Impacts of livestock grazing on blue-eared pheasants (Crossoptilon auritum) survival in subalpine forests of Southwest China
散放家畜对中国西南山地亚高山森林雉类蓝马鸡的影响
Xing Chen and Xiao-Tong Shang contributed equally to this study and are co-first authors.
Editor-in-Chief & Handling Editor: Ahimsa Campos-Arceiz
Abstract
enThe degradation and fragmentation of natural habitats, driven largely by anthropogenic activities such as grazing, represent growing concerns in environmental conservation. We examined the impact of grazing activities on the survival of the blue-eared pheasant Crossoptilon auritum, a ground-nesting bird endemic to subalpine forests. Using camera-trapping and artificial nest experiments, we compared two sites in Sichuan, China: Wanglang (high grazing intensity) and Jiuzhaigou (no grazing) national nature reserves. The study, conducted from 2017 to 2021, evaluated habitat suitability changes for these pheasants and examined the impact of grazing on nesting success by conducting a nest predation experiment. The results of our study showed that (1) since the significant increase of free-ranging livestock post-2014, the area of most suitable and moderately suitable habitats for blue-eared pheasants in Wanglang decreased by 14.28% (net loss 15.12 km2); (2) predominant natural predators of the pheasant, such as yellow-throated martens (Martes flavigula) and leopard cats (Prionailurus bengalensis), were mostly observed to be spatially distant from livestock; (3) the pheasant's nesting failure rate was 2.18 times higher in Wanglang than in Jiuzhaigou; (4) high-intensity livestock disturbance correlated with decreased food resources for pheasants, yet resulted in an increased abundance of Coleopteran insects. These results illustrate a complex dynamic: Although forest pheasants, such as the blue-eared pheasant, may initially benefit from the presence of livestock through increased predator refuge and access to specific food resources, they ultimately face greater risks. These include a significant increase in nest failure rates and remarkable habitat loss and degradation. In light of these results, we advocate for strict control and management of grazing activities inside reserves. Additionally, we recommend the implementation of a systematic monitoring program that focuses on the population dynamics and habitat use of endangered pheasants in Southwest China.
Abstract
zh栖息地是野生动物赖以生存的活动场所,但由于放牧等人为活动,野生动物栖息地的退化和破碎化日益严重。为了解放牧活动对中国特有雉类蓝马鸡(Crossoptilon auritum)生存状况的影响,我们以四川王朗国家级自然保护区为研究区域,于2017-2021年,使用红外相机及样线调查等方法,结合保护区2002-2019年野外巡护监测数据,通过MaxEnt模型和双物种占域模型,分别分析了近20年来保护区内蓝马鸡栖息地适宜度变化和放牧活动对蓝马鸡天敌 (黄喉貂 (Martes flavigula)、豹猫 (Prionailurus bengalensis)) 的空间活动影响。同时,通过模拟实验和样方调查在王朗和九寨沟保护区(无放牧干扰)开展对比研究,,评估不同放牧强度下蓝马鸡营巢及动物性食物资源的差异。结果显示:(1)自2014年以来,王朗保护区散养家畜的活动导致蓝马鸡的潜在适宜栖息地减少了14.28%(15.12 km2);(2)黄喉貂和豹猫在空间上多倾向于远离基础;(3)模拟巢实验表明王朗巢被破坏风险是九寨沟的2.18倍;(4)高强度的放牧活动,降低了雉类的食物资源,但提供了丰度更高的鞘翅目食物。蓝马鸡可能会被家畜活动带来的天敌避难所效应和更丰富的鞘翅目食物资源所吸引,但同时也会面临更大的营巢失败风险以及潜在栖息地的退化;同时,家畜活动给其他哺乳动物带来的负面影响也是不可低估的。基于上述结果,我们建议保护区严格控制区内放牧活动,并持续关注放牧系统的变化对蓝马鸡等濒危雉类种群动态及其天敌的活动情况的影响。
Plain language summary
enIn the northern part of the Minshan Mountains, China, our investigation delved into the effects of human activities, specifically grazing, on the blue-eared pheasant—a ground-nesting bird residing in subalpine forests. Employing camera traps and artificial nests in Wanglang National Nature Reserve (with high grazing) and Jiuzhaigou National Nature Reserve (grazing-free) from 2017 to 2021, our findings unveiled a concerning 14.28% reduction in suitable pheasant habitats in Wanglang since 2014 due to the surge in free-ranging livestock. Intriguingly, natural predators like yellow-throated martens and leopard cats kept their distance from livestock, yet in Wanglang, pheasants experienced a 2.18 times higher nesting failure rate than their counterparts in Jiuzhaigou. Livestock disturbances impacted the pheasants' food resources but led to an increase in Coleopteran insects. Despite potential benefits, such as predator refuge and specific food resources, the study emphasizes the trade-off, revealing heightened risks of nest failure and substantial habitat loss for these forest pheasants. To safeguard these endangered species, we advocate for stringent control and management of grazing activities within reserves, coupled with systematic monitoring of population dynamics and habitat utilization in Southwest China.
Plain Language Summary
zh在中国岷山北部,团队利用红外相机和人工巢实验,深入调查了放牧活动对生存在亚高山森林的地栖雉类蓝马鸡的影响。我们发现,自2014年以来,由于放牧家畜数量的激增,王朗国家级自然保护区蓝马鸡的潜在适宜栖息地减少了14.28%。人工巢实验结果发现,王朗的营巢失败率比九寨沟高出2.18倍。不过,我们也发现,黄喉貂和豹猫等雉类天敌的活动与家畜在空间上保持独立出现。另外,家畜的干扰对蓝马鸡的潜在食物资源产生了消极影响,但也带来了丰度更高的鞘翅目昆虫。尽管家畜的活动可能带来天敌避难所和特点的食物资源等潜在好处,但我们研究同样也揭示了筑巢失败和栖息地丧失等风险的增加。为了更好的保护雉类,我们建议在保护区内对放牧活动进行严格的控制和管理,同时持续关注放牧系统的变化对蓝马鸡等濒危雉类的种群动态的影响。
Practitioner points
en
-
Over a span of 7 years, suitable habitats for forest pheasants decreased by 14.28%, primarily due to livestock disturbances.
-
Activities associated with livestock led to a significant increase in nest failure rates for forest pheasants, with a 2.18-fold rise.
Practitioner Points
zh
-
在过去近10年的时间里,王朗保护区的蓝马鸡潜在适宜栖息地减少了14.28%,这是放牧活动导致的。
-
放牧活动导致王朗的巢被破坏风险是九寨沟保护区的2.18倍。
1 INTRODUCTION
Suitable habitats are places that provide all the environmental conditions that wild animals depend on for survival, food supply, and refuge from predators. Owing to economic and social development, the intensity of anthropogenic activities has been rapidly increasing, putting serious pressure on wildlife habitats by accelerating habitat loss and fragmentation (Loveridge et al., 2020). Among various anthropogenic disturbances, grazing is considered one of the most impactful (Maxwell et al., 2016). For example, intensive grazing activities have led to dramatic changes in the habitats of giant pandas (Ailuropoda melanoleuca) within a short period, rapidly degrading habitats that were once conducive to their survival (Li et al., 2017). In addition, such disturbances can force wildlife to abandon certain parts of their habitats, seeking to avoid interactions with domestic animals (Li et al., 2017). In Baluran National Park, Indonesia, grazing activities reduced the spatial occupancy of both carnivores, such as dholes (Cuon alpinus) and leopard cats (Prionailurus bengalensis), and herbivores, such as the Javan deer (Rusa timorensis) and red muntjac (Muntiacus muntjac) (Pudyatmoko, 2017).
Wild pheasants (Phansianidae), as large ground-dwelling birds inhabiting various environments, often face a trade-off in habitat use due to multiple pressures such as predation risk (Xiong et al., 2017; Zhou et al., 2011), food availability (Jie et al., 2010; Traba et al., 2008), and human disturbance (Winder et al., 2018). Historically, the primary threats to forest pheasants were illegal harvest and habitat loss owing to timber logging (Storch, 2013). However, in recent decades, livestock grazing in forest understories has emerged as a major anthropogenic threat, not only to pheasants but also to other sympatric wildlife (Filazzola et al., 2020; Li et al., 2017; Morand, 2020). Despite its significance, only a few studies have specifically examined the effects of overgrazing on wild pheasant populations (Fan et al., 2020; Winder et al., 2018). High-intensity grazing has been linked to reduced nesting success and higher predation risk, mainly due to reduced vegetation height from livestock foraging (Johnson et al., 2012). For example, the prairie chicken (Tympanuchus cupido) has been observed to adjust its activities based on the intensity of grazing in its habitat (Winder et al., 2018). Contrarily, a recent study found a high degree of overlap in activity rhythms and spatial distribution between blood pheasants (Ithaginis cruentus) and livestock in heavily grazed areas (Fan et al., 2020). This finding could be attributed to the attraction of insects, especially Coleopterans, to livestock droppings, which then become a specific food resource for the birds (Liu et al., 2019). Therefore, the impacts of grazing activities on pheasants are complex, necessitating further research on this relationship and its underlying mechanisms.
The Mountains of Southwest China, recognized as a globally significant biodiversity hotspot (Brooks et al., 2006), have the highest richness and endemism of pheasant species in the country (Zheng, 2015). Among these is the blue-eared pheasant (Crossoptilon auritum), a large and threatened pheasant species mainly found in mixed coniferous forests at 2600 ~ 3600 m and occasionally in subalpine scrub meadows above 3800 m (Lu, 1991). This species, with a highly restricted distribution in the Sichuan, Gansu, Qinghai, and Ningxia provinces, is endemic to China and listed as a Class II Key Protected Species by the state (Zheng, 2017). Their ground nesting behavior (Lu, 1991) and long incubation period (approximately 1 month) (Zheng & Liao, 1983) render them particularly vulnerable to disturbance by livestock. In addition, grazing activities can alter the spatial distribution of the pheasant's natural predators, such as leopard cats (Pudyatmoko, 2017), and affect the habitat use and selection strategies of these pheasants.
In this study, we focused on blue-eared pheasants, a representative of large, threatened pheasant species inhabiting subalpine forests, to examine the impacts of grazing activities. The key research aims were to determine (1) the changes in the habitat suitability of the blue-eared pheasant over the past 20 years, (2) the impact of grazing activities on the species' natural predators, (3) the impact of grazing on nesting success, and (4) the effect of livestock activities on the pheasant's food resources. We hypothesize that livestock activities lead to a reduction in habitat and nesting success for the blue-eared pheasant, while potentially increasing food resources. Livestock presence is also expected to negatively affect the pheasant's natural predators.
2 MATERIALS AND METHODS
2.1 Study area
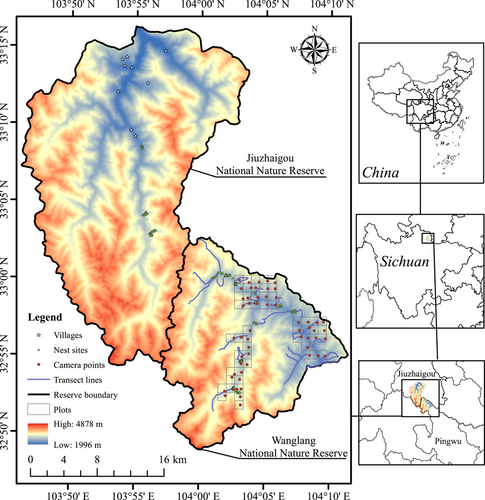
This study was conducted in two adjacent national nature reserves, Wanglang (323 km2, 103°55′–104°10′ E, 32°49′–33°02′ N) and Jiuzhaigou (651 km2, 103°46′–104°03′ E, 32°54′–33°16′ N), located in the northern part of the Minshan Mountains, Sichuan Province, China (Figure 1). Both reserves are characterized by a broad elevation (ELE) range (2000 ~ 4980 m) and share a similar subalpine climate. The predominant vegetation types (VEG) include mixed broadleaf-conifer forests, subalpine coniferous forests, alpine scrublands, alpine meadows, and alpine screes. These areas are home to a diverse community of pheasants, with 11 species recorded in Wanglang (Shang et al., 2020) and 10 in Jiuzhaigou (Li et al., 2020). There are no permanent residents in Wanglang, but there are a few villages in Jiuzhaigou (Figure 1). During the past two decades, Wanglang has experienced a dramatic increase in free-ranging livestock, leading to considerable forest damage and significantly affecting the distribution and habitat use of multiple wildlife species, including the giant panda, Chinese takin (Budorcas tibetana), and tufted deer (Elaphodus cephalophus) (Chen et al., 2019; Li et al., 2017). In contrast to Wanglang, stringent measures were implemented to regulate grazing activities in Jiuzhaigou. Furthermore, Jiuzhaigou has abstained from accommodating visitors since the earthquake in 2017, sustaining this closure until September 27, 2019 (ensuring minimal tourist impact during the experimental period). Consequently, Jiuzhaigou was used as the control area for nesting success experiments and food resource surveys in this study.
2.2 Habitat suitability
2.2.1 Species occurrence data
To assess the impact of livestock grazing on the blue-eared pheasant's habitat suitability in Wanglang, we collected occurrence records from multiple sources. These included sign transect monitoring data from 2002 to 2019, as provided by the reserve, camera-trapping survey data from 2015 to 2020 (Li et al., 2020; Shang et al., 2020), and field observation data collected by the authors from 2018 to 2021. In total, this comprehensive data set encompassed 215 distinct locations. In this context, transect lines denote predetermined monitoring lines within Wanglang, undergoing annual repetitive surveys, amounting to a total of 24 transect lines (Figure 1). The authors' inquiries, conducted from 2018 to 2021, were likewise grounded in examinations along these predefined monitoring transects within the protected area. For analytical purposes, these sites were divided into three groups based on the data collection period: 2002–2007, 2008–2013, and 2014–2021, which were recorded as periods 1–3, respectively, with 102, 52, and 61 sites in each period. For the spatially clumped sites with spacing <250 m apart, we randomly selected one and excluded the rest to avoid overfitting. The final number of sites used for subsequent analysis in the three periods was 63, 37, and 36.
2.2.2 Covariates for habitat modeling
To construct a habitat model for the blue-eared pheasant, we reviewed related studies (Wang et al., 2017; Yang et al., 2020) and identified a set of variables that may affect its habitat suitability. These included (1) climate variables, including 19 bioclimatic variables (WorldClim 2.0 database, 1970–2000, 1 km resolution) (http://www.worldclim.org); (2) topographic variables, including ELE, slope (SLP), terrain roughness index (TRI), and distance to water (DTW), all extracted from the digital ELE model of the reserve (https://www.gscloud.cn/, resolution 30 × 30 m) and calculated using ArcGIS 10.5 (https://support.esri.com/); (3) VEG, including broad-leaved forest, coniferous forest, scrub, meadow, planted forest, and impervious surface (human infrastructure); and (4) disturbance variables, including distance to roads (DTR), the presence probability of cattle (Pc), and the presence probability of horses (Ph). To obtain the Pc and Ph, we built a probability map for each species, respectively, with occurrence data collected in 2018 (cattle: n = 117, horses: n = 202) following the approaches proposed by Li et al. (2017) and Fan et al. (2020). In accordance with the MaxEnt model specifications, we standardized the projection coordinate system for all environmental variables to WGS_1984_UTM_Zone_48N and adjusted the spatial resolution to 30 × 30 m. Before model construction, we first examined the correlations between all variables using Spearman's correlation test and excluded one of them if they were correlated (|r| > 0.7). Ultimately, we got four climatic, three topographic, one vegetation, and three disturbance variables (Table 1).
| Variable | Code | Description | Variable types | Analysis |
|---|---|---|---|---|
| Vegetation factor | VEG | Vegetational form | Categorical | MaxEnt Occupancy |
| Topographic factor | ELE | Elevation | Continuous | MaxEnt Occupancy |
| SLP | Slope | Continuous | MaxEnt Occupancy |
|
| DTW | Distance to water | Continuous | MaxEnt Occupancy |
|
| TRI | Terrain roughness index | Continuous | Occupancy | |
| Disturbance factor | DTR | Distance to roads | Continuous | MaxEnt Occupancy |
| Pc | The presence probability of cattle | Continuous | MaxEnt | |
| Ph | The presence probability of horse | Continuous | MaxEnt | |
| Bioclimatic factor | Bio1 | Annual mean temperature | Continuous | MaxEnt |
| Bio4 | Temperature seasonality (standard deviation ×100) | Continuous | MaxEnt | |
| Bio13 | Precipitation of wettest month | Continuous | MaxEnt | |
| Bio15 | Precipitation seasonality (coefficient of variation) | Continuous | MaxEnt |
- Note: The vegetation layer was divided into six sublayers, namely, coniferous forest, broad-leaved forest, scrub, meadow, planted forest, and impervious surface, all of which are represented in the “0–1” format.
2.2.3 MaxEnt modeling
We used MaxEnt 3.4.1 (https://biodiversityinformatics.amnh.org/open_source/maxent/) to predict the habitat suitability of blue-eared pheasants in each study period. We used 20% of the blue-eared pheasants' presence records for model testing and 80% for training. We used 10 replicates for the cross-validation and used the average as the final result (Phillips et al., 2017). Other optimization parameters were selected by default. The area under the receiver operating characteristic curve (AUC) was used to measure the predictive performance of the model results. Based on the AUC value (0–1), the results were classified as follows: >0.6, poor; 0.7–0.8, fair; 0.8–0.9, good; and >0.9, excellent (Hu et al., 2015; Simpson & Prots, 2013).
Based on the results of the model, the maximum training sensitivity plus specificity threshold (MTSS) and balance training omission, predicted area, and threshold value (BTPT) were used as thresholds to reclassify the potential habitats of the blue-eared pheasant in Wanglang into three classes: most suitable, moderately suitable, and unsuitable (Phillips et al., 2006). According to the results, “permutation importance” was used to identify the most important environmental variables for blue-eared pheasant habitats. Changes in habitat suitability were calculated based on the distribution of habitat suitability (i.e., habitat suitability index distribution) predicted by the MaxEnt model for Periods 1 and 3. The changes were divided into three categories (increasing, stable, and decreasing suitability), and the specific threshold value was determined using the average of the MTSS values in the two periods. The Wilcoxon rank-sum test was used to analyze differences in selecting key variables at different periods. Before reassessing the habitat suitability, the environmental variable of grazing disturbance was added at Period 3 (recorded as Period 3+) to determine whether grazing behavior influenced the habitat suitability of blue-eared pheasants.
2.3 Effect of livestock on the pheasant's predators
2.3.1 Camera traps
To evaluate the effect of livestock on the spatial distribution of blue-eared pheasant's natural predators, we conducted a camera-trapping survey in Wanglang from May to July 2017. We established a sampling array containing 60 (1 × 1 km) grid cells (the data of 55 grid cells met the requirements for analysis) and set up a camera station in each cell. The placement of infrared cameras on a kilometer grid optimizes independent sampling, adhering to assumptions of pertinent models. The cameras were fixed on tree trunks 0.4–0.6 m above the ground and operated 24 h a day with medium sensitivity. Upon each trigger, the camera captured three pictures and one 12-s video, with no delay between consecutive triggers. The two most common meso-carnivore species, that is, yellow-throated martens and leopard cats (Li et al., 2020; Tian et al., 2018), were considered natural predators to blue-eared pheasants in the subsequent analysis.
2.3.2 Two-species occupancy modeling
A two-species occupancy model was used to analyze the effect of livestock on the natural predators of pheasants (yellow-throated martens and leopard cats) (Wang et al., 2015). The two-species occupancy model contained eight parameters (Richmond et al., 2010) (Supporting Information S1: Appendix S1). Based on the model assumptions and related studies (Pudyatmoko, 2017), cattle and horses were considered the dominant species (species A), and leopard cats and yellow-throated martens were subordinate species (species B). Detection histories were established for each species 25 times using 5-day segments for each camera deployment period (from May–July 2017).
Single-species occupancy models were first built separately for each species to simplify candidate models. The occupancy covariates from the optimal model were selected as the best covariates for each species and used to build the two-species occupancy models for each species (Richmond et al., 2010). In accordance with a previous study (Murphy et al., 2019), eight candidate models were constructed for each species. The model results were ranked using the Akaike information criterion (AIC). The model with ΔAIC ≤ 2 and the highest model weight was selected as the optimal model to extract the parameters and calculate the species interaction factor (SIF) estimates, as described by Richmond et al. (2010). When SIF = 1, the spatial distributions of the two species were independent; when SIF was <1, the spatial distributions tended to be distinct; and when SIF was >1, the spatial distributions tended to overlap (Richmond et al., 2010). For single-species occupancy modeling, six variables (ELE, SLP, TRI, DTR, DTW, and VEG) were used as covariates that affected the occupancy probabilities (Table 1), and no variables were assumed to have affected the detection of the camera. We examined the collinearity of variables and selected covariates with a correlation coefficient |r| was <0.7 for later analysis (Fan et al., 2020). The single-species occupancy model was constructed using R 4.0.2 (https://www.r-project.org/), and the two-species occupancy model was constructed using PRESENCE 2.13.11 (https://www.mbr-pwrc.usgs.gov/).
2.4 Effect of livestock on pheasant's nesting success
To determine the impact of free-ranging livestock on the nesting success of blue-eared pheasants, we conducted a nest predation experiment using artificial nests monitored with camera traps at Wanglang (with grazing) and Jiuzhaigou (no grazing) from May to October 2019. We randomly selected line transects (avoiding obvious animal trails) in the five habitat types and set up artificial nests (Wanglang: Open Land n = 11, Shrub n = 48, Broad-leaved Forest n = 26, Broadleaf-conifer Mixed Forest n = 53, and Conifer Forest n = 63; JiuZhaigou: Open Land n = 1, Shrub n = 20, Broad-leaved Forest n = 17, Broadleaf-conifer Mixed Forest n = 34, and Conifer Forest n = 33) at 100 m intervals along the transects. The artificial nests were created as shallow pits on the ground and lined with dry leaves, grass, or moss, in accordance with the real nests of wild pheasants. To simulate the two egg colors of wild pheasants, we placed two fresh chicken eggs (60–70 g) in each nest, which were either in their natural color or painted with reddish-brown spots using odorless acrylic (Supporting Information S2: Appendix S2). We set up a camera at each nest site to record the animals that visited the nest during a 30-day period, with the camera set to take a 30-s video upon each trigger. After each round (30 days) of experiments, we collected data from the camera and recorded the status of the eggs. Then, we set up new sites nearby (10 m) with more colored eggs for subsequent experiments. Three categories were used to describe the fate of each nest: (1) completely destroyed (CD, both eggs lost), (2) partially destroyed (PD, one egg lost or intact (IN) but moved, as well as both eggs IN but both moved), and (3) IN (both eggs intact and not moved). We identified all the nest-visiting events captured by the camera and classified them into three categories based on the animal's behavior in relation to the eggs: predation (ate, moved, or took away the egg), trampling (stepped on the egg), and nest visiting (visited the nest but without physical contact with the egg).
The chi-square test was used to compare the differences in nesting results for different areas and egg colors. Cox's proportional hazards regression model was used to analyze the difference in nesting risk between Wanglang and Jiuzhaigou based on the destruction time of artificial nests recorded by infrared cameras. We obtained statistics on the causes of artificial nest PD and CD events but only for events with clear factors recorded by infrared cameras.
2.5 Effect of livestock on the near-surface soil animals
Previous studies on the diet composition of blue-eared pheasants revealed that they mainly feed on plants (seeds, leaves, and flowers), mosses, and coleopteran insects (especially during the breeding season) (Li & Li, 1981; Zheng & Liao, 1983). Therefore, we conducted a survey of near-ground invertebrates, an important food resource for blue-eared pheasants in the breeding season, concurrently with the nest predation experiment. At each artificial nest site, we set up one 1 × 1 m fixed sampling square approximately 10 m from the nest. We buried four cylindrical plastic jars (6.5 cm diameter, 10 cm height, filled with 75% alcohol at 1/3 depth) as invertebrate traps at the corners of the square. At the end of each 30-day experiment, we collected all the invertebrates from the four traps from each sampling square and combined them to make one site sample. The invertebrates in each sample were counted and identified. We set up 136 sampling squares in Wanglang (11,243 individuals collected) and 101 squares in Jiuzhaigou (16,086 individuals collected).
3 RESULTS
3.1 Habitat suitability
The potential habitat distribution of blue-eared pheasants was obtained using 10 replications for each period. The AUC values for periods 1 (2002–2007), 2 (2008–2013), 3 (2014–2021), and 3+ were 0.911, 0.912, 0.921, and 0.915, respectively, indicating that the predicted results for each period were excellent and could reflect the changes in the habitat distribution of the blue-eared pheasants (Supporting Information S2: Appendix S2).
3.1.1 Factors affecting habitat suitability
The degree of importance of the variables (Table 2) showed that, in Period 1, the variables that explained more than 5% were ELE (78.0%), DTR (9.5%), and SLP (5.9%); in Period 2, the variables that explained more than 5% were ELE (71.1%), SLP (11.1%), and DTW (9.4%); and in Period 3, six variables explained more than 5% of the model (ELE: 31.0%; DTR: 26.4%; Bio13, 16.6%; DTW, 6.9%; Bio4, 6.7%; SLP: 6.5%), with ELE being the highest contributor. The importance of ELE continually decreased across the periods, whereas the importance of VEG gradually increased. Upon the addition of grazing disturbance variables, the importance of key environmental factors, such as ELE, SLP, and DTR, decreased. Pc contributed 28.9% to the predicted results.
| Variables | Period 1 (%) | Period 2 (%) | Period 3 (%) | Period 3+ (%) |
|---|---|---|---|---|
| ELE | 78.0 | 71.1 | 31 | 21.4 |
| DTR | 9.5 | 4.8 | 26.4 | 9.1 |
| SLP | 5.9 | 11.1 | 6.2 | 2.3 |
| DTW | 3.7 | 9.4 | 6.9 | 3.5 |
| Bio13 | 1.0 | 1.8 | 16.6 | 15.7 |
| Bio4 | 0.7 | 0.2 | 6.7 | 5.0 |
| Bio1 | 0.3 | 0.1 | 0.9 | 2.1 |
| Brush | 0.4 | 0.2 | 0 | 0.8 |
| Meadows | 0.3 | 0.3 | 4.7 | 8.9 |
| Bio15 | 0.2 | 0.3 | 0.2 | 0 |
| Broad-leaf forest | 0 | 0 | 0.1 | 0.3 |
| Coniferous forest | 0 | 0 | 0 | 0 |
| Impervious surface | 0 | 0 | 0 | 0 |
| Planted forest | 0 | 0.7 | 0 | 0 |
| Pc | - | - | - | 28.9 |
| Ph | - | - | - | 2 |
- Note: Period 1, 2002–2007; Period 2, 2008–2013; Period 3, 2014–2021; Period 3+, the environmental variable of grazing disturbance was added at Period 3 before reassessing the habitat suitability. The variable importance of VEG is the sum of the percentage contributions of the six sublayers (broad-leaf forest, coniferous forest, brush, meadows, planted forest, and impervious surface).
- Abbreviations: Bio1, annual mean temperature; Bio4, temperature seasonality (standard deviation ×100); Bio13, precipitation of wettest month; Bio15, precipitation seasonality (coefficient of variation); DTR, distance to roads; DTW, distance to water; ELE, elevation; Pc, the presence probability of cattle; Ph, the presence probability of horse; SLP, slope; VEG, vegetational form.
According to the response curves of major environmental factors (Supporting Information S2: Appendix S2) and the threshold value (MTSS, Supporting Information S1: Appendix S1), in Period 1, blue-eared pheasants preferred to use areas with ELE below 3000 m and SLP less than 60°; in Period 2, they preferred to use areas with an ELE of 2550–2850 m, SLP <40°, that were close to water and roads; and in Period 3, they preferred to use areas with ELE below 3000 m. The Wilcoxon rank sum test results showed that compared to Periods 1 and 2, blue-eared pheasants favored higher ELE in Period 3 (Periods 1–3: p = 0.0443; Periods 2–3: p = 0.0443); however, the selection of blue-eared pheasants for SLP (P = 1.0000) and DTR (Periods 1–3: p = 0.3371; Periods 2–3: p = 0.7618; Periods 1–2: p = 0.7617) did not differ significantly between periods (Supporting Information S1: Appendix S1).
3.1.2 Changes in habitat suitability
The model results were reclassified based on the MTSS and BTPT values (Supporting Information S1: Appendix S1), and the habitats of blue-eared pheasants were classified as most suitable (>MTSS), moderately suitable (between BTPT and MTSS), and unsuitable (<BTPT). For Period 1, the area of the potential habitat of the blue-eared pheasant was 105.88 km2 (32.78% of the protected area), of which 43.11 km2 was classified as most suitable habitat and 62.77 km2 was moderately suitable (Figure 2a). For Period 2, the potential habitat area was 99.51 km2 (30.80%), of which 47.16 km2 was most suitable and 52.35 km2 was moderately suitable (Figure 2b). For Period 3, the potential habitat area was 90.76 km2 (28.10%), of which 41.91 km2 was most suitable and 48.85 km2 was moderately suitable (Figure 2c). Compared to that in Period 1, the most suitable habitat area in Period 3 was 1.2 km2 smaller, and the moderately suitable habitat area was 13.92 km2 smaller. Compared to Period 3, in Period 3+ the most suitable habitat area increased by 1.79 km2 (Period 3+: 43.70 km2), and the moderately suitable habitat area decreased by 5.29 km2 (Period 3+: 43.56 km2).
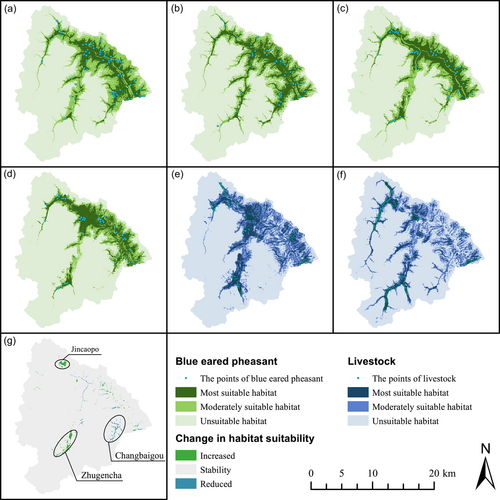
Based on the MTSS values calculated using the MaxEnt model for Periods 1 and 3, the suitability indices of the blue-eared pheasant were classified into three categories: increasing suitability (>0.2893), stable suitability (−0.2893 to 0.2893), and decreasing suitability (<−0.2893). The results showed that the habitat suitability of the blue-eared pheasant increased in some areas of Wanglang (2.81 km2) but decreased in others (2.05 km2); the suitability of most areas was stable (318.14 km2) (Figure 2g). Increases in suitability were mainly observed in Jincaopo and Zhugencha, whereas decreases were mainly observed in Changbaigou.
3.2 Effect of livestock on pheasant's predators
The results of the single-species occupancy models for livestock and natural predators of blue-eared pheasants (Supporting Information S1: Appendix S1) showed that the covariates affecting occupancy in the optimal model were ELE, DTW, SLP, and VEG for domestic cattle; ELE and DTW for domestic horses; and ELE for yellow-throated martens. The probability of occupancy for leopard cats was not affected by the covariates. These environmental variables were included in the analysis of the respective two-species occupancy models, and the results are presented in Supporting Information S1: Appendix S1.
The SIF values were calculated based on the optimal parameters of the two-species occupancy model. They showed that (1) there was a tendency for yellow-throated martens to exhibit spatial segregation with domestic cattle (SIF = 0.87 ± 0.05) while maintaining independence from domestic horses (SIF = 1 ± 0); (2) leopard cats showed spatial overlap with domestic cattle (SIF = 1.04), whereas they tended to be separated from the distribution of domestic horses (SIF = 0.59 ± 0.00).
3.3 Impact of livestock on pheasant nesting success
Among the 306 artificial nests, 250 (146 in Wanglang and 104 in Jiuzhaigou) were selected for further analysis because the eggs in these nests lasted for at least 30 days. Of these, 171 were categorized as IN, 22 as PD, and 57 as CD, with a nest success rate (nIN/Nall) of 68.40%. The nesting success rate was significantly lower in Wanglang (59.59%) than in Jiuzhaigou (80.77%) (χ2 = 15.341, p < 0.001). There was a significant difference between the nest results of artificial nests with the same egg color in different regions (original color: χ2 = 8.007, p = 0.018; spotted: χ2 = 7.715, p = 0.021) (Table 3). The difference in nesting success between the original-colored (n = 123, 66.67%) and spotted eggs (n = 127, 70.08%) was not significant (χ2 = 1.031, p = 0.597). No significant differences were observed in nesting success between egg colors in the same area (Wanglang: χ2 = 0.174, p = 0.971; Jiuzhaigou: χ2 = 1.243, p = 0.537).
| Chi-square test term | Intact | Partially damaged | Completely destroyed | Chi-square | Expected lower limit | p Value | |
|---|---|---|---|---|---|---|---|
| Original colored | Wanglang (n = 72) | 42 (58.33%) | 7 | 23 | 8.007 | 5.48 | 0.018 |
| Jiuzhaihou (n = 51) | 40 (78.43%) | 6 | 5 | ||||
| Spotted | Wanglang (n = 74) | 45 (60.81%) | 6 | 23 | 7.715 | 3.76 | 0.021 |
| Jiuzhaihou (n = 53) | 44 (83.02%) | 3 | 6 | ||||
| Total of Wanglang (n = 146) | 87 (59.59%) | 13 | 46 | 15.341 | 9.23 | 0.000 | |
| Total of Jiuzhaigou (n = 104) | 84 (80.77%) | 9 | 11 | ||||
| Wanglang | Original colored (n = 72) | 42 (58.33%) | 7 | 23 | 0.174 | 6.32 | 0.917 |
| Spotted (n = 74) | 45 (60.81%) | 6 | 23 | ||||
| Jiuzhaigou | Original colored (n = 51) | 40 (78.43%) | 6 | 5 | 1.243 | 4.41 | 0.537 |
| Spotted (n = 53) | 44 (83.02) | 3 | 6 | ||||
| Total of primary (n = 123) | 82 (66.67%) | 13 | 28 | 1.031 | 10.73 | 0.597 | |
| Total of speckle (n = 127) | 89 (70.08%) | 9 | 29 | ||||
The results of the artificial nest survival analysis showed that five nests survived for <1 day, and 24 nests survived for <10 days at Wanglang; only three nests survived for <10 days at Jiuzhaigou (Supporting Information S2: Appendix S2). The artificial nest (286 resultant nests, including 171 IN,22 PD, 57 CD, 36 censored data could be used in COX proportional hazards model) destruction risk in Wanglang was 2.18 times higher than that in Jiuzhaigou (95% confidence interval: 1.128–4.203; p = 0.021).
Among all the nest encounter events in Wanglang, 60.87% were identified as nest predation, 32.61% as trampling (80% by cattle and horses), and 6.52% as neutral visits. However, in Jiuzhaigou, nest predation accounted for 40% and nest visits for 60% (Supporting Information S1: Appendix S1).
3.4 Evaluation of near-surface soil animals
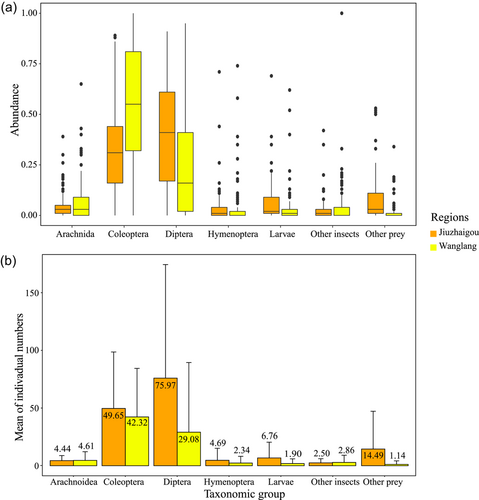
A total of 237 near-surface soil animal samples were collected throughout the survey period (Wanglang, n = 136; Jiuzhaigou, n = 101). A total of 27,329 individuals from both areas were counted (Wanglang: n = 11,243; Jiuzhaigou: n = 16,086). Among the seven main categories, Diptera and Coleoptera were the most abundant. Hymenoptera, Diptera, Coleoptera, larvae, and other prey were less abundant in Wanglang than in Jiuzhaigou (Supporting Information S2: Appendix S2).
According to the relative abundance calculations (Figure 3), pheasant faunal food resources in Jiuzhaigou were more abundant in Diptera and Coleoptera, whereas Wanglang was dominated by Coleoptera. The relative abundances of Coleoptera were significantly higher in Wanglang than in Jiuzhaigou (U = 3,676.500, Z = −5.927, p < 0.001), whereas those of Hymenoptera (U = 8,137.000, Z = 2.989, p = 0.003), Diptera (U = 9,253.500, Z = 4.954, p < 0.001), larvae (U = 8,998.500, Z = 4.531, p < 0.001), and other prey (U = 10,432.000, Z = 7.508, p < 0.001) were significantly lower in Wanglang than in Jiuzhaigou.
4 DISCUSSION
Grazing behavior has been shown to disturb many animals (Li et al., 2017; Maxwell et al., 2016). This study found a large reduction in suitable habitat areas for blue-eared pheasants in Wanglang over the last 20 years (16.12 km2, Figure 2). This decrease in the habitat suitability index indicates that the survival prospects of blue-eared pheasants may be greatly affected. Previous studies have shown that certain disturbed habitats are abandoned when they exceed the tolerance threshold of a species or when the resources required for survival are destroyed (Li et al., 2017; Lowe et al., 2014). Our analysis revealed that (1) blue-eared pheasants gradually shifted to higher ELE areas (Supporting Information S1: Appendix S1), and (2) the VEG (meadows and brush) became more influential in predicting habitat suitability (from Periods 1 to 3, Table 2).
In this study, we found that the area of most suitable habitat of the blue-eared pheasants was reduced by 1.2 km2, while the moderately suitable habitat was reduced by 13.92 km2 (comparing Periods 1 and 3, Figure 2a,c). Interestingly, there was no discernible reduction in habitat suitability following the inclusion of Pc and Ph in the model. However, the overall habitat structure for blue-eared pheasants in the entire region exhibited increased fragmentation. This suggests that grazing may have had a strong negative effect on blue-eared pheasants. As shown in Figure 2g, the change in habitat suitability for blue-eared pheasants mainly occurred in three areas of the reserve; habitat suitability increased in Jincaopo and Zhugencha, yet decreased in Changbaigou. We found that the three areas with increased habitat suitability also had frequent cattle and horse activity (Figure 2e,f). Habitat selection is largely based on food supply and risk of predation. Our study on the natural predators and food resources of blue-eared pheasants confirmed an increase in natural predator disturbance and relative abundance of Coleopterans caused by cattle and horse activity. Results from the two-species occupancy model indicated that yellow-throated martens and leopard cats remained spatially distant from domestic livestock (cattle-yellow-throated marten: SIF = 0.87 ± 0.05; horse-yellow-throated marten: SIF = 1; horse-leopard cat: SIF = 0.59; cattle-leopard cat: SIF = 1.04). Although the effects of livestock on raptors, the pheasant's main natural enemy, were not explored in this research, many studies have shown that increased grazing intensity can lead to reduced raptor presence in such areas (Johnson & Horn, 2008; Piana & Marsden, 2014). This suggests that predators spatially avoid cattle and horses, forming areas that act as refuges for pheasants. However, we still need to further explore the role of grazing as a refuge for pheasants.
We found distinct differences between both sites, which might be due to livestock grazing in Wanglang (vs. no grazing in Jiuzhaigou). A key finding was the increase in the relative abundance of Coleopterans in grazing areas, providing highly nutritious food for blue-eared pheasants. Coleopterans are known to be favored by terrestrial birds due to their nutritional value. For instance, a study in Yunnan, China, found that green peafowl (Pavo muticus) frequent areas where domestic cattle gather to eat more Coleoptera from cow dung (Gu et al., 2022). This behavior aligns with findings that blue-eared pheasants increase their animal intake during the breeding season (Zheng & Liao, 1983). Therefore, the increased attraction of Coleopterans to livestock feces could, to some extent, compensate for the disadvantage of lower relative abundance of other food sources (Liu et al., 2019; Traba et al., 2008). Thus, we hypothesize that the dual factors of predator avoidance and the increased relative abundance of Coleopterans are likely responsible for the presence of blue-eared pheasants in areas where cattle and horses are active.
Despite the potential benefits of grazing activities, our artificial nesting experiments revealed that cattle and horse activity resulted in more nest predators and was also the main cause of nest trampling (Supporting Information S1: Appendix S1). The presence of cattle and horses significantly reduced the nesting success of the blue-eared pheasants in Wanglang. Given that nesting success is directly linked to the survival of the entire population of blue-eared pheasants, these findings are particularly concerning. According to the nest predator survey (Supporting Information S1: Appendix S1), leopard cats and yellow-throated martens not only targeted blue-eared pheasants but also their eggs. These species were more frequently observed in Wanglang, which suggests that grazing does not adversely affect the natural predators of blue-eared pheasants. Although our initial findings indicated spatial avoidance of leopard cats and yellow-throated martens in relation to cattle and horse activity, it appears that these predators are capable of adapting to grazed environments. In other words, changes in the relationship between blue-eared pheasants and their natural predators in response to grazing activity are complex and synergistic. This complexity was also evident in a study of greater prairie chickens, where these birds were found to adjust their activities in habitats disturbed by different grazing intensities based on survival and reproductive needs (Winder et al., 2018). Our findings suggest that blue-eared pheasants may employ similar survival strategies, potentially using different foraging and breeding sites to adapt to environmental changes. Further supporting this notion, a study by Chen et al. (2019) on cattle and horse activity in Wanglang showed that activity was concentrated in areas below 3200 m. Correlating with this, our findings show a gradual increase in the activity of blue-eared pheasants (Supporting Information S1: Appendix S1). This pattern could be interpreted as a strategic response, where they increasingly nest at higher ELEs to avoid the influence of cattle, horses, and predators on their nest sites during the breeding season.
Based on our results, we propose that livestock in the reserve have a significant negative impact on the survival of blue-eared pheasants. To mitigate this impact, the reserve authorities should strictly control illegal grazing practices and continuously monitor the distribution of existing habitats to avoid further loss. We also recommend that the reserve continue to monitor the effects changes in grazing systems have on the population dynamics of endangered pheasants and the activities of their natural predators.
AUTHOR CONTRIBUTIONS
Xing Chen: Conceptualization; methodology; software; formal analysis; investigation; data curation; writing—original draft; visualization. Xiao-Tong Shang: Conceptualization; methodology; formal analysis; investigation; data curation; visualization; writing—review and editing; supervision. Fan Fan: Conceptualization; investigation. Yong Zheng: Investigation. Lian-Jun Zhao: Resources; project administration. Hong-Ou Sun: Investigation; project administration. Sheng Li: Conceptualization; methodology; resources; data curation; writing—review and editing; supervision; project administration. Li Zhang: Conceptualization; methodology; resources; data curation; writing—review and editing; supervision; project administration; funding acquisition.
ACKNOWLEDGMENTS
The survey was supported by the Wanglang Nature Reserve Administration and Jiuzhaigou Nature Reserve Administration. We thank the research group of ornithology and plant taxonomy of Beijing Normal University for their help in related experiments, and all the volunteers in the field work for their contributions. We thank Associate Professor Xiaoli Shen of the Institute of Botany, Chinese Academy of Sciences for her support in experimental design. This work was supported by Wanglang National Nature Reserve, Sichuan (grant #: Ping Gong Jiao Cai Tan [2017] No. 1-1), and the Ministry of Ecology and Environment (grant #: 2019HJ2096001006, MM-2017-026; 2018-02-06-M2019-43/44).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data sets analyzed during the current study are available from the corresponding author on reasonable request.




