In vitro evaluation of photodynamic activity of methylene blue against Trichophyton verrucosum azole-susceptible and -resistant strains
Corresponding Author
Sebastian Gnat
Faculty of Veterinary Medicine, Department of Veterinary Microbiology, University of Life Sciences, Lublin, Poland
Correspondence
Sebastian Gnat, Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Life Sciences, Lublin, Poland.
Email: [email protected]
Search for more papers by this authorDominik Łagowski
Faculty of Veterinary Medicine, Department of Veterinary Microbiology, University of Life Sciences, Lublin, Poland
Search for more papers by this authorMariusz Dyląg
Faculty of Biological Sciences, Department of Mycology and Genetics, University of Wroclaw, Wroclaw, Poland
Search for more papers by this authorJessica Zielinski
Hollings Cancer Center, Medical University of South Carolina, Charleston, South Carolina, USA
Search for more papers by this authorAneta Nowakiewicz
Faculty of Veterinary Medicine, Department of Veterinary Microbiology, University of Life Sciences, Lublin, Poland
Search for more papers by this authorCorresponding Author
Sebastian Gnat
Faculty of Veterinary Medicine, Department of Veterinary Microbiology, University of Life Sciences, Lublin, Poland
Correspondence
Sebastian Gnat, Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Life Sciences, Lublin, Poland.
Email: [email protected]
Search for more papers by this authorDominik Łagowski
Faculty of Veterinary Medicine, Department of Veterinary Microbiology, University of Life Sciences, Lublin, Poland
Search for more papers by this authorMariusz Dyląg
Faculty of Biological Sciences, Department of Mycology and Genetics, University of Wroclaw, Wroclaw, Poland
Search for more papers by this authorJessica Zielinski
Hollings Cancer Center, Medical University of South Carolina, Charleston, South Carolina, USA
Search for more papers by this authorAneta Nowakiewicz
Faculty of Veterinary Medicine, Department of Veterinary Microbiology, University of Life Sciences, Lublin, Poland
Search for more papers by this authorAbstract
The intense search for the “Holy Grail” of antifungal therapy can be observed today. The searches are not limited only to discovery of potential antifungal drugs, but also new therapeutic strategies involving the use of chemosensitizers to achieve synergistic effect or physicochemical factors inducing stress conditions in fungal cells. In this study was examined in vitro effectiveness of photodynamic antifungal strategy with methylene blue using a light beam with a wavelength equal to 635 nm toward the Trichophyton verrucosum susceptible and itraconazole- and/or fluconazole-resistant strains. Methylene blue used at concentration equal to 5 μg/mL and in the presence of 40 J/cm2 of light energy showed fungicidal effect toward the susceptible strains. However, for azole-resistant isolates, only the energy dose equal to 60 J/cm2 at 5 μg/mL of methylene blue allowed to kill the pathogen. This study confirms that methylene blue induced by red light has a definite inhibitory effect on zoophilic dermatophytes.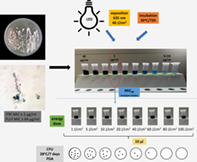
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1S. Gnat, D. Łagowski, A. Nowakiewicz, J. Appl. Microbiol. 2020, 129, 212.
- 2S. Gnat, D. Łagowski, A. Nowakiewicz, M. Dyląg, J. Appl. Microbiol. 2021, 15032.
- 3P. G. Pappas, Am. J. Med. Sci. 2010, 340, 253.
- 4S. Gnat, D. Łagowski, A. Nowakiewicz, M. Dyląg, Infection 2020, 48, 429.
- 5V. L. Burstein, I. Beccacece, L. Guasconi, C. J. Mena, L. Cervi, L. S. Chiapello, Front. Immunol. 2020, 11, 605644.
- 6P. Nenoff, S. B. Verma, R. Vasani, A. Burmester, U. C. Hipler, F. Wittig, C. Krüger, K. Nenoff, C. Wiegand, A. Saraswat, R. Madhu, S. Panda, A. Das, M. Kura, A. Jain, D. Koch, Y. Gräser, S. Uhrlaß, Mycoses 2019, 62, 336.
- 7D. Bouazzi, S. Fabricius, G. B. Jemec, D. M. Lindhardt Saunte, Med Mycol Case Rep 2019, 26, 67.
- 8S. Gnat, D. Łagowski, A. Nowakiewicz, M. Osińska, Ł. Kopiński, Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2099.
- 9A. Singh, A. Masih, J. Monroy-Nieto, P. K. Singh, J. Bowers, J. Travis, A. Khurana, D. M. Engelthaler, J. F. Meis, A. Chowdhary, Fungal Genet. Biol. 2019, 133, 103266.
- 10D. Łagowski, S. Gnat, A. Nowakiewicz, M. Osińska, A. Trościańczyk, P. Zięba, Zoonoses Public Health 2019, 66, 982.
- 11S. Gnat, D. Łagowski, A. Nowakiewicz, A. Trościańczyk, P. Zięba, Mycoses 2018, 61, 681.
- 12L. Courtellemont, S. Chevrier, B. Degeilh, S. Belaz, J. P. Gangneux, F. Robert-Gangneux, Med. Mycol. 2017, 55, 720.
- 13S. Gnat, D. Łagowski, A. Nowakiewicz, M. Dyląg, Postępy Mikrobiol - Adv Microbiol 2020, 59, 63.
- 14A. Khurana, K. Sardana, A. Chowdhary, Fungal Genet. Biol. 2019, 132, 103255.
- 15D. Łagowski, S. Gnat, A. Nowakiewicz, Postępy Mikrobiol - Adv Microbiol 2020, 59, 153.
- 16S. Dogra, D. Shaw, S. Rudramurthy, Indian Dermatol. Online J. 2019, 10, 225.
- 17A. K. Gupta, K. A. Foley, S. G. Versteeg, Mycopathologia 2017, 182, 127.
- 18A. Alkeswani, W. Cantrell, B. Elewski, Ski Appendage Disord 2019, 5, 201.
- 19A. Bhatia, B. Kanish, D. Badyal, P. Kate, S. Choudhary, Indian J. Pharm. 2019, 51, 116.
- 20D. Łagowski, S. Gnat, A. Nowakiewicz, M. Osińska, M. Dyląg, Infection 2020, 48, 889.
- 21H. A. S. M. M. Al, B. C. Sil, F. Iliopoulos, R. Lever, J. Hadgraft, M. E. Lane, A. H. ASMM, B. C. Sil, F. Iliopoulos, R. Lever, J. Hadgraft, M. E. Lane, Pharmaceutics 2019, 11, 548.
- 22V. Pai, A. Ganavalli, N. N. Kikkeri, Indian J. Dermatol. 2018, 63, 361.
- 23M. Abastabar, T. Hosseini, R. Valadan, M. Lagzian, I. Haghani, N. Aslani, H. Badali, S. Nouripour-Sisakht, M. Nazeri, S. Gholami, M. Vakili, P. Bowyer, T. Shokohi, M. T. Hedayati, Microb. Drug Resist. 2019, 25, 652.
- 24J. Zhang, L. Li, Q. Lv, L. Yan, Y. Wang, Y. Jiang, Front. Microbiol. 2019, 10, 691.
- 25M. Monod, M. Feuermann, K. Salamin, M. Fratti, M. Makino, M. M. Alshahni, K. Makimura, T. Yamada, Antimicrob. Agents Chemother. 2019, 63, e00863.
- 26N. Parrish, S. L. Fisher, A. Gartling, D. Craig, N. Boire, J. Khuvis, S. Riedel, S. Zhang, Front. Cell. Infect. Microbiol. 2020, 10, 545913.
- 27S. W. Chapman, D. C. Sullivan, J. D. Cleary, Trans. Am. Clin. Climatol. Assoc. 2008, 119, 197.
- 28R. Falk-Mahapatra, S. O. Gollnick, Photochem. Photobiol. 2020, 96, 550.
- 29J. Brasch, V. Beck-Jendroschek, V. Mahn, Mycoses 2018, 61, 393.
- 30P. López-Chicón, N. S. Gulías, M. Agut, Actas Dermosifiliogr. 2016, 107, 765.
- 31L. W. F. Souza, S. V. T. Souza, A. C. Botelho, An. Bras. Dermatol. 2014, 89, 184.
- 32J. S. Guffey, W. Payne, W. Roegge, Mycoses 2017, 60, 723.
- 33C. Li, X. Jia, Y. Bian, D. Qi, J. Wu, Mycoses 2021, 64, 48.
- 34J. P. Tardivo, A. Del Giglio, C. S. De Oliveira, D. S. Gabrielli, H. C. Junqueira, D. B. Tada, D. Severino, T. R. De Fátima, M. S. Baptista, Photodiagn. Photodyn. Ther. 2005, 2, 175.
- 35M. Tanaka, M. Kinoshita, Y. Yoshihara, N. Shinomiya, S. Seki, K. Nemoto, T. Hirayama, T. Dai, L. Huang, M. R. Hamblin, Y. Morimoto, Photochem. Photobiol. 2012, 88, 227.
- 36B. T. White, S. Lee, J. Taylor, T. J. White, T. D. Bruns, S. B. Lee, J. W. Taylor, PCR Protocol 2007, 315.
- 37 Clinical and Laboratory Standards Institute (CLSI M38-2A), Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed., Wayne, PA, Clinical and Laboratory Standards Institute, 2008.
- 38N. Robbins, G. D. Wright, L. E. Cowen, Fungal Kingdom 2016, 4, 903.
- 39S. B. Verma, A. Bishnoi, K. Vinay, S. Dogra, Lancet Infect. Dis. 2018, 18, 250.
- 40E. M. Tuite, J. M. Kelly, J. Photochem. Photobiol. B Biol. 1993, 21, 103.
- 41H. C. Junqueira, D. Severino, L. G. Dias, M. S. Gugliotti, M. S. Baptista, Phys. Chem. Chem. Phys. 2002, 4, 2320.
- 42J. P. Tardivo, A. Del Giglio, L. H. C. Paschoal, A. S. Ito, M. S. Baptista, Photodiagn. Photodyn. Ther. 2004, 1, 345.
- 43L. Via, S. Magno, Curr. Med. Chem. 2001, 8, 1405.
- 44A. R. Scwingel, A. R. P. Barcessat, S. C. Núñez, M. S. Ribeiro, Photomed. Laser Surg. 2012, 30, 429.
- 45G. B. Rodrigues, L. K. S. Ferreira, M. Wainwright, G. U. L. Braga, J. Photochem. Photobiol. B 2012, 116, 89.
- 46S. C. de Souza, J. C. Junqueira, I. Balducci, C. Y. Koga-Ito, E. Munin, A. O. C. Jorge, J. Photochem. Photobiol. B 2006, 83, 34.
- 47T. Dai, V. J. Bil de Arce, G. P. Tegos, M. R. Hamblin, Antimicrob. Agents Chemother. 2011, 55, 5710.
- 48T. Spezzia-Mazzocco, S. A. Torres-Hurtado, J. C. Ramírez-San-Juan, R. Ramos-García, Photonics Lasers Med 2016, 5, 203.
- 49N. M. Martinez-Rossi, T. A. Bitencourt, N. T. A. Peres, E. A. S. Lang, E. V. Gomes, N. R. Quaresemin, M. P. Martins, L. Lopes, A. Rossi, Front. Microbiol. 2018, 9, 1108.
- 50B. Geißel, V. Loiko, I. Klugherz, Z. Zhu, N. Wagener, O. Kurzai, C. A. M. J. J. van den Hondel, J. Wagener, Nat. Commun. 2018, 9, 3098.
- 51A. Singal, D. Khanna, Indian J. Dermatol. Venereol. Leprol. 2011, 77, 659.