The near-infrared autofluorescence fingerprint of the brain
Correction(s) for this article
-
Corrigendum: The near-infrared autofluorescence fingerprint of the brain
- Volume 14Issue 9Journal of Biophotonics
- First Published online: August 3, 2021
José Lifante
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Search for more papers by this authorBlanca del Rosal
ARC Centre of Excellence for Nanoscale BioPhotonics, School of Science, RMIT University, Melbourne, Victoria, Australia
Search for more papers by this authorIrene Chaves-Coira
Department of Anatomy, Histology and Neuroscience, Faculty of Medicine, Universidad Autónoma de Madrid, Madrid, Spain
Search for more papers by this authorNuria Fernández
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Search for more papers by this authorCorresponding Author
Daniel Jaque
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Correspondence
Dr Daniel Jaque, Faculty of Sciences, Department of Physics of Materials, Universidad Autonoma de Madrid, Madrid 28049, Spain.
Email: [email protected]
Search for more papers by this authorErving Ximendes
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Search for more papers by this authorJosé Lifante
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Search for more papers by this authorBlanca del Rosal
ARC Centre of Excellence for Nanoscale BioPhotonics, School of Science, RMIT University, Melbourne, Victoria, Australia
Search for more papers by this authorIrene Chaves-Coira
Department of Anatomy, Histology and Neuroscience, Faculty of Medicine, Universidad Autónoma de Madrid, Madrid, Spain
Search for more papers by this authorNuria Fernández
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Search for more papers by this authorCorresponding Author
Daniel Jaque
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Correspondence
Dr Daniel Jaque, Faculty of Sciences, Department of Physics of Materials, Universidad Autonoma de Madrid, Madrid 28049, Spain.
Email: [email protected]
Search for more papers by this authorErving Ximendes
Fluorescence Imaging Group, Universidad Autonoma de Madrid, Madrid, Spain
Nanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain
Search for more papers by this authorFunding information: Comunidad de Madrid, Grant/Award Number: B2017/BMD-3867RENIMCM; European Cooperation in Science and Technology, Grant/Award Number: CA17140; Fundación para la Investigación Biomédica del Hospital Universitario Ramón y Cajal, Grant/Award Number: IMP18_38(2018/0265); Horizon 2020 Framework Programme, Grant/Award Number: 801305; Instituto de Salud Carlos III, Grant/Award Number: PI16/00812; Ministerio de Ciencia, Innovación y Universidades, Grant/Award Number: FJC2018-036734-I; Ministerio de Economía y Competitividad, Grant/Award Numbers: MAT2016-75362-C3-1-R, MAT2017-83111R, MAT2017-85617-R
Abstract
The brain is a vital organ involved in most of the central nervous system disorders. Their diagnosis and treatment require fast, cost-effective, high-resolution and high-sensitivity imaging. The combination of a new generation of luminescent nanoparticles and imaging systems working in the second biological window (near-infrared II [NIR-II]) is emerging as a reliable alternative. For NIR-II imaging to become a robust technique at the preclinical level, full knowledge of the NIR-II brain autofluorescence, responsible for the loss of image resolution and contrast, is required. This work demonstrates that the brain shows a peculiar infrared autofluorescence spectrum that can be correlated with specific molecular components. The existence of particular structures within the brain with well-defined NIR autofluorescence fingerprints is also evidenced, opening the door to in vivo anatomical imaging. Finally, we propose a rational selection of NIR luminescent probes suitable for low-noise brain imaging based on their spectral overlap with brain autofluorescence.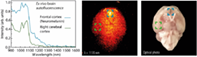
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
Supporting Information
| Filename | Description |
|---|---|
| jbio202000154-sup-0001-Supinfo.docxWord 2007 document , 1.7 MB | Figure S1 Hyperspectral Imaging. (a) Schematic representation of the hyperspectral imaging system used in this work. Figure S2 Optical effects induced by the skull. (a) Narrowband fluorescence image of a brain half covered by a piece of skull (left) and a piece of skull isolated (right). (b) Comparison of autofluorescence spectra of the isolated brain and skull. (c) Transmittance of the skull in the 900–1600 nm wavelength range according to previously published data [22]. (d) Comparison between the autofluorescence provided by the isolated brain (black), the skull mounted on the brain (red) and the simulation using the data of [22] for the values of transmittance. Figure S3 In vivo hyperspectral analysis of brain under a cranial window. Figure S4 Residual spectra for reported NIR-II liver autofluorescence and the data obtained in this work. (a) Residual spectra was calculated for both reported and experimentally obtained curves after applying the Savitzky–Golay smoothing method (Polynomial order 5, 9 points of window). (b) original spectra from which the noise was estimated [23]. Figure S5 Ex vivo ratiometric images of different tissues and organs. Figure S III: Ratiometric images of the different tissues. (a) Ratiometric image as obtained by calculating the ratio between emitted intensities at 1080/1125 nm. (b) Ratiometric image as obtained by calculating the ratio between emitted intensities at 1020/990 nm. (c) Intensity ratio between 1080 and 1125 nm. (d) Intensity ratio between 1020 and 990 nm. Figure S6 Optical frontal view of the brain of a C57BL/6J mouse. The olfactory bulbs have been removed to expose the frontal area in which meningeal melanocytes enriched in neuromelanin can be observed by naked-eye examination (gray square). |
| jbio202000154-sup-0002-TableS1.pdfPDF document, 386.5 KB | Table S1 Recent advances in NIR-II fluorophores for transcranial brain imaging. An updated list of some of the most relevant NIR-II nanoprobes and their in vivo applications. (Wt = wild type, MCAO = Middle cerebral artery occlusion). |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1V. L. Feigin, A. A. Abajobir, K. H. Abate, F. Abd-Allah, A. M. Abdulle, S. F. Abera, G. Y. Abyu, M. B. Ahmed, A. N. Aichour, I. Aichour, M. T. E. Aichour, R. O. Akinyemi, S. Alabed, R. Al-Raddadi, N. Alvis-Guzman, A. T. Amare, H. Ansari, P. Anwari, J. Ärnlöv, H. Asayesh, S. W. Asgedom, T. M. Atey, L. Avila-Burgos, E. Frinel, G. A. Avokpaho, M. R. Azarpazhooh, A. Barac, M. Barboza, S. L. Barker-Collo, T. Bärnighausen, N. Bedi, E. Beghi, D. A. Bennett, I. M. Bensenor, A. Berhane, B. D. Betsu, S. Bhaumik, S. M. Birlik, S. Biryukov, D. J. Boneya, L. N. B. Bulto, H. Carabin, D. Casey, C. A. Castañeda-Orjuela, F. Catalá-López, H. Chen, A. A. Chitheer, R. Chowdhury, H. Christensen, L. Dandona, R. Dandona, G. A. de Veber, S. D. Dharmaratne, H. P. Do, K. Dokova, E. R. Dorsey, R. G. Ellenbogen, S. Eskandarieh, M. S. Farvid, S.-M. Fereshtehnejad, F. Fischer, K. J. Foreman, J. M. Geleijnse, R. F. Gillum, G. Giussani, E. M. Goldberg, P. N. Gona, A. C. Goulart, H. C. Gugnani, R. Gupta, V. Hachinski, R. Gupta, R. R. Hamadeh, M. Hambisa, G. J. Hankey, H. A. Hareri, R. Havmoeller, S. I. Hay, P. Heydarpour, P. J. Hotez, M. B. Jakovljevic, M. Javanbakht, P. Jeemon, J. B. Jonas, Y. Kalkonde, A. Kandel, A. Karch, A. Kasaeian, A. Kastor, P. N. Keiyoro, Y. S. Khader, I. A. Khalil, E. A. Khan, Y.-H. Khang, A. Tawfih, A. Khoja, J. Khubchandani, C. Kulkarni, D. Kim, Y. J. Kim, M. Kivimaki, Y. Kokubo, S. Kosen, M. Kravchenko, R. V. Krishnamurthi, B. K. Defo, G. A. Kumar, R. Kumar, H. H. Kyu, A. Larsson, P. M. Lavados, Y. Li, X. Liang, M. L. Liben, W. D. Lo, G. Logroscino, P. A. Lotufo, C. T. Loy, M. T. Mackay, H. M. A. El Razek, M. M. A. El Razek, A. Majeed, R. Malekzadeh, T. Manhertz, L. G. Mantovani, J. Massano, M. Mazidi, C. McAlinden, S. Mehata, M. M. Mehndiratta, Z. A. Memish, W. Mendoza, M. A. Mengistie, G. A. Mensah, A. Meretoja, H. B. Mezgebe, T. R. Miller, S. R. Mishra, N. M. Ibrahim, A. Mohammadi, K. E. Mohammed, S. Mohammed, A. H. Mokdad, M. Moradi-Lakeh, I. M. Velasquez, K. I. Musa, M. Naghavi, J. W. Ngunjiri, C. T. Nguyen, G. Nguyen, Q. Le Nguyen, T. H. Nguyen, E. Nichols, D. N. A. Ningrum, V. M. Nong, B. Norrving, J. J. N. Noubiap, F. A. Ogbo, M. O. Owolabi, J. D. Pandian, P. G. Parmar, D. M. Pereira, M. Petzold, M. R. Phillips, M. A. Piradov, R. G. Poulton, F. Pourmalek, M. Qorbani, A. Rafay, M. Rahman, M. H. Rahman, R. K. Rai, S. Rajsic, A. Ranta, S. Rawaf, A. M. N. Renzaho, M. S. Rezai, G. A. Roth, G. Roshandel, E. Rubagotti, P. Sachdev, S. Safiri, R. Sahathevan, M. A. Sahraian, A. M. Samy, P. Santalucia, I. S. Santos, B. Sartorius, M. Satpathy, M. Sawhney, M. I. Saylan, S. G. Sepanlou, M. A. Shaikh, R. Shakir, M. Shamsizadeh, K. N. Sheth, M. Shigematsu, H. Shoman, D. A. S. Silva, M. Smith, E. Sobngwi, L. A. Sposato, J. D. Stanaway, D. J. Stein, T. J. Steiner, L. J. Stovner, R. S. Abdulkader, C. E. Szoeke, R. Tabarés-Seisdedos, D. Tanne, A. M. Theadom, A. G. Thrift, D. L. Tirschwell, R. Topor-Madry, B. X. Tran, T. Truelsen, K. B. Tuem, K. N. Ukwaja, O. A. Uthman, Y. Y. Varakin, T. Vasankari, N. Venketasubramanian, V. V. Vlassov, F. Wadilo, T. Wakayo, M. T. Wallin, E. Weiderpass, R. Westerman, T. Wijeratne, C. S. Wiysonge, M. A. Woldu, C. D. A. Wolfe, D. Xavier, G. Xu, Y. Yano, H. H. Yimam, N. Yonemoto, C. Yu, Z. Zaidi, M. E. S. Zaki, J. R. Zunt, C. J. L. Murray, T. Vos, Lancet Neurol. 2017, 16, 877.
- 2M. Katsuno, K. Sahashi, Y. Iguchi, A. Hashizume, Nagoya J. Med. Sci. 2018, 80, 289.
- 3J. N. D. Kerr, W. Denk, Nat. Rev. Neurosci. 2008, 9, 195.
- 4E. Hemmer, A. Benayas, F. Légaré, F. Vetrone, Nanoscale Horiz. 2016, 1, 168.
- 5R. R. Anderson, J. A. Parrish, J. Invest. Dermatol. 1981, 77, 13.
- 6G. Hong, A. L. Antaris, H. Dai, Nature Biomed. Eng. 2017, 1, 10.
- 7G. Chen, Y. Zhang, C. Li, D. Huang, Q. Wang, Q. Wang, Adv. Healthc. Mater. 2018, 7, 497.
- 8B. del Rosal, D. Ruiz, I. Chaves-Coira, B. H. Juárez, L. Monge, G. Hong, N. Fernández, D. Jaque, Adv. Funct. Mater. 2018, 28, 88.
- 9I. Villa, A. Vedda, I. X. Cantarelli, M. Pedroni, F. Piccinelli, M. Bettinelli, A. Speghini, M. Quintanilla, F. Vetrone, U. Rocha, C. Jacinto, E. Carrasco, F. S. Rodríguez, Á. Juarranz, B. del Rosal, D. H. Ortgies, P. H. Gonzalez, J. G. Solé, D. J. García, Nano Res. 2015, 8, 649.
- 10B. del Rosal, I. Villa, D. Jaque, F. Sanz-Rodríguez, J. Biophotonics 2016, 9, 1059.
- 11B. del Rosal, A. Benayas, Small Methods 2018, 2, 75.
- 12B. del Rosal, D. H. Ortgies, N. Fernández, F. Sanz-Rodríguez, D. Jaque, E. M. Rodríguez, Adv. Mater. 2016, 28, 88.
- 13X. Zheng, X. Zhu, Y. Lu, J. Zhao, W. Feng, G. Jia, F. Wang, F. Li, D. Jin, Anal. Chem. 2016, 88, 3449.
- 14M. Tan, B. del Rosal, Y. Zhang, E. Martín Rodríguez, J. Hu, Z. Zhou, R. Fan, D. H. Ortgies, N. Fernández, I. Chaves-Coira, Á. Núñez, D. Jaque, G. Chen, Nanoscale 2018, 10, 771.
- 15C. Bouzigues, T. Gacoin, A. Alexandrou, ACS Nano 2011, 5, 8488.
- 16Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnology annual review 2005, 11, 227.
- 17N. M. Htun, Y. C. Chen, B. Lim, T. Schiller, G. J. Maghzal, A. L. Huang, K. D. Elgass, J. Rivera, H. G. Schneider, B. R. Wood, R. Stocker, K. Peter, Nat. Commun. 2017, 8, 75.
- 18G. Gopalakrishnan, A. Awasthi, W. Belkaid, O. De Faria Jr, D. Liazoghli, D. R. Colman, A. S. Dhaunchak, J. Neurosci. Res. 2013, 91, 321.
- 19A. Ishii, R. Dutta, G. M. Wark, S.-I. Hwang, D. K. Han, B. D. Trapp, S. E. Pfeiffer, R. Bansal, Proc. Natl. Acad. Sci. 2009, 106, 14605.
- 20K. Bozek, Y. Wei, Z. Yan, X. Liu, J. Xiong, M. Sugimoto, M. Tomita, S. Pääbo, C. C. Sherwood, P. R. Hof, J. J. Ely, Y. Li, D. Steinhauser, L. Willmitzer, P. Giavalisco, P. Khaitovich, Neuron 2015, 85, 695.
- 21S. S. Seehafer, D. A. Pearce, Neurobiol. Aging 2006, 27, 576.
- 22H. Barden, S. Levine, Brain Res. Bull. 1983, 10, 847.
- 23S. A. H. Gudjohnsen, D. A. M. Atacho, F. Gesbert, G. Raposo, I. Hurbain, L. Larue, E. Steingrimsson, P. H. Petersen, Front Neuroanat. 2015, 9, 149.
- 24S. S. Joshi, B. Tandukar, L. Pan, J. M. Huang, F. Livak, B. J. Smith, T. Hodges, A. A. Mahurkar, T. J. Hornyak, PLoS Genet. 2019, 15, e1008034.
- 25L. Zecca, P. Costi, C. Mecacci, S. Ito, M. Terreni, S. Sonnino, J. Neurochem. 2000, 74, 1758.
- 26L. Zecca, D. Tampellini, M. Gerlach, P. Riederer, R. G. Fariello, D. Sulzer, Mol. Pathol. 2001, 54, 414.
- 27R. L. Haining, C. Achat-Mendes, Neural Regen. Res. 2017, 12, 372.
- 28D. Sulzer, C. Cassidy, G. Horga, U. J. Kang, S. Fahn, L. Casella, G. Pezzoli, J. Langley, X. P. Hu, F. A. Zucca, I. U. Isaias, L. Zecca, NPJ Parkinsons Dis. 2018, 4, 11.
- 29E. Vosoughi, J. M. Lee, J. R. Miller, M. Nosrati, D. R. Minor, R. Abendroth, J. W. Lee, B. T. Andrews, L. Z. Leng, M. Wu, S. P. Leong, M. Kashani-Sabet, K. B. Kim, BMC Cancer 2018, 18, 490.
- 30A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng, H. Dai, Nat. Mater. 2016, 15, 235.
- 31G. Hong, S. Diao, J. Chang, A. L. Antaris, C. Chen, B. Zhang, S. Zhao, D. N. Atochin, P. L. Huang, K. I. Andreasson, C. J. Kuo, H. Dai, Nat. Photonics. 2014, 8, 723.
- 32Y. Zhong, Z. Ma, S. Zhu, J. Yue, M. Zhang, A. L. Antaris, J. Yuan, R. Cui, H. Wan, Y. Zhou, W. Wang, N. F. Huang, J. Luo, Z. Hu, H. Dai Nat, Commun. Theory 2017, 8, 737.




