Early prognosis biomarkers of severe fever with thrombocytopenia syndrome
Mingrong Ou, Aofan Wang, and Jie Yu contributed equally to this work and shared the co-first authorship.
Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease that results from SFTS bunyavirus (SFTSV) infection. Infection with SFTSV can activate the immune system, producing a series of inflammatory factors. Some patients, particularly those with pre-existing conditions or at an advanced age, may experience an excessive inflammatory response, triggering systemic multi-organ failure progressing to severe disease and, potentially, death. In recent years, extensive research has been conducted on the mechanism of SFTSV infection and its interaction with host immune responses. Additionally, a range of biomarkers with significant prognostic value for SFTS have been identified. This review provides a comprehensive summary of the latest advancements in understanding the interplay between SFTSV and host immune responses, and elucidates the role of these biomarkers in the early detection of severe cases and fatal outcomes. The insights presented aim to inform strategies for early intervention, clinical treatment, and prognostic assessment of patients with SFTS.
Abbreviations
-
- ASC
-
- apoptosis-associated speck-like proteins
-
- CCB
-
- calcium channel blocker
-
- DAMPs
-
- danger-associated molecular patterns
-
- DCs
-
- dendritic cells
-
- IBs
-
- inclusion bodies
-
- IFN-I
-
- type I interferon
-
- IFN-α
-
- interferon-alpha
-
- IFN-β
-
- interferon-beta
-
- IP-10
-
- IFN-γ-induced protein-10
-
- IRFs
-
- interferon regulatory factors
-
- ISGF3
-
- interferon-stimulated gene factor 3
-
- mDCs
-
- myeloid dendritic cells
-
- mtDNA
-
- mitochondrial DNA
-
- NK
-
- natural killer
-
- NLRP3
-
- pyrin-domain protein 3
-
- NP
-
- nucleocapsid proteins
-
- NSs
-
- nonstructural proteins
-
- PAMPs
-
- pathogen-associated molecular patterns
-
- PDGF
-
- platelet-derived growth factor
-
- RANTES
-
- regulated on activation in normal T cell expressed and secreted
-
- RdRp
-
- RNA-dependent RNA polymerase
-
- SFTS
-
- severe fever with thrombocytopenia syndrome
-
- SFTSV
-
- severe fever with thrombocytopenia syndrome bunyavirus
-
- TBK1
-
- TANK-binding kinase 1
-
- TLRs
-
- toll-like receptors
1 INTRODUCTION
Severe fever with thrombocytopenia syndrome bunyavirus (SFTSV) was renamed Dabie bandavirus in 2019 by the International Committee on Taxonomy of Viruses [1]. This change was part of a broader effort to classify the virus more accurately within the scientific community. Despite the official name change, the term SFTSV is still frequently used because of its widespread recognition and historical context. SFTSV is an emerging pathogen within the Phlebovirus family and is classified as a novel bunyavirus [2]. It is predominantly transmitted through tick bites, with additional modes of transmission including blood, body fluids, and respiratory droplets, with documented instances of human-to-human transmission [3, 4]. Initially identified in China in 2010, SFTSV has since been associated with cases in Japan, Southeast Asia, and Republic of Korea [5-8]. Patients infected with SFTSV often exhibit a range of clinical symptoms such as fever, thrombocytopenia, leukopenia, gastrointestinal problems, and lymphadenopathy. Some individuals may present with central nervous system symptoms and signs of bleeding. In severe cases, it can lead to multi-organ dysfunction, disseminated intravascular coagulation, and potentially progress to systemic multi-organ dysfunction, carrying a mortality rate as high as 30% [9-12]. The risk factors for SFTS fatality include advanced age, altered mental status, elevated serum lactate dehydrogenase and aspartate aminotransferase levels, prolonged activated partial thromboplastin time, and high blood viral RNA load [9].
SFTSV is a single negative-stranded enveloped RNA virus, and its genome is composed of three segments of RNA: large (L), medium (M), and small (S) [13, 14]. The L segment encodes a crucial enzyme known as viral RNA-dependent RNA polymerase (RdRp), which plays a pivotal role in the replication of viral RNA and the synthesis of viral mRNA, essential processes for viral replication and protein production. The M segment encodes a glycoprotein precursor, which is processed by host cell proteases into two distinct glycoproteins, Gn and Gc; these glycoproteins are essential for viral assembly and facilitate virus entry into host cells. Gn is used in the early stages of SFTSV infection by binding to non-muscle myosin heavy chain IIA on the cell surface [5, 15-17]. The S segment encodes nucleocapsid proteins (NPs) and nonstructural proteins (NSs). NP proteins encapsulate the three RNA genome segments of SFTSV, forming a ribonucleoprotein complex along with the RdRp that shields the virus from nuclease and host immune system degradation; this highlights that NP plays a critical role in viral transcription and replication. It is speculated that NSs are the primary virulence factors of SFTSV, known to modulate the host's innate immune response and promote viral replication [18].
Although The World Health Organization has designated SFTS as a disease that urgently requires research [19] and the development of treatment methods, there are currently no effective treatment methods, resulting in a distressingly high mortality rate. Consequently, developing strategies for the prevention and treatment of SFTS has become an urgent challenge. Identifying biomarkers associated with the disease is beneficial in deepening our understanding of the complex interplay between the host immune system and SFTSV, aiding in evaluating disease severity and potentially reducing the mortality rate. This review summarizes the biomarkers that can serve as early indicators of severe disease progression and fatal outcomes in SFTS, aiming to lay the foundation for identifying novel therapeutic targets.
2 HOST INNATE IMMUNE RESPONSE DURING SFTSV INFECTION
The innate immune system serves as the first line of defense, capable of preventing viral invasion with an immediate response. Innate immune cells are equipped with pattern recognition receptors that can identify both pathogen-associated (PAMPs) and danger-associated molecular patterns (DAMPs) in viruses. This recognition process is crucial for the detection of viral invasion and initiating an antiviral response [20] (Figure 1).
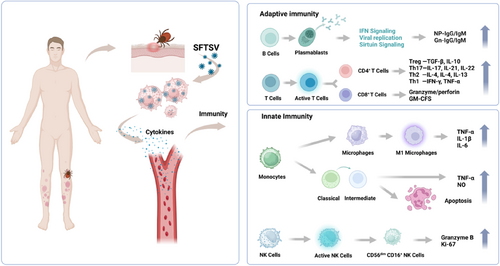
SFTSV infection initiates the activation of the human immune syste. GM-CFS, granulocyte-macrophage colony stimulating factor; IFN, interferon; IL, interleukin; NK, natural killer; NP, nucleocapsid proteins; SFTSV, severe fever with thrombocytopenia syndrome bunyavirus; TGF, transforming growth factor; TNF, tumor necrosis factor. Images were made using the biorender.
Monocytes, widely present in the bloodstream, constitute a vital component of the innate immune system. Toll-like receptors (TLRs) recognize pathogenic microorganisms by recognizing infected monocytes. This recognition induces changes in their cytokine and chemokine profiles, as well as in their coordinated stimulus molecular patterns, leading to the differentiation of macrophages and their migration to the infected tissue, where they become resident cells. In patients with SFTS, mononuclear cell counts are significantly lower than in convalescent patients or healthy controls, and there is a positive correlation between viral load and the reduction of these cells [21]. This indicates that the mononuclear cells may be the primary target cells during SFTSV infection, which could be induced to become dysfunctional and/or undergo cell death after infection [21]. The reduction in mononuclear cells and their associated dysfunction are linked to the SFTS progression.
In the innate immune system, macrophages regulate adaptive immune responses to pathogens by processing and presenting antigens [22]. They are also primary target cells for terminal SFTS infection, facilitating active replication of SFTSV [23, 24]. SFTSV is capable of replicating within monocytes and averting apoptosis by suppressing the TNF and NF-κB signaling pathways [25]. Infection with SFTSV upregulates the expression of IL-10 and activates the STAT3 pathway. This pathway is intricately linked with the production of miR-146-b, which inhibits the differentiation of M1 macrophages and drives them toward an M2 phenotypic by targeting STAT1 and facilitating viral dissemination [26].
Dendritic cells (DCs) are among the most crucial antigen-presenting cells, forming a pivotal link between innate and adaptive immunity [27]. DCs in human peripheral blood are primarily categorized into two types: myeloid (mDCs) and plasmacytoid DCs [28]. Elevated circulating mDCs may serve as a protective factor for patients with SFTS [29]. In cases of fatal SFTSV infection, the rate of mDC apoptosis was significantly increased post-infection [30]. There is also a negative correlation between mDCs and the levels of IL-6, IL-10, TNF-α, and viral load [29]. In patients who succumb to SFTS, significant mononuclear cell apoptosis and a deficiency in IL-4 and GM-CSF are observed, which impede the maturation of mDCs and lead to a substantial reduction in the adequate number of DCs. A scarcity of mDCs can inhibit the differentiation of naive T cells into T follicular helper cells, potentially leading to an ineffective humoral immune response or immune paralysis. Consequently, the measurement of circulating mDCs can be considered as a valuable predictive biomarker for these conditions.
Natural killer (NK) cells are essential components of the antiviral immune response; the depletion of NK cells may contribute to an increase in viral load, as they play a vital role in defense and immune surveillance [31]. Human peripheral blood NK cells can be categorized into five distinct subsets based on the expression levels of the surface markers CD16 and CD56: CD56brightCD16−, CD56brightCD16+, CD56dimCD16−, CD56dimCD16+, and CD56−CD16+. The CD56dimCD16+ is predominant, constituting no less than 90% of all NK cells in peripheral blood [32]. During the initial phase of SFTSV infection, there is a significant decrease in the prevalence of CD56dimCD16+ NK cells, which is inversely associated with disease severity [33]. Additionally, acute infection with SFTSV leads to increased expression of Ki-67 and granzyme B, as well as decreased expression of NKG2A, in CD56dimCD16+ NK cells. Compared with the recovery phase, the efficacy of CD56dimCD16+ NK cells is heightened during the acute phase of SFTS [34]. Despite the depletion of CD56dimCD16+ NK cells, their activation and functional enhancement indicate a protective role against early SFTSV infection [34]. The loss of NK cells may lead to a rise in viral load. Studies have demonstrated that alterations in Siglec-9 expression on peripheral NK cells in patients with SFTS can significantly impact NK cell function; the number of NK cells was significantly reduced in peripheral blood compared with healthy controls, and the activation of receptors on NK cells was also diminished. Additionally, the expression of Siglec-9 and the frequency of Siglec-9+ NK cells are both significantly decreased in patients with SFTS. Interestingly, the expression of Siglec-9(which belongs to the CD33-related Siglec family, one major group of the Siglecs) on CD56dimCD16+ NK cells is negatively correlated with SFTSV viral load. Siglec-9+ NK cells exhibited higher levels of activated receptors and stronger effector functions than Siglec-9− NK cells. The decrease in Siglec-9 expression on NK cells predicts NK cell dysfunction in patients with SFTS, suggesting that it may serve as a potential marker for identifying functional NK cell subsets in these patients [35].
3 INFLAMMATORY PATHWAYS
Cytokines are a group of soluble, small molecular-weight proteins secreted by both immune and tissue cells. They are essential in cell development, differentiation, and immune responses, as well as in the regulation of immune cells. The activation of the immune system initiates the transmission of cellular signaling pathways, which in turn activate downstream signaling complexes and cytokines. The intricate interplay among various cytokines and their interactions with inflammatory complexes can modulate inflammatory pathways. They are also involved in pathogen recognition and in orchestrating the initiation and regulation of inflammatory responses. Cytokine secretion is modulated by various cellular signaling pathways, including JAK/STAT3, MAPK, NF-κB, mTOR, and TLR4. The interplay among these signaling pathways can influence cytokine expression.
Interferons are potent antiviral cytokines that modulate both innate and adaptive immune responses to inhibit viral replication [34]. They are categorized into Types I, II, and III. Type I interferons (IFN-Is), which include interferon-alpha (IFN-α) and interferon-beta (IFN-β), are integral to the innate immune response and are secreted by immune cells such as macrophages and DCs [36]. RIG-I has been identified as a critical viral RNA sensor in the recognition of cells infected with SFTSV by recruiting the IFN-β promoter stimulator 1, also known as mitochondrial antiviral signaling protein (MAVS). MAVS then relays the signal to TANK-binding kinase 1 (TBK1) and the NF-κB kinase inhibitor, leading to the activation of the phosphorylation of interferon regulatory factors (IRFs) −3 and −7, which in turn induces the secretion of IFN-I [37, 38]. Both Type I and III interferons utilize the same receptor and share the final activation of the interferon-stimulated gene factor 3 (ISGF3) signaling pathway. ISGF3 activates the STAT1 and STAT2 heterodimers along with IRF9 [39], stimulates the JAK-STAT signaling pathway, and induces the expression of interferon-stimulated genes through both autocrine and paracrine mechanisms.
SFTSV infection can result in remarkable inflammation, with abnormal activation of pro-inflammatory cytokines such as IL-6, IL-10, IFN-γ, IL-8, and monocyte chemotactic protein 1 in serum samples [40, 41]. In addition, elevated levels of IFN-α have been correlated with SFTS severity [42]. Studies have reported that mice with a gene deletion of the IFN-α and IFN-β receptor α chain, as well as hamsters with a STAT2 gene deletion, exhibit high serum viral loads and hematological profiles similar to human infections. These animals are highly susceptible to SFTSV infection, which can lead to death. This underscores the crucial role of the IFN-I signaling pathway in preventing lethal SFTSV infection [43] (Figure 2).
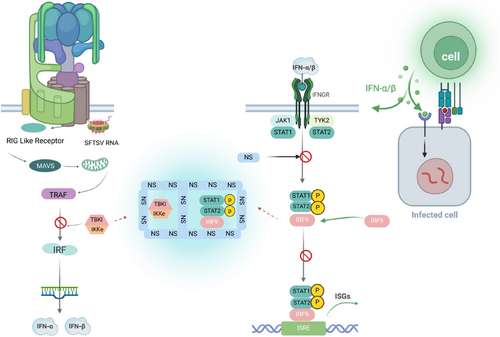
The immunological interplay between SFTSV and its host. IFN, interferon; IFNGR, interferon gamma receptor; IKK, inhibitor of kappa B kinase; IRF, interferon regulatory factor; ISRE, interferon stimulated response element; JAK, janus kinase; MAVS, mitochondrial antiviral signaling protein; NS, nonstructural protein; SFTSV, severe fever with thrombocytopenia syndrome bunyavirus; STAT, signal transducer and activator of transcription; TBK1, TANK binding kinase 1; TRAF, TNF receptor associated factor. Images were made using the biorender.
Viruses, in their ongoing struggle with their hosts, have evolved various strategies to evade the innate immune system, including avoiding host recognition, disrupting interferon signaling pathways, and impeding interferon production and autophagy pathways. Current studies on SFTSV evasion from host innate immune response mainly focus on the inhibition of SFTSV NSs on IFN-I production [44]. The modulation of IFN-β promoter activity by MAVS requires interaction with NSs of SFTSV and TBK1. The NSs can bind to TBK1, preventing the activation of downstream IRF and NF-κB signaling [45]. This interaction results in the suppression of TBK1 phosphorylation, which is essential for the activation of the MAVS-mediated IFN-β promoter [46]. In addition, it has been shown that SFTSV can use autophagy to evade the antiviral innate immune response; NSs interact with the CCD domain of BECN1 to induce complete macrophage/autophagy, promoting the formation of a BECN1-dependent autophagy initiation complex [47]. NSs sequestrate antiviral proteins such as TBK1 into autophagic vesicles, promoting their degradation. Recent studies have shown that SFTSV NP can induce TUFM-mediated mitophagy to degrade MAVS and escape the host immune response [48].
Researchers have explored the relationship between SFTSV infection and the cGAS-STING signaling pathway, finding that SFTSV infection prompts the release of mitochondrial DNA (mtDNA) into the cytoplasm, which subsequently activates the cytoplasmic DNA receptor cGAS, leading to the suppression of downstream innate immune signaling pathways. A key discovery is the role of SFTSV's envelope glycoprotein, Gn, which acts as a potent inhibitor of the cGAS-STING pathway. Gn hinders the nuclear accumulation of IRFs 3 and p65, impeding downstream innate immune signaling. Additionally, Gn interacts with STING, preventing its dimerization and K27 ubiquitination. This interference disrupts the assembly and downstream signaling of the STING-TANK-binding kinase 1 complex. Additionally, Gn has been found to facilitate STING degradation, which further suppresses immune responses. Overall, this study underscores the significant role of the glycoprotein Gn in modulating the antiviral innate immune response and reveals a novel mechanism by which SFTSV evades immune detection [49].
As a crucial transcription factor, NF-κB exerts multiple biological functions, acting in pro-inflammatory, antiviral, and apoptotic responses. The activation of NF-κB involves both canonical and non-canonical pathways. In SFTSV-infected THP-1 cells during the early stages of infection, the NF-κB signaling pathway may be temporarily activated, promoting viral replication. Pretreatment of THP-1 cells with NF-κB inhibitors before SFTSV infection significantly reduced viral load [50]. The NSs can trigger cytokine storms following SFTSV infection, and TBK1 can suppress the NF-κB signaling pathway and cytokine/chemokine production in a kinase activation-dependent manner. In contrast, NSs can sequester TBK1, preventing its inhibitory effect on NF-κB and promoting NF-κB activation and the transcription of its downstream targets [45]. Further experiments revealed that the activity of NF-κB gradually diminished after SFTSV infection, and the SFTSV NSs and NPs can inhibit NF-κB signaling [51]. Additionally, pretreatment of HepG2 cells with NF-κB inhibitors not only significantly reduced the expression of pro-inflammatory cytokines but also decreased the copy number of the S gene within the cells, which confirmed the inhibitory effect of the SFTSV NSs protein on NF-κB signaling [18].
The inflammasome is a complex of multiprotein platforms, predominantly composed of polymeric protein complexes that include sensor, junction, and effector caspase components, such as NACHT, leucine-rich, and pyrin-containing proteins, apoptosis-associated speck-like proteins (ASC), and pro-caspase-1 [52-54]. The pyrin-domain protein 3 (NLRP3) inflammasome stands out as it recognizes a variety of PAMPs and DAMPs, such as RNA, ATP, ion flux, and lysosomal damage, playing a crucial role in the inflammatory response following SFTSV infection. Mitochondria are essential organelles that perform multiple biological functions, including energy metabolism, programmed cell death, and maintaining cellular homeostasis [55]. Activation of the NLRP3 inflammasome is a two-step process involving initiation and maturation. Briefly, the TLR-NF-κB signaling pathway enhances the transcription of IL-1β and inflammasome-related components. Once activated, NLRP3 recruits the adapter protein ASC and pro-caspase-1, leading to the cleavage of pro-caspase-1 into its active form, caspase-1. This promotes the maturation and secretion of the pro-inflammatory cytokines IL-1β and IL-18 and triggers gasdermin D-dependent pyroptosis [56]. The permeability of the mitochondrial membrane, mediated by BAX and BAK, plays a significant role in the translocation of mtDNA from the mitochondria to the cytosol [57]. The binding of mtDNA to NLRP3 leads to inflammasome activation and an amplified inflammatory response, confirming that SFTSV infection facilitates the maturation and secretion of the pro-inflammatory cytokine IL-1β via the NLRP3 inflammasome. BAK expression levels have been correlated with disease progression and fatal outcomes in SFTS. The exact composition and mechanism of the inflammasome are intricate, necessitating further research to clarify its role. Nonetheless, these findings shed light on the clinical manifestations and molecular underpinnings of severe and fatal cases of SFTS.
Cytokines can stimulate immune cells and bolster the immune response. A deficiency or overabundance of cytokines can disrupt the delicate balance between pro-inflammatory and anti-inflammatory factors. Cytokine storm-induced immune activation and subsequent organ damage are vital in the pathogenesis and severity of SFTS [58]. Compared with healthy controls, patients with SFTS exhibit a significant upregulation of certain inflammatory mediators, regardless of disease severity or patient survival status [59], including IL-6, IL-10, IL-8, IL-15, IL-1-RA, TNF-α, IFN-γ, IFN-γ-induced protein-10 (IP-10), heat shock protein 70, granulocyte colony-stimulating factor, granzyme B, caspase-8, and C-C motif chemokine ligand 7, among others. Conversely, the downregulation of other cytokines or chemokines, such as tissue polypeptide antigen, platelet-derived growth factor, growth-related oncogenes, transforming growth factor-β, and regulated on activation in normal T cell expressed and secreted (RANTES) [60-63], suggests their potential involvement in SFTS progression. IL-6, a pro-inflammatory cytokine, is essential for hastening cellular responses to curtail persistent viral infections. IL-10, an anti-inflammatory cytokine, is markedly elevated in SFTS patients, particularly in those with fatal outcomes [9]. The overproduction of IL-6 and IL-10 can lead to cytokine storms, exacerbating the pathological effects of SFTSV infection [64]. Compared with healthy individuals, reduced levels of platelet-derived growth factor and RANTES in patients with SFTS imply that diminished levels of these mediators may signal a higher mortality risk [64]. Additionally, during the acute phase, both survivors and deceased patients exhibited elevated levels of multiple cytokines, including IL-6, IL-10, IFN-γ, IP-10, and TNF-α [65, 66]. Even with repeated plasmapheresis, the levels of TNF-α, IL-6, IP-10, and granzyme B remain high in fatal cases [65].
These insights reveal that the aberrant secretion of cytokines and chemokines at different stages of SFTS may induce pathological organ damage and dysfunction, potentially leading to disease progression and death. Although cytokine secretion profiles in patients with SFTS vary across studies, the identified distinct cytokine pattern can serve as a biomarker for the early detection of severe SFTS and potentially fatal outcomes. Targeting IFN signaling, suppressing NF-κB signaling, and blocking the cytokine storm could significantly alleviate the severity of the disease and aid in the therapeutic management of SFTS.
4 EARLY DETECTION OF BIOMARKERS AND THEIR ASSOCIATION WITH THE PROGNOSIS OF SFTS
In addition to the biomarkers associated with the innate immune response and inflammatory pathways, ongoing research is continually refining our understanding of biomarkers pertinent to the early detection and prognostic evaluation of SFTS.
Among these studies, some have successfully grouped samples based on infection time points. They suggest that PHGDH and NLRP12 may offer valuable insights into the early stages of SFTSV pathogenesis and enhance our understanding of host-virus interactions [67]. In the early stages of SFTS, deceased patients exhibited significantly lower counts of Th1,Th2 and Treg cells compared with survivors, while the count of Th17 cells was notably higher [68]. Th17 cells can hinder T cell-mediated killing of target cells, thus impeding the antiviral response [69]. The reduction in Th1,Th2 and Treg cells, coupled with the relative increase in Th17 cells, affects the balance of cellular and humoral immunity, leading to immune dysregulation and an excessive release of inflammatory cytokines [68]. Overall, studies showed that CD4+ T cell deficiency along with Th2 and Th17 bias were strongly associated with the severity of SFTS, suggesting the therapeutic potential of early immune intervention.
Some studies have compared gene expression profiles between patients infected with SFTSV and healthy controls. By analyzing differentially expressed genes and conducting weighted gene co-expression network analysis, they identified a set of genes—ADIPOR1, CENPO, E2F2, and H2AC17—that may serve as a diagnostic signature for the acute phase of SFTSV infection [70]. T lymphocytes, particularly CD4+ T lymphocytes, are significantly diminished in both surviving and deceased patients during the acute phase of SFTS, with a more severe reduction observed in those who succumb to the disease [71]. Although the CD8+ T cell count also decreases, the difference between survivors and non-survivors is not statistically significant; the reduction in CD4+ T cells is a more meaningful indicator for assessing disease severity and prognosis [68].
Patients with SFTS have been found to exhibit elevated levels of certain cytokines, with the non-surviving group having significantly higher levels than the surviving group. Among these, monocyte chemoattractant protein-3 emerged as the most significant predictor of poor prognosis in SFTS patients; its predictive role was further validated using receiver operating characteristic analysis [72]. Distinct plasma proteomic profiles have been linked to varying outcomes in SFTS cases. CCL20 has been identified as a novel biomarker that offers high sensitivity, accuracy, and specificity for predicting the prognosis of SFTS, highlighting its potential to advance diagnostic and prognostic capabilities for the disease [73].
Single-cell RNA sequencing of Peripheral Blood Mononuclear Cells from patients with SFTS has revealed a significant expansion of the B cell population, correlating with disease severity [24, 28, 29]. Deceased patients' sera lack SFTSV NP-specific IgM and IgG, as well as Gn-specific IgG. However, survivors of the acute phase of infection exhibit SFTSV NP-specific IgM antibodies, with symptoms emerging 2–3 weeks post-infection, coincident with the appearance of NP-specific IgG antibodies [30]. These results indicate that in fatal cases of SFTS, there is a failure in antibody class switching, PB proliferation dysfunction, and an ineffective humoral immune response, all of which contribute to damage to the immune system [30, 74]. SFTSV infection may inhibit antibody secretion and B cell maturation, thus hindering an effective humoral immune response. Patients with acute SFTS who lack NP-specific IgM responses have more adverse clinical outcomes and a higher risk of death [75]. Considering the close association of NP-specific IgG antibodies with viral clearance, humoral immune responses are linked to the prognosis of SFTS [33].
One study meticulously screened the library of FDA-approved drugs and identified a calcium channel blocker (CCB), benidipine hydrochloride, that could control viral infections by impeding viral internalization and curtail the replication of the SFTSV in vitro. Additionally, an extensive array of CCBs, such as nifedipine, have also demonstrated an inhibitory effect on SFTSV infection [76]. Using machine learning, certain studies have successfully developed a prognostic model for SFTS encephalitis, leveraging traditional clinical parameters to predict life-threatening conditions associated with SFTS [77]. Its ability to significantly enhance the accuracy of early prognosis is of great value. Additionally, its potential for widespread application in underdeveloped areas with limited medical resources underscores its practical significance [77]. Recently, we identified elevated expression of PD-1/PD-L1 in a range of immune cells post-SFTSV infection. The anti-PD-1 nanobody NbP45 can effectively inhibit SFTSV infection in peripheral blood mononuclear cells. Additionally, in a humanized mouse model infected with SFTSV, subcutaneous administration of NbP45 exhibited superior efficacy compared with the approved anti-PD-1 antibody [78]. These findings emphasize the engagement of the PD-1/PD-L1 pathway during the acute phase of SFTSV infection, indicating its promise as a biomarker for potential immunotherapeutic strategies.
5 CONCLUSIONS
This review encapsulates the pivotal biomarkers that mediate the interaction between SFTSV and the host immune system, including IFN-γ, TNF-α, PD-1, NLRP3, and IP-10. These biomarkers serve as critical indicators in patients with SFTS, signaling the potential progression to severe disease or even fatal outcomes. A profound comprehension of their regulatory roles within the immune response is essential for the timely prevention, clinical intervention, and accurate predictive assessment of patients with SFTS.
AUTHOR CONTRIBUTIONS
Yuxin Chen: Writing—review & editing (supporting). Mingrong Ou: Writing—original draft (lead). Aofan Wang: Writing—review & editing (equal). Jie Yu: Writing—review & editing (equal). Liwei Zhao: Writing—review & editing (equal). Chuang Li: Writing—review & editing (equal). Yuanyuan Wu: Writing—review & editing (equal).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This article is a practice-oriented case study description that made extensive use of secondary information sources and also drew upon the professional knowledge of the co-authors. As such, the creation of this case study article did not involve any formal research study, nor did it involve human participation in a research study. As such, IRB review was not required for this article.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed.




