Magnetic resonance contrast agents in optical clearing: Prospects for multimodal tissue imaging
Daria K. Tuchina
Saratov State University, Saratov, Russia
Tomsk State University, Tomsk, Russia
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Search for more papers by this authorCorresponding Author
Irina G. Meerovich
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Correspondence
Irina G. Meerovich, А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, 33 (build. 2) Leninsky prospect, Moscow 119071, Russia.
Email: [email protected]
Search for more papers by this authorVictoria V. Zherdeva
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Search for more papers by this authorAlexander P. Savitsky
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Search for more papers by this authorAlexei A. Bogdanov Jr
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
University of Massachusetts Medical School, Worcester, Massachusetts, USA
Department of Bioengineering and Bioinformatics, Moscow State University, Moscow, Russia
Search for more papers by this authorValery V. Tuchin
Saratov State University, Saratov, Russia
Tomsk State University, Tomsk, Russia
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Institute of Precision Mechanics and Control of the Russian Academy of Sciences, Saratov, Russia
Search for more papers by this authorDaria K. Tuchina
Saratov State University, Saratov, Russia
Tomsk State University, Tomsk, Russia
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Search for more papers by this authorCorresponding Author
Irina G. Meerovich
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Correspondence
Irina G. Meerovich, А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, 33 (build. 2) Leninsky prospect, Moscow 119071, Russia.
Email: [email protected]
Search for more papers by this authorVictoria V. Zherdeva
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Search for more papers by this authorAlexander P. Savitsky
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Search for more papers by this authorAlexei A. Bogdanov Jr
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
University of Massachusetts Medical School, Worcester, Massachusetts, USA
Department of Bioengineering and Bioinformatics, Moscow State University, Moscow, Russia
Search for more papers by this authorValery V. Tuchin
Saratov State University, Saratov, Russia
Tomsk State University, Tomsk, Russia
А.N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow, Russia
Institute of Precision Mechanics and Control of the Russian Academy of Sciences, Saratov, Russia
Search for more papers by this authorFunding information: Government of the Russian Federation, Grant/Award Number: 14.W03.31.0023
Abstract
Skin optical clearing effect ex vivo and in vivo was achieved by topical application of low molecular weight paramagnetic magnetic resonance contrast agents. This novel feature has not been explored before. By using collimated transmittance the diffusion coefficients of three clinically used magnetic resonance contrast agents, that is Gadovist, Magnevist and Dotarem as well as X-ray contrast agent Visipaque in mouse skin were determined ex vivo as (4.29 ± 0.39) × 10−7 cm2/s, (5.00 ± 0.72) × 10−7 cm2/s, (3.72 ± 0.67) × 10−7 cm2/s and (1.64 ± 0.18) × 10−7 cm2/s, respectively. The application of gadobutrol (Gadovist) resulted in efficient optical clearing that in general, was superior to other contrast agents tested and allowed to achieve: (a) more than 12-fold increase of transmittance over 10 minutes after application ex vivo; (b) markedly improved images of skin architecture obtained with optical coherence tomography; (c) an increase of the fluorescence intensity/background ratio in TagRFP-red fluorescent marker protein expressing tumor by five times after 15 minutes application into the skin in vivo. The obtained results have immediate implications for multimodality imaging because many contrast agents are capable of simultaneously enhancing the contrast of multiple imaging modalities.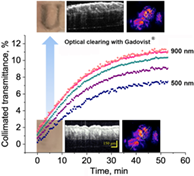
CONFLICT OF INTEREST
The authors declare no competing financial interest.
REFERENCES
- 1A. Akselrod-Ballin, H. Dafni, Y. Addadi, I. Biton, R. Avni, Y. Brenner, M. Neeman, Sci. Rep. 2016, 6, 27940.
- 2R. A. Leitgeb, B. Baumann, Front. Phys. 2018, 6, 114.
- 3V. V. Tuchin, Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnostics, Society of Photo-Optical Instrumentation Engineers (SPIE), Bellingham 2015, p. 988.
- 4 V. V. Tuchin Ed., Handbook of Optical Biomedical Diagnostics. Light-Tissue Interaction. Methods, Society of Photo-Optical Instrumentation Engineers (SPIE), Bellingham 2016, p. 1552.
- 5D. Calle, P. Ballesteros, S. Cerdán, Advanced Contrast Agents for Multimodal Biomedical Imaging Based on Nanotechnology. in Preclinical MRI. Methods in Molecular Biology (Eds: M. L. García-Martín, P. López-Larrubia), Humana Press, New York, NY 2018, p. 441.
10.1007/978-1-4939-7531-0_26 Google Scholar
- 6V. V. Tuchin, I. L. Maksimova, D. A. Zimnyakov, I. L. Kon, A. H. Mavlutov, A. A. Mishin, J. Biomed. Opt. 1997, 2, 304.
- 7M. H. Khan, B. Choi, S. Chess, K. M. Kelly, J. McCullough, J. S. Nelson, Lasers Surg. Med. 2004, 34, 83.
- 8V. V. Tuchin, Optical Clearing of Tissues and Blood, Society of Photo-Optical Instrumentation Engineers (SPIE), Bellingham 2005, p. 256.
- 9V. V. Tuchin, IEEE J. Select. Tops Quant. Electr. 2007, 13, 1621.
- 10E. A. Genina, A. N. Bashkatov, V. V. Tuchin, Expert Rev. Med. Devices 2010, 7, 825.
- 11H. Hama, H. Kurokawa, H. Kawano, R. Ando, T. Shimogori, H. Noda, K. Fukami, A. Sakaue-Sawano, A. Miyawaki, Nat. Neurosci. 2011, 14, 1481.
- 12A. Erturk, C. P. Mauch, F. Hellal, F. Forstner, T. Keck, K. Becker, N. Jahrling, H. Steffens, M. Richter, M. Hubener, E. Kramer, F. Kirchhoff, H. U. Dodt, F. Bradke, Nat. Med. 2012, 18, 166.
- 13A. Erturk, K. Becker, N. Jahrling, C. P. Mauch, C. D. Hojer, J. G. Egen, F. Hellal, F. Bradke, M. Sheng, H. U. Dodt, Nat. Prot. 2012, 7, 1983.
- 14D. Zhu, K. V. Larin, Q. Luo, V. V. Tuchin, Laser Photon. Rev. 2013, 7, 732.
- 15E. A. Susaki, K. Tainaka, D. Perrin, F. Kishino, T. Tawara, T. M. Watanabe, C. Yokoyama, H. Onoe, M. Eguchi, S. Yamaguchi, T. Abe, H. Kiyonari, Y. Shimizu, A. Miyawaki, H. Yokota, H. R. Ueda, Cell 2014, 157, 726.
- 16D. S. Richardson, J. W. Lichtman, Cell 2015, 162, 246.
- 17E. A. Genina, A. N. Bashkatov, Y. P. Sinichkin, I. Y. Yanina, V. V. Tuchin, J. Biomed. Photon. Eng. 2015, 1, 22.
10.18287/JBPE-2015-1-1-22 Google Scholar
- 18C. Pan, R. Cai, F. P. Quacquarelli, A. Ghasemigharagoz, A. Lourbopoulos, P. Matryba, N. Plesnila, M. Dichgans, F. Hellal, A. Erturk, Nat. Methods 2016, 13, 859.
- 19Q. Zhao, C. Dai, S. Fan, J. Lv, L. Nie, Sci. Rep. 2016, 6, 34954.
- 20M.-T. Ke, Y. Nakai, S. Fujimoto, R. Takayama, S. Yoshida, T. S. Kitajima, M. Sato, T. Imai, Cell Rep. 2016, 14, 2718.
- 21F. Perbellini, A. K. L. Liu, S. A. Watson, I. Bardi, S. M. Rothery, C. M. Terracciano, Sci. Rep. 2017, 7, 5188.
- 22Y. Qi, T. Yu, J. Xu, P. Wan, Y. Ma, J. Zhu, Y. Li, H. Gong, Q. Luo, D. Zhu, Sci. Adv. 2019, 5, eaau8355.
- 23A. N. Bashkatov, K. V. Berezin, K. N. Dvoretskiy, M. L. Chernavina, E. A. Genina, V. D. Genin, V. I. Kochubey, E. N. Lazareva, A. B. Pravdin, M. E. Shvachkina, P. A. Timoshina, D. K. Tuchina, D. D. Yakovlev, D. A. Yakovlev, I. Y. Yanina, O. S. Zhernovaya, V. V. Tuchin, J. Biomed. Opt. 2018, 23, 091416.
- 24J. H. Kim, M. J. Jang, J. Choi, E. Lee, K.-D. Song, J. Cho, K.-T. Kim, H.-J. Cha, W. Sun, Sci. Rep. 2018, 8, 12815.
- 25L. F. Ochoa, A. Kholodnykh, P. Villarreal, B. Tian, R. Pal, A. N. Freiberg, A. R. Brasier, M. Motamedi, G. Vargas, Sci. Rep. 2018, 8, 13348.
- 26P. Matryba, L. Kaczmarek, J. Gołąb, Laser Photon. Rev. 2019, 13, 1800292.
- 27L. M. Oliveira, V. V. Tuchin, The Optical Clearing Method – A New Tool for Clinical Practice and Biomedical Engineering, Springer Nature, Switzerland AG, Basel 2019, p. 177.
- 28P. Wan, J. Zhu, J. Xu, Y. Li, T. Yu, D. Zhu, Neurophoton 2018, 5, 035007.
- 29I. Costantini, R. Cicchi, L. Silvestri, F. Vanzi, F. S. Pavone, Biomed. Opt. Express 2019, 10, 5251.
- 30M. Inyushin, D. Meshalkina, L. Zueva, A. Zayas-Santiago, Molecules 2019, 24, E2388.
- 31K. Tainaka, T. C. Murakami, E. A. Susaki, C. Shimizu, R. Saito, K. Takahashi, A. Hayashi-Takagi, H. Sekiya, Y. Arima, S. Nojima, M. Ikemura, T. Ushiku, Y. Shimizu, M. Murakami, K. F. Tanaka, M. Iino, H. Kasai, T. Sasaoka, K. Kobayashi, K. Miyazono, E. Morii, T. Isa, M. Fukayama, A. Kakita, H. R. Ueda, Cell Rep. 2018, 24, 2196.
- 32K. Matsumoto, T. T. Mitani, S. A. Horiguchi, J. Kaneshiro, T. C. Murakami, T. Mano, H. Fujishima, A. Konno, T. M. Watanabe, H. Hirai, H. R. Ueda, Nat. Prot. 2019, 14, 3506.
- 33A. Bykov, T. Hautala, M. Kinnunen, A. Popov, S. Karhula, S. Saarakkala, M. T. Nieminen, V. Tuchin, I. Meglinski, J. Biophotonics 2016, 9, 270.
- 34A. Y. Sdobnov, M. E. Darvin, J. Lademann, V. V. Tuchin, J. Biophotonics 2017, 10, 1115.
- 35A. Y. Sdobnov, V. V. Tuchin, J. Lademann, M. E. Darvin, J. Phys. D Appl. Phys. 2017, 50, 285401.
- 36Y. M. Alexandrovskaya, E. G. Evtushenko, M. M. Obrezkova, V. V. Tuchin, E. N. Sobol, J. Biophotonics 2018, 11, e201800195.
- 37A. Y. Sdobnov, M. E. Darvin, J. Schleusener, J. Lademann, V. V. Tuchin, J. Biophotonics 2019, 12, e201800283.
- 38P. Caravan, J. Ellison, T. McMurry, R. Lauffer, Chem. Rev. 1999, 99, 2293.
- 39S. P. Lin, J. J. Brown, J. Magn. Reson. Imaging 2007, 25, 884.
- 40S. S. Alshowiman, A. K. Alswailem, O. A. Almohizy, A. A. Alfawaz, A. A. Ibn Alshaikh, Int. J. Pharmac. Sci. Inv. 2018, 7, 11.
- 41V. C. Pierre, M. J. Allen, P. Caravan, J. Biol. Inorg. Chem. 2014, 19, 127.
- 42Z. Chen, Y. Li, R. Airan, Z. Han, J. Xu, K. W. Y. Chan, Y. Xu, J. W. M. Bulte, P. C. M. van Zijl, M. T. McMahon, S. Zhou, G. Liu, Quant. Imaging Med. Surg. 2019, 9, 1579.
- 43P. Pandit, S. M. Johnston, Y. Qi, J. Story, R. Nelson, G. A. Johnson, Acad. Radiol. 2013, 20, 430.
- 44Thorlabs catalog "Microscopy and Laser Imaging: Spectral Radar OCT Systems," https://www.thorlabs.com/catalogpages/595.pdf
- 45 “Spectrometer Operating Software Installation and Operation Manual,” SpectraSuite®, Document Number 000-20000-300-02-201205, 2009.
- 46A. N. Bashkatov, E. A. Genina, V. V. Tuchin, Measurement of Glucose Diffusion Coefficients in Human Tissues. in Handbook of Optical Sensing of Glucose in Biological Fluids and Tissues (Ed: V. V. Tuchin), CRC Press, Boca Raton 2009, p. 587.
- 47D. K. Tuchina, R. Shi, A. N. Bashkatov, E. A. Genina, D. Zhu, Q. Luo, V. V. Tuchin, J. Biophotonics 2015, 8, 332.
- 48S. Mériaux, A. Conti, B. Larrat, Front. Phys. 2018, 6, 38.
10.3389/fphy.2018.00038 Google Scholar
- 49G. E. Hagberg, I. Mamedov, A. Power, M. Beyerlein, H. Merkle, V. G. Kiselev, K. Dhingra, V. Kubìček, G. Angelovski, N. K. Logothetis, Contrast Media Mol. Imaging 2014, 9, 71.
- 50A. Conti, R. Magnin, M. Gerstenmayer, N. Tsapis, E. Dumont, O. Tillement, F. Lux, D. Le Bihan, S. Mériaux, S. D. Penna, B. Larrat, Contrast Media Mol. Imaging 2019, 2019, 6341545.
- 51https://www.glossary.oilfield.slb.com/en/Terms/t/tortuosity.aspx (viewed on December 23, 2019).
- 52I. Carneiro, S. Carvalho, V. Silva, R. Henrique, L. Oliveira, V. V. Tuchin, J. Biomed. Opt. 2018, 23, 121620.
- 53R. Graaff, J. G. Aarnoudse, J. R. Zijp, P. M. A. Sloot, F. F. M. de Mul, J. Greve, M. H. Koelink, Appl. Optics 1992, 31, 1370.
- 54V. Zherdeva, N. I. Kazachkina, V. Shcheslavskiy, A. P. Savitsky, J. Biomed. Opt. 2018, 23, 035002.
- 55K. S. Sarkisyan, A. S. Goryashchenko, P. V. Lidsky, D. A. Gorbachev, N. G. Bozhanova, A. Y. Gorokhovatsky, A. R. Pereverzeva, A. P. Ryumina, V. V. Zherdeva, A. P. Savitsky, K. M. Solntsev, A. S. Bommarius, G. V. Sharonov, J. R. Lindquist, M. Drobizhev, T. E. Hughes, A. Rebane, K. A. Lukyanov, A. S. Mishin, Biophys. J. 2015, 109, 380.
- 56https://www.becker-hickl.com/wp-content/uploads/2019/06/dcs-hb-8ed-v04f.pdf
- 57L. M. Oliveira, M. I. Carvalho, E. M. Nogueira, V. V. Tuchin, J. Biophotonics 2018, 11, e201700094.
- 58https://refractiveindex.info/?shelf=3d&book=liquids&page=water
- 59I. O. Jelescu, M. D. Budde, Front. Phys. 2017, 5, 61.
- 60H. Singh, K. A. Bishen, D. Garg, H. Sukhija, D. Sharma, U. Tomar, Dent. J. Adv. Stud. 2019, 7, 51.
10.1055/s-0039-1693098 Google Scholar
- 61B. W. Pogue, J. Feng, E. P. LaRochelle, P. Bruža, H. Lin, R. Zhang, J. R. Shell, H. Dehghani, S. C. Davis, S. A. Vinogradov, D. J. Gladstone, L. A. Jarvis, Nat. Biomed. Eng. 2018, 2, 254.
- 62K. Calabro, A. Curtis, J.-R. Galarneau, T. Krucker, I. J. Bigio, J. Biomed. Opt. 2011, 16, 011008.
- 63R.-Z. Lin, Y.-C. Chen, R. Moreno-Luna, A. Khademhosseini, J. M. Melero-Martin, Biomaterials 2013, 34, 6785.
- 64D. L. Longo, F. Michelotti, L. Consolino, P. Bardini, G. Digilio, G. Xiao, P. Z. Sun, S. Aime, Invest. Radiol. 2016, 51, 155.
- 65S. Laurent, L. Vander Elst, R. N. Muller, Contrast Med. Mol. Imaging 2006, 1, 128.
- 66L. J. Scott, Clin. Drug Invest. 2018, 38, 773.
- 67https://pubchem.ncbi.nlm.nih.gov/compound/Gadovist (accessed: December 2019).
- 68https://pubchem.ncbi.nlm.nih.gov/compound/Magnevist (accessed: December 2019).
- 69https://pubchem.ncbi.nlm.nih.gov/compound/Dotarem (accessed: December 2019).
- 70https://www.bayer.ca/omr/online/magnevist-pm-en.pdf (accessed: December 2019).
- 71https://www.rxlist.com/dotarem-drug.htm (accessed: December 2019).
- 72https://www.umassmed.edu/globalassets/radiology/documents/dotarem-info-1.pdf (accessed: December 2019).
- 73https://pubchem.ncbi.nlm.nih.gov/compound/Iodixanol; https://www.rxlist.com/visipaque-drug.htm#clinpharm (accessed: April 2019).
- 74 C. Surber, C. Abels, H. Maibach Eds., pH of the Skin: Issues and Challenges, Karger, Basel, New York 2018.
10.1159/isbn.978-3-318-06385-1 Google Scholar
- 75S. Papadopoulos, K. D. Juergens, G. Gros, Biophys. J. 2000, 79, 2084.
- 76M. Meyer, Biomed. Eng. Online 2019, 18, 1 10.1186/s12938-019-0647-0.
- 77R. LaComb, O. Nadiarnykh, S. Carey, P. J. Campagnola, J. Biomed. Opt. 2008, 13, 021109.
- 78E. V. Migacheva, A. B. Pravdin, V. V. Tuchin, J. Innov. Opt. Health Sci. 2010, 3, 147.




