Combination therapy with chitosan/siRNA nanoplexes targeting PDGF-D and PDGFR-β reveals anticancer effect in breast cancer
Funding information: Marmara University Scientific Research Projects Association, Grant/Award Number: SAG-B-110412-0075
Abstract
Background
Platelet derived growth factors (PDGF)-D and the expression of its receptor increase in neoplastic progression of cancer. Co-silencing of growth factor and receptor can be suggested as an important strategy for effective cancer therapy. In the present study, we hypothesized that suppression of PDGF-D signaling pathway with small interfering RNAs (siRNAs) targeting both PDGF-D and PDGF receptor (PDGFR)-β is a promising strategy for anticancer therapy.
Methods
Chitosan nanoplexes containing dual and single siRNA were prepared at different weight ratios and controlled by gel retardation assay. Characterization, cellular uptake, gene silencing and invasion studies were performed. The effect of nanoplexes on breast tumor growth, PDGF expression and apoptosis was investigated.
Results
We have shown that downregulation of PDGF-D and PDGFR-β with chitosan/siRNA nanoplex formulations reduced proliferation and invasion in breast cancer cells. In the in vivo breast tumor model, it was determined that the intratumoral administration of chitosan/siPDGF-D/siPDGFR-β nanoplexes markedly decreased the tumor volume and PDGF-D and PDGFR-β mRNA and protein expression levels and increased apoptosis.
Conclusions
According to the results obtained, we evaluated the effect of PDGF-D and PDGFR-β on breast tumor development and showed that RNAi-mediated inhibition of this pathway formulated with chitosan nanoplexes can be considered as a new breast cancer therapy strategy.
1 INTRODUCTION
Angiogenesis is a vital process in solid tumor development and invasion. Treatment approaches that target angiogenesis-related growth factors or their receptors can lead to tumor regression.1 Platelet derived growth factors (PDGFs) and their receptors are one of those targets. They regulate different cellular processes such as cell proliferation, transformation, migration and survival during tumor development and pathogenesis.2
PDGF family has four members (-A, -B, -C and -D) and is also referred as the PDGF/vascular endothelial growth factor (VEGF) family because of the high sequence similarity between PDGF and VEGF. Besides PDGF, PDGF receptors (PDGFR) expression has been shown in many solid tumors, endothelial and perivascular cells.3 It has been reported that PDGFRs are more abundant, especially in the aggressive period of breast cancer.4 PDGF isoforms (-A, -B, -C and -D) show biological activity through PDGFR-α and -β. PDGF-A binds PDGFR-α; PDGF-B binds PDGFR-α, -α/-β and -β; PDGF-C binds PDGFR-α and -α/-β; PDGF-D binds PDGFR-β.3 PDGFR-β has been associated with increased angiogenesis in breast cancer tissue.5 Because signaling through PDGFR promote tumor angiogenesis, pericyte migration and vascular maturation, they are considered to be suitable targets for cancer gene therapy.2, 6, 7 As newly stated, the anti-angiogenic effect of PDGF-D activates both the autocrine and paracrine signaling pathway between tumor cells and surrounding stromal cells.8-12 Effects of PDGF-D signaling on proliferation and invasion of breast cancer have only been investigated in few studies.5, 13, 14 In these studies, endothelial expression of PDGF receptors and the role of PDGF-D in progression, metastasis and response to chemotherapy in breast tumor models were reported.5, 14 Gene silencing effect of chitosan/short hairpin RNA PDGF-D nanoparticles has been studied breast cancer cells in vitro.13 In different studies, the inhibition effect of PDGF-D on tumor growth was investigated in lung, stomach and esophagus cancers; however, there is no detailed paper on effectiveness in breast cancer and usage PDGF-D and PDGFR-β in the same formula. The exact molecular mechanisms of PDGF-D for its role in breast cancer have not been completely elucidated.
Suppression of gene expression by RNA interference (RNAi) technology has been investigated in many preclinical and clinical studies.15-18 The success of cancer treatment with small interfering RNAs (siRNAs) may only be possible when the stability is maintained in physiological conditions. The plasma half-life of naked siRNAs is very short and they are rapidly degraded before reaching the target tissue or cell.19 Therefore, a suitable siRNA delivery system is necessary for successful cancer therapy. Lipid and polymer-based delivery systems have been developed to efficiently and safely deliver siRNAs to target cancer cells. Until now, chitosan-based delivery systems have been preferred to increase the stability and delivery of siRNA as a result of the biocompatibility and safety of chitosan. The nano-sized chitosan-siRNA complexes can easily be formed by electrostatic interaction between cationic chitosan and anionic siRNA molecules.20
Because of the complex nature of cancer, treatment effectiveness can be enhanced and resistance to therapy can be reduced by simultaneous silencing of several genes that are involved in cancer.21, 22 In particular, co-silencing the target gene and its receptor will provide a better therapeutic effect because it will suppress the whole signaling pathway. Our previous study has also shown that co-targeting of VEGF-A and its receptors was an efficient breast cancer therapy.20 It is known that the RNAi-based strategies targeting PDGF-D cause regression of breast tumor.3, 6, 11 However, no study has investigated the therapeutic effects of PDGF-D and PDGFR-β dual inhibition in breast cancer. In this regard, given that overexpression of PDGF-D and its receptor promote breast cancer growth, we hypothesized that co-silencing these two targets would decrease the malignancy of breast cancer cells. In the present study, an effective therapy in breast cancer is devised by using chitosan nanoplexes containing siRNAs targeting both PDGF-D and its receptor PDGFR-β. PDGF-D signaling pathway suppression resulted in decreased cell invasion in in vitro breast cancer cells, regressed tumor volume and increased apoptosis in in vivo breast cancer model. Thus, we concluded that it is possible to inhibit angiogenesis and regress tumor growing by silencing PDGF-D signaling pathway.
2 MATERIALS AND METHODS
2.1 Materials
siRNAs were purchased from Qiagen (Valencia, CA, USA) and GE Healthcare Dharmacon (Chicago, IL, USA). Low molecular weight (LMW) chitosan (70 kDa, 75–85% deacetylated) was purchased from Sigma-Aldrich (St Louis, MO, USA). Dulbecco's Modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco Invitrogen (Grand Island, NY, USA). The anti-PDGF-D and anti-PDGFR-β and mouse and rabbit specific horseradish peroxidase (HRP)/3,3′-diaminobenzidine tetrahydrochloride (DAB) detection kit were purchased from AbCam (Cambridge, MA, USA). cDNA synthesis kit, master mix and Taqman gene expression assays were obtained from Applied Biosystems (Foster City, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits were purchased from USCN Life Science (Wuhan, China). An ApopTag Peroxidase In Situ Apoptosis Detection Kit was purchased from Millipore (Burlington, MA, USA).
2.2 Preparation and control of chitosan nanoplexes
To prepare chitosan/siRNA nanoplexes, LMW chitosan solution in 1% acetic acid was prepared at 0.25% (w/v) concentration. siRNA was slowly added into chitosan solution at different weight ratios (+/−) and mixed by vortexing. The mixture was incubated for 1 h at room temperature. Nanoplexes containing dual and single siRNA were prepared at 5:1, 10:1, 20:1 and 50:1 ratio (w/w). The nanoplex formation was controlled by gel retardation assay by using 2% agarose gel electrophoresis.
2.3 Characterization of nanoplexes
Particle size and zeta potentials of chitosan nanoplexes were measured by Zetasizer (NanoZS, ZEN3500; Malvern Instruments, Malvern, UK) in phosphate-buffered saline (PBS) (pH:7.4). All measurements were performed in triplicate.
The morphologies of nanoplexes were observed using transmission electron microscopy (TEM) (JEM 1200 EXII; Jeol, Tokyo, Japan). The nanoplexes were dispersed in PBS and samples were placed on top of carbon-coated copper grids. Grids were allowed to dry at room temperature.
In vitro serum stability of chitosan nanoplexes containing siRNA was investigated. For this purpose, 150 mM NaCl solution with 10% fetal bovine serum was added to naked siRNA and chitosan/siRNA (20:1) nanoplexes and incubated at 37°C. Samples were collected at different time intervals (0 and 30 min; 1, 2, 4, 24, 48 and 72 h). The siRNA decomplexation of chitosan nanoplexes was evaluated by gel retardation assay using 2% agarose gel electrophoresis.
2.4 In vitro transfection
MCF-7 (HTB-22; ATCC, Manassas, VA, USA) and MDA-MB-231 (HTB-26; ATCC) breast cancer cell lines expressing PDGF-D and PDGFR-β were used in transfection studies. Cells were cultured in DMEM supplemented with 10% FBS at 37°C, 5% CO2. For transfection, 2x106 cells per well were seeded in a six-well plate and nanoplexes containing siRNA were added to the wells in DMEM without serum. After 4 h of incubation, fresh complete medium was added to wells and, after 48 h of transfection, cells were collected. PDGF-D and PDGFR-β protein expression levels were investigated by ELISA.
The observation of transfection efficiency was performed by nanoplexes prepared with 5′-fluorescein isothiocyanate (FITC) siRNAs. Cells were seeded and transfected as mentioned above and examined at different time intervals with fluorescence microscopy (Axiovert; Zeiss, Oberkochen, Germany) for cell entry and localization detection.
2.5 Quantification of protein expression
PDGF-D and PDGFR-β protein expression levels in naked siRNAs and chitosan/siRNA nanoplexes treated MCF-7 and MDA-MB-231 cells were determined using an ELISA in accordance with the manufacturer's instructions. Cells treated with lipogen/siRNAs served as a positive control. All experiments were performed in triplicate and the mean ± SD values were calculated accordingly.
2.6 Invasion assay
MDA-MB-231 cells were transfected with chitosan nanoplexes containing siRNA. After 24 h, cells were detached from the tissue culture plate and 2 × 105 cells per well added on the top of matrigel coated on the 8.0 μm pore sized inserts. After 24 h incubation, non-invading cells are removed with cotton swab and cells on the inserts were fixed with 10% formaldehyde solution and then stained with 0.1% crystal violet. Invading cells were counted and photographed under the light microscope (BX51; Olympus, Tokyo, Japan).
2.7 In vivo studies
Thirty day-old female Sprague–Dawley rats were used in in vivo studies. N-nitroso-N-methylurea was injected intraperitoneally into rats at a dose of 50 mg/kg. Animals were kept in a standard light/dark cycle with access to food and water ad libitum. Tumor formation was monitored twice a week by palpation and tumor size was determined by caliper measurement. Tumor size was measured using a caliper across its length (L) and weight (W) and height (H) was calculated using the formula of V = 0.52 × L × W × H. Treatment started when the tumor volumes reached approximately 100 mm3 and rats were separated into six groups; tumor (untreated positive control group), naked siPDGF-D, naked siPDGFR-β, chitosan/siPDGF-D, chitosan/siPDGFR-β and chitosan/siPDGF-D/siPDGFR-β nanoplex groups (each group, n = 6). The untreated control group received intratumoral injections of PBS (pH 7.0). Formulations (w/w ratio of 50:1, 25 μg siRNA in PBS) were administered intratumorally twice a week and rats were killed after 30 days and samples were collected. Animal experiments were performed in accordance with regulations of local ethical committee of the Marmara University of Medical Faculty (36.2012.mar).
2.8 Real-time quantitative polymerase chain reaction (RT-qPCR)
RNA was isolated using RNA isolation kit and controlled spectrophotometrically. The cDNA was synthesized using a cDNA Synthesis Kit as the template for the qPCR reaction. The gene of interest (PDGF-D and PDGFR-β) was amplified using Taqman gene expression assay master mix (MgCl, dNTP, PCR buffer) in the RT-qPCR instrument (StepOne Plus; Applied Biosystems) and specific primers (PDGF-D Assay ID: Rn01447937_m1, PDGFR-β Assay ID: Rn00709573_m1, Actb Assay ID: Rn00667869_m1). All samples were tested in duplicate and the mean Ct values were calculated. The relative mRNA level of the target gene was normalized to endogenous ‘housekeeping’ gene (β-actin). Levels of PDGF-D and PDGFR-β mRNAs were compared between siRNA-treated groups and non-treated tumor group.
2.9 Immunohistochemistry
The streptavidin–biotin–peroxidase staining method was used to investigate PDGFR-β and CD-31 expression in tumor tissue. Tissue sections were deparaffinized and rehydrated through xylene and graded alcohols to water. Sections were incubated with hydrogen peroxide/methanol to block endogen peroxidase activity. After the antigen retrieval stage, protein blocking was performed to eliminate non-specific background staining. Following protein blocking, the primary anti-PDGFR-β (ab32570; Abcam, Cambridge, UK) and anti-CD-31 (sc-1,506-R; Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies were incubated with tissue sections for 1 h at room temperature. After that, sections were incubated with mouse and rabbit specific HRP/DAB micro-polymer detection kit (ab236466; Abcam) To show PDGFR-β and CD-31 immunostainings, diaminobenzidinetetrahydrochloride (DAB) was used as a chromogen, with Mayer hematoxylin as a counterstain. The staining intensity in stromal cells scored 0–3 (0 = negative, 1 = weak, 2 = intermediate and 3 = strong).23 The MVD intensity scoring for CD31 staining was obtained as the count for immunoreactive endothelial cells or clusters.
2.10 TUNEL assay
DNA fragmentation was determined by the terminal deoxynucleotide transferase mediated deoxyuridine triphosphate nick-end labeling (TUNEL) method using an ApopTag Plus Peroxidase In Situ Apoptosis Kit (S7101; Milipore). Following the deparaffinization and rehydration process, slides were incubated with proteinase K solution and inactivated endogenous peroxidase. Slides were incubated with TdT equilibration buffer. TdT labeling reaction mixture was applied onto slide and then stop buffer was added. All tumor slides were visualized using DAB solution and counterstained using hematoxylin solution. The apoptotic cell number was counted in 5–10 random fields at 20× magnification.
2.11 Interferon analysis
Serum interferon (IFN)-α levels were measured using an ELISA kit (YLBiont, China) to determine whether naked siRNAs and chitosan/siRNA nanoplexes induced the interferon response. The study was performed in accordance with the manufacturer's instructions. All experiments were performed in triplicate and the mean ± SD values were calculated accordingly.
2.12 Statistical analysis
All values were expressed as the mean ± SD. SPSS, version 12.0 (SPSS Inc., Chicago, IL, USA) was used to analyze data. A t test or analysis of variance was used to compare the results. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Control of chitosan nanoplexes
Nanoplexes were prepared by polyelectrolyte complexation between positively charged chitosan and negatively charged siRNAs with mechanical stirring at room temperature. A gel retardation assay was used to show the complexation capability of siRNA with chitosan. According to the electrophoresis results of chitosan/siRNA nanoplexes, partial complexation at 5:1 and 10:1 ratios and full complexation from 20:1 and 50:1 were observed (Figure 1A). It has thus been shown that chitosan nanoplexes can condense siRNA efficiently.

3.2 Control of zeta potential, particle size and morphology
Complex formation occurred successfully with protonation of the amine group between (+) charged chitosan and (−) charged siRNA molecules. Zeta potential values of chitosan/siPDGFR-β formulations changed between −0.29 ± 0.05 mV and +24.03 ± 2.13 mV and the particle size values were between 174.58 ± 4.63 nm and 347.25 ± 3.81 nm. Zeta potential values of chitosan/siPDGF-D formulations changed between −0.19 ± 0.02 mV and +23.79 ± 1.92 mV and the particle sizes were between 179.13 ± 5.14 nm and 342.02 ± 3.96 nm. The zeta potential values were between +3.14 ± 0.92 and +18.03 ± 0.79 mV and particle size values were between 130.92 ± 1.61 and 349.72 ± 4.82 nm in the chitosan/siRNA formulations containing both PDGF-D and PDGFR-β siRNAs together (Figure 1B).
Zeta potential measurement results of chitosan/siPDGF-D and siPDGFR-β nanoplexes were compatible with agarose gel electrophoresis profiles. Zeta potential values of 5:1 and 10:1 chitosan/PDGFR-β and chitosan/PDGF-D siRNA formulations were near neutral, which supports the partial complexation shown in agarose gel electrophoresis. Zeta potential and particle size values were increased at 10:1, 20:1 and 50:1 chitosan/siRNA formulations in direct proportion to the chitosan amount (p < 0.001) (Figure 1B).
As shown in Figure 1C, nanoplexes with regular shape were obtained by TEM.
3.3 Serum stability of nanoplexes
Naked siRNA treated with FBS was degraded by binding serum proteins from the 30 min and could not be detected by agarose gel electrophoresis after 24 h. However, chitosan/siRNA nanoplexes did not degrade up to 72 h. siRNAs which were not bound to nanoplexes, interact with FBS components and move slower at the agarose gel, which results smear shaped bands. A small amount of siRNA was released at 48 h, but no degradation in the nanoplex structure was observed. It was determined that the complex structures formed by siRNA and chitosan were stable and protected siRNA from serum effect during 72 h (Figure 1D).
3.4 In vitro cellular uptake by chitosan nanoplexes
Delivery of siRNA to breast cancer cells with chitosan nanoplexes was examined by transfection studies. After transfection of MCF-7 and MDA-MB-231 cell lines with chitosan nanoplexes prepared with FITC labeled siPDGF-D (chitosan/FITC-siRNA; 20:1), localization of siRNA in the cytoplasm was observed by fluorescence microscopy (Figure 2A). However, naked siRNA could not be observed at the same duration. This indicates that free siRNAs can not pass through the cell membrane in the absence of delivery systems.
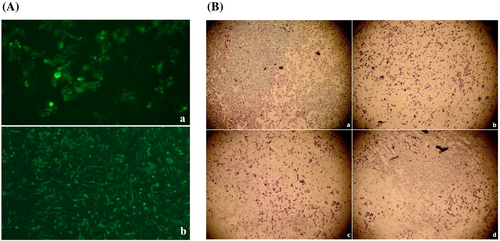
3.5 Decrease of tumor cell invasion by nanoplexes
The effect on the invasion ability of MDA-MB-231 cells, which are highly invasive, by chitosan/siPDGF-D + siPDGFR-β nanoplexes was investigated using a Matrigel invasion assay (Figure 2B). It was shown that the invasion of cells was significantly decreased after treatment with nanoplexes compared to the extent of the invasion of untreated control cells. The chitosan nanoplexes containing dual siRNA were more effective than chitosan nanoplex single siRNAs with respect to decreasing cell invasion (Figure 2B, b–d). These results showed that PDGF-D and its receptor play an important role in preventing breast cancer cell invasion.
3.6 Determination of protein expression by an ELISA
The rate of suppression of protein expression by simultaneous cellular delivery of PDGF-D and PDGFR-β siRNAs was investigated by a sandwich ELISA. The dual gene silencing efficacy of chitosan/siRNA nanoplexes in MCF-7 and MDA-MB-231 cell lines was demonstrated by an ELISA at 48 h post-transfection (Figure 3).
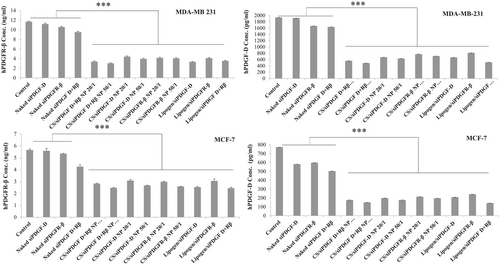
In the in vitro inhibition study conducted in MCF-7 cells, the highest PDGF-D gene silencing was obtained with combined nanoplexes and the silencing ratios in the following order (starting from the highest): 50:1 chitosan/siRNA formulations containing both siPDGF-D and siPDGFR-β 80.21 ± 0.11% > individual nanoplexes of chitosan/siPDGF-D 76.72 ± 0.13% > chitosan/siPDGFR-β siRNA 74.20 ± 0.04%. When siPDGF-D and siPDGFR-β were co-administered with commercial carrier lipogen, 81.17 ± 0.07% silencing was obtained, which was higher than silencing (68.35% and 72.65%) when siPDGF-D and siPDGFR-β were administered alone. Nanoplexes with chitosan showed similar silencing with lipogen.
In the study with MDA-MB-231 cells, the highest PDGF-D gene silencing was obtained with combined chitosan complexes of 74.59 ± 0.05%. When siPDGF-D and siPDGFR-β were applied alone, gene inhibition of 63.19 ± 0.71% with chitosan/siPDGFR-β and 67.23 ± 0.20% with chitosan/siPDGF-D was found. As can be seen in Figure 3, chitosan nanoplexes showed a similar inhibition rate as commercial lipogen (73.29 ± 0.30%) (p < 0.001).
We also determined PDGFR-β protein levels in both cells. In chitosan/siRNA nanoplexes containing both siPDGF-D and siPDGFR-β (50:1, w/w) treated cells, the expression of PDGFR-β protein (2.47 ng/ml and 3.02 ng/ml) was significantly reduced compared to untreated cells (5.65 ng/ml and 11.68 ng/ml), respectively, for MCF-7 and MDA-MB-231. Naked siPDGF-D and siPDGFR-β did not cause any statistical differences compared to untreated cells. The commercial lipogen/siPDGF-D/siPDGFR-β treated cells (58.7% and 70%) showed a similar silencing effect with chitosan/siPDGF-D/siPDGFR-β nanoplexes (56.3% and 74.2%), respectively, for MCF-7 and MDA-MB-231.
3.7 In vivo gene silencing and therapeutic effect of chitosan nanoplexes
Effect of chitosan/siPDGF-D + siPDGFR-β dual nanoplexes on tumor growth was monitored during 30 days. When the tumor volumes reached 100 mm3, the formulations were administered intratumorally once every three days during 4 weeks (naked siPDGF-D, naked siPDGFR-β, chitosan/siPDGF-D nanoplex, chitosan/siPDGFR-β, chitosan/siPDGF-D + siPDGFR-β). Tumor size was regularly measured every 7 days. After administration of formulations for 30 days, there were visible differences in tumor size. Tumor volume in the tumor control group was approximately 593 ± 79 mm3. Tumor volume decreased significantly after chitosan nanoplexes administration containing siPDGF-D and siPDGFR-β compared to the naked siRNA group. The nanoplexes containing both siPDGF-D and siPDGFR-β had the highest suppression of tumor growth; in other words, the highest reduction in tumor volumes is obtained with formula containing siPDGF-D and siPDGFR-β together (11 ± 5 mm3, 97.81%) (p < 0.001) (Figure 4A).
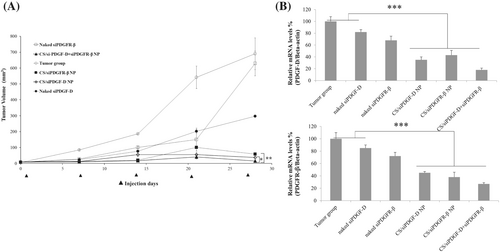
Assessment of suppression of PDGF-D pathway by siPDGF-D and siPDGFR-β containing nanoplexes was investigated by RT-qPCR assay (Figure 4B). The PDGF-D and PDGFR-β mRNA expression after chitosan/siPDGF-D + siPDGFR-β nanoplexes administration was decreased by 82% and 73% compared to the control group, respectively. qPCR results showed a markedly reduced expression of PDGF-D and PDGFR-β by combination therapy in a rat breast cancer model (p < 0.001). Single siRNAs also suppressed the mRNA expression of their corresponding targets.
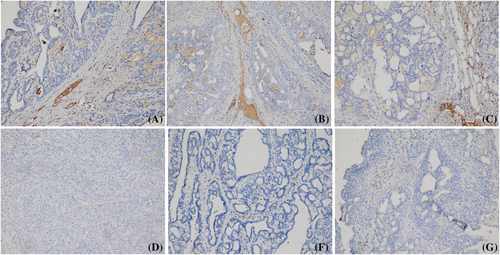
Immunohistochemical analysis was performed to detect PDGFR-β expression in breast tumor tissues. It was observed chitosan/siPDGF-D + siPDGFR-β nanoplexes caused a significant reduction in PDGFR-β expression compared to the tumor control group. In addition, PDGFR-β expression in the tumor stromal cells treated with dual nanoplexes decreased compared to individual nanoplex groups (Figure 5). When breast tumor-bearing rats were treated with chitosan/siPDGF-D + siPDGFR-β nanoplexes, vessel density was significantly reduced compared to the tumor control group (Figure 6).
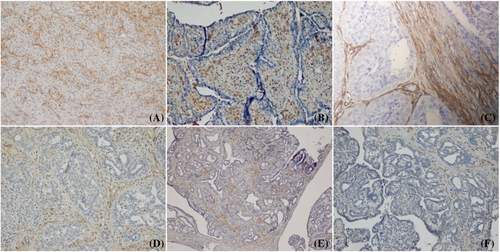
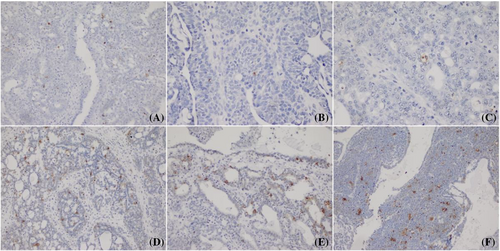
The induction of apoptosis in breast tumors was showed by TUNEL staining. Although the highest apoptotic cell staining was observed in the chitosan/siPDGF-D + siPDGFR-β group, staining intensity was low at single siRNA groups (p < 0.05) (Figure 7). Furthermore, there was almost no apoptotic staining in the tumor control and naked siRNA groups.
3.8 Interferon analysis
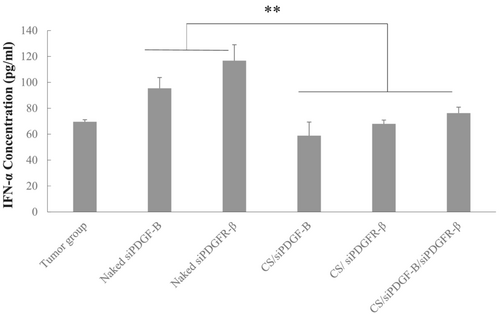
To determine whether IFN-α expression was induced following given of naked siRNAs and siRNA containing nanoplexes, serum samples were collected from tumor control and therapy groups. IFN-α levels were measured by an ELISA. Naked siRNAs induced higher levels of IFN-α expression (p < 0,01) compared to the tumor control group; however, chitosan/siRNA nanoplexes did not induce immunresponse (p > 0.05) (Figure 8).
4 DISCUSSION
Breast cancer is the leading cause of cancer-related morbidity and mortality in women. One of the most important tumor-promoting process is the aberrant induction of angiogenesis. PDGFs and their tyrosine kinase receptors are known to play an important role in the pathogenesis and angiogenesis of many tumor types.3 PDGFs and their receptors are highly expressed and activated in cancer, and they have shown to promote tumor growth. From the PDGF family of growth factors, PDGF-D has recently been detected in human breast cancer tissues.14 PDGF-D can regulate many cellular processes, including cell proliferation, apoptosis, transformation, migration, invasion, angiogenesis and metastasis.24 Liu et al. reported that overexpression of PDGF-D and PDGFR-β enhances breast tumor growth and metastasis.14 However, there is no study on the therapeutic effects dual inhibition of PDGF-D and PDGFR-β in breast cancer. The knockdown of PDGF-D and PDGFR-β in tumor stroma and tumor endothelial cell can be proposed as an important strategy for cancer therapy. Especially, silencing of genes and their receptors can be evaluated as alternative treatment approach in cancer. In our previous in vitro and in vivo studies about VEGF inhibition, it was demonstrated that combination therapy for knocking down both the growth factor and its associated receptor increase therapeutic efficiency.22 In the present study, we showed for the first time that co-inhibition of PDGF-D and its receptor PDGFR-β using chitosan-based gene delivery system reduces tumor growth and angiogenesis in breast cancer.
RNAi is a highly promising strategy for cancer treatment because of its high specificity and efficiency, low cost, and broad gene targeting properties. Especially, silencing of genes, which play an important role in cancer cell growth, proliferation, invasion, angiogenesis and metastasis, by RNAi can be considered as a way to increase the effectiveness of treatment.25 Knocking down a single tumor-associated gene may not be sufficient for eradicating the heterogeneous cancer population. Therefore, targeting multiple pathways or multiple members of the same pathway could be more effective. Doan et al.26 reported that siRNA cocktail targeting both VEGF (involved in angiogenesis) and kinesin spindle protein (involved in cell proliferation) inhibited proliferation and invasion of hepatocellular carcinoma cells and exhibited multigene silencing efficiency better than single siRNA individually. Multi-targeted siRNAs are known to show more effective silencing than single-target siRNA.27 Lee et al.21 developed chemically modified siRNA polymer from siRNAs that target two proteins, namely, VEGF and B-cell lymphoma 2 (Bcl-2), and this dual siRNA polymer, in turn, formed a complex with glycol chitosan carrier system. As a result, effective multi-gene silencing with synergistic effect in prostate cancer therapy was obtained with dual-poly-siRNA chitosan nanoparticles.
Even though siRNAs are important therapeutic molecules for cancer therapy, they cannot cross extracellular and intracellular barriers by themselves because of their negative surface charge and low stability. The use of nanocarriers to deliver siRNA prevents both fast renal clearence and RNase degradation by protecting siRNA chains and increasing their half-life in blood. Many studies have shown that chitosan is an effective biopolymer for siRNA delivery.28-30 In the present study, chitosan was chosen for nanoplex formulations because of its favorable properties, such as biocompatibility, biodegradability, non-toxicity and non-immunogenicity.31-33 In the preliminary study by our group, it was observed that siPDGF-D/chitosan nanoplexes can be effective against a breast cancer model.34 In another in vitro study, the silencing by chitosan nanoparticles containing different siRNA sequences of PDGF-D led to a significant decrease in PDGF-D protein expression in breast cancer cells.13 In the present study, we developed chitosan/siRNA nanoplexes for suppression of PDGF-D signaling pathway by targeting both PDGF-D and PDGFR-β.
In the first part of this study, in vitro characterization and transfection efficiency were investigated. At an w/w ratio of 20:1 ratio, there was complete complexation of siRNA with chitosan. Particle size and charge of nanoparticles are important parameters in formulations in terms of reaching to tumor, cellular uptake and intracellular trafficking.35 Small nanoparticles can reach to the tumor more effectively and easily with heterogeneous structure of the tumor vascularization and enhanced and permeability retention effect.36 Wu et al.37 showed that 200 nm in size, positive charged trimethyl chitosan nanoplexes encapsulate both doxorubicin and rhIL-2 had significant anti-tumor effect. In the present study, a prominent anti-tumor effect was obtained with approximately 300 nm sized and +18 mV surface charged chitosan/siPDGF-D + siPDGFR-β nanoplexes. In addition, TEM results of chitosan/siRNA nanoplexes indicated that the nanoplexes had spherical morphology (Figure 1C). Nanoplexes improved the stability of siRNA to serum for 72 h. In the uptake study with nanoplexes containing fluorescence-labeled siRNA, it was observed that the nanoplexes entered the cell at the end of 4 h. Naked siPDGF-D and siPDGFR-β had no obvious gene silencing effect, whereas chitosan/siPDGF-D and chitosan/siPDGFR-β nanoplexes showed significantly decreased protein expression, compared to naked siRNAs (p < 0.001). The highest gene silencing effect was provided with the w/w ratio of 50:1 chitosan/siRNA formulations containing both siPDGF-D and siPDGFR-β in MCF-7 and MDA-MB-231 cells (Figure 3). The results indicated that the expression of PDGF-D and PDGFR-β proteins was significantly decreased at an almost equal level to the lipogen/siPDGF-D/siPDGFR-β group in both breast cancer cell lines. This shows that the chitosan-based nanoplexes is a very efficient system for carrying multiple siRNAs. Our data also indicated that gene silencing effect vary depending on the type of breast cancer cells. In the in vitro cell culture study, approximately 68–80% silencing efficiency was obtained in the cells studied, and especially the combined use showed a better effect. Similar results with PDGF-D silencing were also observed in lung, colorectal and breast cancer studies.6, 13, 24, 38
In the invasion study, the invasion of cells was significantly decreased after treatment with nanoplexes compared to invasion of untreated control cells (Figure 2B). The chitosan nanoplexes containing dual siRNA were more effective than chitosan nanoplexes containing siPDGF-D or siPDGFR-β alone in reducing cell invasion. Because the invasive capacity of the tumor cells affects prognosis significantly, these findings support the rationale for dually targeting these two molecules to dampen invasiveness. Huang et al.6 also showed that knockdown of PDGF-D using siRNA significantly reduced cell invasion in lung cancer cells. In another study that demonstrated a mechanistic link between PDGF-D and invasion, PDGF-D was shown to play an important role in triggering epithelial–mesenchymal transition, a process essential for tumor invasion and migration.39
In the in vivo rat breast cancer model, the tumor volume was measured during the experiment. Whole volume in tumor-bearing rats without siRNA administration reached 593 ± 100 mm3 at the end of day 28, 581 ± 78 mm3 and 354 ± 16 mm3 in those administrated naked siPDGFR-β and siPDGF-D, respectively. Tumor volume was significantly decreased in the groups treated with chitosan/siPDGF-D and chitosan/siPDGFR-β nanoplexes compared to the naked siRNAs after intratumoral administration every 3 days. We observed that co-inhibition of PDGF-D and PDGFR-β has shown an improved anti-tumor effect compared to siRNAs targeting only one gene. In our previous study, it was shown that RNAi gene therapy with chitosan nanoplexes targeting both VEGF-A and VEGFRs (VEGFR1, VEGFR2 and NRP-1) provide a strong additive effect in tumor growth inhibition (97%).20 The efficacy of the naked siRNAs may be reduced because siRNA does not reach the tumor cells and microenvironment. siRNA from chitosan nanoplexes would be expected to be delivered and released more efficiently in the tumor.40 Therefore, chitosan/siRNA nanoplexes increased therapy efficiency. Our results are consistent with this notion.
For the in vivo gene silencing efficacy, RT-PCR results revealed that chitosan nanoplexes containing siPDGF-D and siPDGFR-β together decreased the mRNA expression of PDGF-D and PDGFR-β (82% and 73%, respectively). It was observed chitosan/siPDGF-D/siPDGFR-β nanoplexes caused a significantly decreased PDGF-D expression compared to the tumor control group and individual nanoplex groups (Figure 5). Lee et al.21 showed more effective knockdown of VEGF and Bcl-2 mRNA with dual chitosan nanoparticles compared to both siVEGF and siBcl-2 nanoparticles.
The overexpression of PDGF-D has been known to stimulate angiogenesis and inhibit apoptosis in cancer.41 Liu et al.14 reported that overexpression of PDGF-D led to tumor growth and metastasis through increased proliferation and decreased apoptosis in breast cancer. Some studies have also shown that siRNA-mediated knockdown of PDGF-D expression led to inhibition of angiogenesis and breast tumor growth.24 We found that dual siRNA containing chitosan nanoplexes decreased angiogenesis by CD-31 immunostaining and increase apoptosis by TUNEL staining. In addition, immunohistochemical analysis was performed to detect PDGFR-β expression in breast tumor tissues. It was observed chitosan/siPDGF-D + siPDGFR-β nanoplexes caused significantly decreased PDGFR-β expression compared to the tumor control group and individual nanoplex groups (Figure 5). Treatment of breast tumor-bearing rats with chitosan/siPDGF-D + siPDGFR-β nanoplexes markedly decreased vessel density compared to the tumor control group (Figure 6). The induction of apoptosis in breast tumors was showed by TUNEL staining. Although the highest apoptotic cell staining was observed in the chitosan/siPDGF-D + siPDGFR-β group, staining intensity was low for single siRNA groups (p < 0.05) (Figure 7). Furthermore, there was almost no apoptotic staining in tumor control and naked siRNA groups. These results show that tumor volume reduction by the dual siRNA nanoplexes can at least partially be explained by increased apoptosis of tumor cells and decreased angiogenesis that hinders tumor cell growth.
Many studies have shown that siRNA could induce cytokine production and the innate immune responses.42, 43 The sequence, structure and chemistry of siRNA can influence this immunostimulation.44 For effective silencing of target genes, the IFN response must be avoided. To examine whether naked siRNAs and chitosan/siRNA nanoplexes induced interferon responses, IFN-α levels in serum were assessed. The IFN-α level was not changed after treatment with chitosan/siPDGF-D/siPDGFR-β nanoplexes (Figure 8). On the other hand, introduction of naked siRNAs induced IFN-α response. RNAi pathway is negatively regulated by innate response. This shows that complexing with chitosan protected siRNAs form immune recognition, which contributed to the safety and efficiency of the treatment.
5 CONCLUSIONS
Upon investigating the effect of RNAi-mediated inhibition of PDGF-D and PDGFR-β on breast cancer, it was found that dual targeting of these molecules by the siRNAs complexed with chitosan vector decreased breast cancer malignancy and growth for both in vitro and in vivo settings. Overall, RNAi mediated gene suppression of PDGF-D and its receptor could be an effective therapeutic approach for breast cancer gene therapy. Therefore, this approach and formulation is a promising new candidate for an effective breast cancer treatment strategy.
ACKNOWLEDGEMENTS
This study was supported by Marmara University Scientific Research Projects Association (grant number SAG-B-110412-0075).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
E. Şalva, J. Akbuğa: Conceptualization, data curation, formal analysis, investigation, project administration, writing – original draft. S. Özbaş: Data curation, formal analysis, investigation. S. Alan: Formal analysis, data curation. N. Özkan, L. Kabasakal: Data curation. C. Ekentok-Atıcı: Writing – review and editing, data curation. All authors have read and agreed to the published version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




