked, and non-contiguous areas removed where >0.5 cm from the pre-chemoradiotherapy contour or clearly an adjacent anatomical structure, such as bladder. This step was to maintain geographic correlative assessment accuracy for treatment response and avoid influence of local tissue background activity.
Response assessment
The percentage reduction in each SUV metric (SUVmax, SUVmean, SUVmedian, TLG and volume) also described as the response index (RI)14 was calculated for each time point post-chemoradiotherapy relative to pre-chemoradiotherapy (TP1) values and used to score response. Patients were classified as having treatment response when RI was >66.2%.14 Resultant change in the above quantitative response values were assessed at each time point for statistical significance against TP1 using a linear mixed effects model.
Results
Eleven AC patients were referred to our centre over a 2-year period. All 11 met study criteria and were enrolled in this study. One patient was excluded from analysis because of their inability to attend the 1-month post-chemoradiotherapy 18F-FDG-PET scan. Therefore, nine female and one male with a median (range) age of 60 (50–74) years at the time of enrolment were available for analysis (Table 1). Nine of the 10 eligible patients were qualitatively classified by the senior nuclear medicine specialist as having complete metabolic response at TP2. A significant (P < 0.05) reduction in tumour volume, SUVmax, SUVmean and TLG, was observed in all patients on both contouring methods at TP2 relative to TP1 (Table 2). These reductions were greater at TP3, with all patients considered complete responders by the senior nuclear medicine specialist. Metabolic response was persistent at TP4, and all patients were classified as having complete clinical response at 12 months of follow-up (Table 3). Further, all patients remain recurrence-free at a minimum of 4 years post-chemoradiotherapy. Figure 3 demonstrates the resultant contours for one patient with T2N2M0 AC.
| Patient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Age | 74 | 59 | 60 | 57 | 65 | 50 | 63 | 63 | 55 | 60 |
| Gender | F | F | M | F | F | F | F | F | F | F |
| Tumour staging | ||||||||||
| T | 2 | 3 | 3 | 2 | 4 | 2 | 2 | 4 | 4 | 2 |
| N | 0 | 1 | 1 | 2 | 3 | 0 | 1 | 0 | 2 | 0 |
| M | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pre-chemoradiotherapy values | RI (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| TP1 | TP2 | TP3 | TP4 | |||||
| Manual | Threshold | Manual | Threshold | Manual | Threshold | Manual | Threshold | |
| median (min, max) | median (min, max) | median (min, max) | median (min, max) | median (min, max) | median (min, max) | median (min, max) | median (min, max) | |
| Datasets (n) | 10 | 10 | 8 | 9 | ||||
| Contoured datasets | 10 | 10 | 1 | 7 | 0 | 5 | 0 | 3 |
| Volume (cc) | 6.4 (1.9, 39.8) | 8.5 (2.9, 30.9) | 100 (63.1, 100) | 90.3 (1.2, 100) | 100 (100, 100) | 97.6 (58.9, 100) | 100 (100, 100) | 100 (−74.6, 100) |
| P | – | – | <0.001 | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 |
| SUVmax | 10.4 (4.7, 36.1) | 10.4 (4.7, 36.1) | 100 (45.9, 100) | 52.9 (34.8, 100) | 100 (100, 100) | 56.1 (39.5, 100) | 100 (100, 100) | 100 (25.6, 100) |
| P | – | – | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| SUVmean | 5.2 (3.3, 17.4) | 5.9 (3.0, 24.1) | 100 (37.6, 100) | 29.9 (8.7, 100) | 100 (100, 100) | 33.5 (15.4, 100) | 100 (100100) | 100 (19.5, 100) |
| P | – | – | <0.001 | <0.01 | <0.001 | <0.01 | <0.0001 | <0.001 |
| SUVmedian | 5.2 (3.4, 16.1) | 5.4 (2.7, 24.4) | 100 (34.1, 100) | 27.3 (2.1, 100) | 100 (100, 100) | 30.5 (8.6, 100) | 100 (100, 100) | 100 (12.3, 100) |
| P | – | – | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| TLG | 43.8 (6.1, 691.3) | 47.4 (9.7, 531) | 100 (77.0, 100) | 92.8 (10.0, 100) | 100 (100, 100) | 98.4 (58.9, 100) | 100 (100, 100) | 100 (−74.6, 100) |
| P | – | – | 0.02 | 0.018 | 0.03 | 0.033 | 0.02 | 0.02 |
| TP2 | TP3 | TP4 | ||||
|---|---|---|---|---|---|---|
| Manual | Threshold | Manual | Threshold | Manual | Threshold | |
| Datasets (n) | 10 | 8 | 9 | |||
| PMV | 9 | 8 | 9 | |||
| Volume (cc) | 9 | 7 | 8 | 6 | 9 | 9 |
| SUVmax | 9 | 4 | 8 | 3 | 9 | 7 |
| SUVmean | 9 | 4 | 8 | 3 | 9 | 7 |
| SUVmedian | 9 | 4 | 8 | 3 | 9 | 7 |
| TLG | 9 | 8 | 8 | 7 | 9 | 9 |
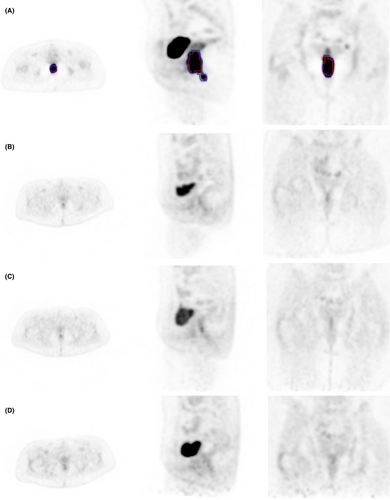
Only one patient had persistent metabolic disease at TP2 as per the Manual method. A volume RI of 63.1% indicated a partial metabolic response; however, all 10 were considered as having complete metabolic response at TP3. The SUV-based Threshold method created contours and, therefore, persistent disease on seven patients at TP2, five at TP3 and three at TP4. The percentage change in measured PET values post-chemoradiotherapy are presented in Table 3.
The Manual method demonstrated larger reductions in all quantitative measurements than the Threshold method at each time point post-chemoradiotherapy (Table 3). The largest difference in measured values between the two methods was seen for the SUVmedian, with a median (range) reduction of 27.3% (2.1, 100) for the Threshold method versus 100% (34.1, 100) for the Threshold method at TP2, and 30.5 (8.6, 100) versus 100 (100, 100) at TP3. TLG demonstrated the largest percentage reduction in both methods for all time points, 100% (77, 100) and 92.8% (10, 100) for Manual and Threshold methods, respectively, at TP2.
Using a RI > 66.2%, volume and TLG classified the most responders at each post-chemoradiotherapy time point for Threshold-based contours (Table 3). Each other SUV-based metric identified responders at each time point, with TP4 seeing a minimum of seven of nine imaged patients classified as responders (Table 3).
Discussion
In this pilot study, 18F-FDG-PET treatment response assessment of AC at 1-month post-chemoradiotherapy appears assessable by both qualitative and quantitative methods. Only one patient exhibited persistent 18F-FDG-PET avid primary disease at 1-month (TP2) using qualitative measures. All patients exhibited measurable treatment response by a quantitative SUV-based contour method, with a median reduction in tumour volume of 90.3% (1.3–100). All metrics of the resultant contours reduced by a minimum median value of 27.3% (2.1–100) at TP2, with all increasing to 100% reduction at the TP4 time point indicative of complete metabolic response. Response classification using a RI of >66.2% identified most responders at each time point using TLG and volume, and these appear the most sensitive qualitative measures. All patients remain recurrence-free at a minimum of 4 years post-chemoradiotherapy.
One-month post-chemoradiotherapy (TP2) has generally been considered too early to perform 18F-FDG-PET imaging within our health service and many others due to treatment-associated inflammation and ulceration creating false positives.5, 23, 24 However, we have demonstrated in our pilot study, this may not be the case. The lack of a prominent epithelial lining, prone to inflammation, may in part explain this finding, when compared to other anatomical sites, including rectum, oesophagus and head and neck, on which the imaging recommendation is based. The only patient assessed by the senior nuclear medicine specialist as having persistent disease a month post-chemoradiotherapy had measured pre-chemoradiotherapy values that were unremarkable relative to the cohort median, specifically SUVmax 9.3 versus 10.4, SUVmean 5.5 versus 5.2, SUVmedian 5.3 versus 5.2, TLG 34.2 versus 43.8, Volume 6.2 cc versus 6.4 cc. This patient progressed to complete response status by TP4 and as with all patients is recurrence-free at 4 years post-chemoradiotherapy.
We have confirmed the ability to detect metabolic response at 1-month post-chemoradiotherapy with 18F-FDG-PET. Larger studies are required to identify whether these changes are indicative of long-term outcomes, so that we may use this time point as a predictor of outcomes and clinical decision making. 18F-FDG-PET exhibits high specificity and using quantitative measures of metabolic activity may provide that clear distinction between initial response and overall disease-free survival.
The spatial resolution of PET is low, between 0.4 and 1 cm,25, 26 meaning minute residual disease may not be accurately visualised. However, the relative change in tumour activity and volume post-chemoradiotherapy is likely more important in the assessment and prediction of long-term tumour response than the potential presence of a small amounts of residual disease. Hatt et al.12 examined repeated weekly scans to identify changes post-chemoradiotherapy for rectal cancer using PET-based metrics. They found the changes in SUV-based metrics, particularly TLG at 2 weeks post-chemoradiotherapy, were more predictive of outcomes than pre-chemoradiotherapy values. TLG showed the greatest reduction 1-month post-chemoradiotherapy in our study cohort, 100% (77, 100) and 92.8% (10, 100) for Manual and Threshold methods, respectively. The percentage reduction in TLG increased at subsequent time points.
Auto-segmentation, based on quantitative measures of PET, may reduce the observer variation seen with manual definition of tumour. All study semi-automated methods proved useful for identifying response at 1-month post-chemoradiotherapy in our cohort. A limitation of the study is that it has a small patient number but given the rarity of this cancer, it nevertheless provides useful pilot information on AC tumour response. All patients in our study have remained recurrence-free at a minimum of 4 years post-chemoradiotherapy. We cannot reliably confirm which method is most predictive of long-term recurrence-free survival until we evaluate a larger sample size than feasible in our pilot study and will be the focus of future collaborative research.
Further, background metabolic activity within the surrounding tissues may be patient-dependent and influence the definition of the gross tumour based solely on a single SUV-based value. In such cases, an experienced clinical observer may avoid such false positives for persistent disease. More reliable measures, less prone to partial voxel influence, particularly TLG, may be more robust. Within our cohort, TLG decreased by a median of 92.8% at TP2, increasing to 98.4 at TP3 and 100% at TP4 using the Threshold method and is consistent with the literature.27 SUVmedian and SUVmean show the lowest change at one and 3–6 months, increasing to a median of 100% reduction at the final 9–12-month time point. Further investigation is required to assess which method is the most reliable and indicative of long-term outcomes at this early time point of 1-month post-chemoradiotherapy. Large changes were observed within patients and across the Manual and Threshold contouring methods, a larger study cohort observed over a longer period of time is warranted.
Our pilot study accrued a small cohort over 2 years, which subsequently limits statistical assessment and generalisability of the results. The study results do, however, provide an interesting observation and indication that further investigation of early assessment of response is warranted, particularly in the context of response detection at 1-month post-chemoradiotherapy and its persistence up to 4 years in our cohort. As AC is a rare disease, a multi-centre approach is required for adequate statistical power to provide the appropriate evidence required to change practice.
Conclusion
While qualitative assessment of response is the current clinical standard, quantitative assessment appears acceptable, particularly using a Threshold method and TLG. The identification of treatment response with 18F-FDG-PET imaging at 1-month post-chemoradiotherapy is possible. It has the potential to impact the follow-up and treatment of patients with AC that may have otherwise waited months for clear clinical examination findings. Those with persistent disease could be considered for intensification of follow-up and treatment including radiotherapy dose escalation or surgical salvage. The optimisation of response assessment and further individualisation of a patient's care has the potential for improved clinical outcomes.
Acknowledgments
Clinical trial registration: ANZCTR, ACTRN12620000066987. Retrospectively registered 28 January 2020 – https://www.anzctr.org.au/ACTRN12620000066987.aspx.
Funding
This study was conducted as part of a larger study that received funding via a 2015 Royal Australian and New Zealand College of Radiologists (RANZCR) Research Grant. RANZCR had no role in the design or analysis for this manuscript.
Conflict of Interest
The author(s) declare no conflicts of interest.
Ethical Approval
All procedures within this manuscript were in accordance with the ethical standards of the Austin Health Research Ethics Committee, reference number HREC/14/Austin/643 and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patient Consent to Participate
All patients were only enrolled into the study after full informed consent.
Open Research
Data Availability Statement
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.




