Fluorescent polymer markers photoconvertible with a 532 nm laser for individual cell labeling
Corresponding Author
P. A. Demina
Science Medical Center, Saratov State University, Saratov, Russia
Correspondence
P. A. Demina, Science Medical Center, Saratov State University, Saratov 410012, Russia.
Email: [email protected]
Search for more papers by this authorO. A. Sindeeva
A.V. Zelmann Center for Neurobiology and Brain Rehabilitation, Skolkovo Institute of Science and Technology, Moscow, Russia
Search for more papers by this authorA. M. Abramova
Science Medical Center, Saratov State University, Saratov, Russia
Search for more papers by this authorM. S. Saveleva
Science Medical Center, Saratov State University, Saratov, Russia
Search for more papers by this authorG. B. Sukhorukov
A.V. Zelmann Center for Neurobiology and Brain Rehabilitation, Skolkovo Institute of Science and Technology, Moscow, Russia
School of Engineering and Materials Science, Queen Mary University of London, London, UK
Search for more papers by this authorI. Y. Goryacheva
Science Medical Center, Saratov State University, Saratov, Russia
Search for more papers by this authorCorresponding Author
P. A. Demina
Science Medical Center, Saratov State University, Saratov, Russia
Correspondence
P. A. Demina, Science Medical Center, Saratov State University, Saratov 410012, Russia.
Email: [email protected]
Search for more papers by this authorO. A. Sindeeva
A.V. Zelmann Center for Neurobiology and Brain Rehabilitation, Skolkovo Institute of Science and Technology, Moscow, Russia
Search for more papers by this authorA. M. Abramova
Science Medical Center, Saratov State University, Saratov, Russia
Search for more papers by this authorM. S. Saveleva
Science Medical Center, Saratov State University, Saratov, Russia
Search for more papers by this authorG. B. Sukhorukov
A.V. Zelmann Center for Neurobiology and Brain Rehabilitation, Skolkovo Institute of Science and Technology, Moscow, Russia
School of Engineering and Materials Science, Queen Mary University of London, London, UK
Search for more papers by this authorI. Y. Goryacheva
Science Medical Center, Saratov State University, Saratov, Russia
Search for more papers by this authorAbstract
Fluorescent photoconvertible materials and molecules have been successfully exploited as bioimaging markers and cell trackers. Recently, the novel fluorescent photoconvertible polymer markers have been developed that allow the long-term tracking of individual labeled cells. However, it is still necessary to study the functionality of this type of fluorescent labels for various operating conditions, in particular for commonly used discrete wavelength lasers. In this article, the photoconversion of fluorescent polymer labels with both pulsed and continuous-wave lasers with 532 nm-irradiation wavelength, and under different laser power densities were studied. The photoconversion process was described and its possible mechanism was proposed. The peculiarities of fluorescent polymer capsules performance as an aqueous suspension and as a single capsule were described. We performed the successful nondestructivity marker photoconversion inside RAW 264.7 monocyte/macrophage cells under continuous-wave laser with 532 nm-irradiation wavelength, showing prospects of these fluorescent markers for long-term live cell labeling.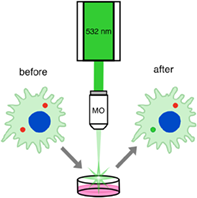
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1J. Haseloff, Methods Cell Biol. 1998, 58, 139.
- 2E. Belykh, K. V. Shaffer, C. Lin, V. A. Byvaltsev, M. C. Preul, L. Chen, Front. Oncol. 2020, 10, 10.
- 3Y. Fan, S. Wang, F. Zhang, Angew. Chem. 2019, 131, 13342.
10.1002/ange.201901964 Google Scholar
- 4A. Terskikh, A. Fradkov, G. Ermakova, A. Zaraisky, P. Tan, A. V. Kajava, X. Zhao, S. Lukyanov, M. Matz, S. Kim, I. Weissman, P. Siebert, Science 2000, 290, 1585.
- 5J. Wiedenmann, S. Ivanchenko, F. Oswald, F. Schmitt, C. Röcker, A. Salih, K.-D. Spindler, G. U. Nienhaus, Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 15905.
- 6S. M. Baker, R. W. Buckheit, M. M. Falk, BMC Cell Biol. 2010, 11, 15.
- 7V. Adam, R. Berardozzi, M. Byrdin, D. Bourgeois, Curr. Opin. Chem. Biol. 2014, 20, 92.
- 8S. Pletnev, D. M. Shcherbakova, O. M. Subach, N. V. Pletneva, V. N. Malashkevich, S. C. Almo, Z. Dauter, V. V. Verkhusha, PLoS One 2014, 9, e99136.
- 9R. Wachter, Int. J. Mol. Sci. 2017, 18, 1792.
- 10J. Wiedenmann, F. Oswald, G. U. Nienhaus, IUBMB Life 2009, 61, 1029.
- 11S. Guo, X. Cai, S. Zhou, Q. Zhu, H. Feng, Z. Qian, Dyes Pigm. 2022, 201, 110235.
- 12S. Shashkova, M. C. Leake, Biosci. Rep. 2017, 37, BSR20170031.
- 13S. Schildknecht, C. Karreman, D. Pöltl, L. Efremova, C. Kullmann, S. Gutbier, A.-K. Krug, D. Scholz, H. R. Gerding, M. Leist, ALTEX 2013, 30, 427.
- 14K. Tamada, D. Geng, Y. Sakoda, N. Bansal, R. Srivastava, Z. Li, E. Davila, Clin. Cancer Res. 2012, 18, 6436.
- 15T. Li, Q. Chen, Y. Zheng, P. Zhang, X. Chen, J. Lu, Y. Lv, S. Sun, W. Zeng, Stem Cell Res. Ther. 2019, 10, 399.
- 16I. A. Simpson, A. Carruthers, S. J. Vannucci, J. Cereb. Blood Flow Metab. 2007, 27, 1766.
- 17M. Asheuer, F. Pflumio, S. Benhamida, A. Dubart-Kupperschmitt, F. Fouquet, Y. Imai, P. Aubourg, N. Cartier, Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 3557.
- 18S. P. Quenneville, P. Chapdelaine, D. Skuk, M. Paradis, M. Goulet, J. Rousseau, X. Xiao, L. Garcia, J. P. Tremblay, Mol. Ther. 2007, 15, 431.
- 19S. Zhu, Y. Lu, J. Zhu, J. Xu, H. Huang, M. Zhu, Y. Chen, Y. Zhou, X. Fan, Z. Wang, J. Surg. Res. 2011, 168, 213.
- 20J. Bloch, J.-F. Brunet, C. R. S. McEntire, D. E. Redmond, J. Comp. Neurol. 2014, 522, 2729.
- 21M. Bujold, N. Caron, G. Camiran, S. Mukherjee, P. D. Allen, J. P. Tremblay, Y. Wang, Cell Transpl. 2002, 11, 759.
- 22D. Kami, Y. Suzuki, M. Yamanami, T. Tsukimura, T. Togawa, H. Sakuraba, S. Gojo, Cell Transpl. 2021, 30, 096368972110602.
- 23H. Tranchart, M. Gaillard, P. S. Diop, S. Goulinet, P. Lainas, I. Dagher, J. Surg. Res. 2018, 224, 23.
- 24G. C. Wilson, J. M. Sutton, D. E. Abbott, M. T. Smith, A. M. Lowy, J. B. Matthews, H. L. R. Rilo, N. Schmulewitz, M. Salehi, K. Choe, J. Brunner, D. J. Hanseman, J. J. Sussman, M. J. Edwards, S. A. Ahmad, Ann. Surg. 2014, 260, 659.
- 25K. M. Turner, A. M. Delman, E. C. Donovan, J. Brunner, S. A. Wahab, Y. Dai, K. A. Choe, M. T. Smith, S. H. Patel, S. A. Ahmad, G. C. Wilson, HPB 2013, 2022, 24.
- 26Y. Nasu, Y. Shen, L. Kramer, R. E. Campbell, Nat. Chem. Biol. 2021, 17, 509.
- 27R. M. Lackner, C. L. Johnny, D. M. Chenoweth, Methods Enzymol. 2020, 639, 379.
- 28E. A. Halabi, J. Arasa, S. Püntener, V. Collado-Diaz, C. Halin, P. Rivera-Fuentes, ACS Chem. Biol. 2020, 15, 1613.
- 29R. Turcotte, J. W. Wu, C. P. Lin, J. Biophoton. 2017, 10, 206.
- 30P. A. Demina, O. A. Sindeeva, A. M. Abramova, E. S. Prikhozhdenko, R. A. Verkhovskii, E. V. Lengert, A. V. Sapelkin, I. Y. Goryacheva, G. B. Sukhorukov, ACS Appl. Mater. Interfaces 2021, 13, 19701.
- 31M. J. Petrasiunas, M. I. Hussain, J. Canning, M. Stevenson, D. Kielpinski, Opt. Express 2014, 22, 17716.
- 32 NT250 series. Tunable wavelength UV-NIR range DPSS lasers (homepage on the internet), https://ekspla.com/wp-content/uploads/products/tunable-lasers/NT250/NT250-datasheet-20220913.pdf (accessed: January 20, 2023).
- 33V. Kapoor, F. V. Subach, V. G. Kozlov, A. Grudinin, V. V. Verkhusha, W. G. Telford, Nat. Methods 2007, 4, 678.
- 34S. Kellnberger, D. Soliman, G. J. Tserevelakis, M. Seeger, H. Yang, A. Karlas, L. Prade, M. Omar, V. Ntziachristos, Light Sci. Appl. 2018, 7, 109.
- 35H. M. Shapiro, W. G. Telford, Curr. Protoc. Cytom. 2009, 49, 17.
- 36W. G. Telford, Methods Cell Biol. 2011, 102, 373.
- 37 Amnis ImageStreamX Mk II imaging flow cytometer (homepage on the internet). https://www.luminexcorp.com/imagestreamx-mk-ii/#overview (accessed: January 20, 2023).
- 38K. M. McKinnon, Curr. Protoc. Immunol. 2018, 120, 11.
10.1002/cpim.40 Google Scholar
- 39M. Hashemi Shabestari, A. E. C. Meijering, W. H. Roos, G. J. L. Wuite, E. J. G. Peterman, Methods Enzymol. 2017, 582, 85.
- 40G. S. Pai, A. H. Pai, Aesthet. Dermatol. Surg. 2017, 1, 2.
10.1159/000454913 Google Scholar
- 41B. Taciak, M. Białasek, A. Braniewska, Z. Sas, P. Sawicka, Ł. Kiraga, T. Rygiel, M. Król, PLoS One 2018, 13, e0198943.
- 42X. Cao, T. Tan, D. Zhu, H. Yu, Y. Liu, H. Zhou, Y. Jin, Q. Xia, Int. J. Nanomed. 1915, 2020, 15.
- 43T. Liang, R. Zhang, X. Liu, Q. Ding, S. Wu, C. Li, Y. Lin, Y. Ye, Z. Zhong, M. Zhou, Int. J. Nanomed. 2021, 16, 2703.
- 44A. Y. Sapach, O. A. Sindeeva, M. V. Nesterchuk, A. A. Tsitrina, O. A. Mayorova, E. S. Prikhozhdenko, R. A. Verkhovskii, A. S. Mikaelyan, Y. V. Kotelevtsev, G. B. Sukhorukov, ACS Appl. Mater. Interfaces 2022, 14, 51579.
- 45D. V. Volodkin, A. I. Petrov, M. Prevot, G. B. Sukhorukov, Langmuir 2004, 20, 3398.
- 46G. B. Sukhorukov, E. Donath, H. Lichtenfeld, E. Knippel, M. Knippel, A. Budde, H. Möhwald, Colloids Surf. A: Physicochem. Eng. Asp 1998, 137, 253.
- 47E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis, H. Möhwald, Angew. Chem. Int. Ed. 1998, 37, 2201.
10.1002/(SICI)1521-3773(19980904)37:16<2201::AID-ANIE2201>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 48G. Decher, Science 1997, 277, 1232.
- 49D. Hirayama, T. Iida, H. Nakase, Int. J. Mol. Sci. 2017, 19, 92.
- 50X. Geeraerts, E. Bolli, S.-M. Fendt, J. A. Van Ginderachter, Front. Immunol. 2017, 8, 289.
- 51S. Rutkowski, T. Si, M. Gai, M. Sun, J. Frueh, Q. He, J. Colloid Interface Sci. 2019, 541, 407.
- 52D. B. Trushina, T. V. Bukreeva, T. N. Borodina, D. D. Belova, S. Belyakov, M. N. Antipina, Colloids Surf. B Biointerfaces 2018, 170, 312.
- 53K. Köhler, D. G. Shchukin, H. Möhwald, G. B. Sukhorukov, J. Phys. Chem. B 2005, 109, 18250.
- 54W. Kang, Y. Gao, X. Tang, C. Cao, L. Hu, H. Yang, J. Appl. Polym. Sci. 2019, 136, 47468.
- 55I. Estrela-Lopis, J. J. Iturri Ramos, E. Donath, S. E. Moya, J. Phys. Chem. B 2010, 114, 84.
- 56M. Alba, P. Formentín, J. Ferré-Borrull, J. Pallarès, L. F. Marsal, Nanoscale Res. Lett. 2014, 9, 411.




