Pseudomonas aeruginosa infections and improper storage conditions influence the performance of 1,3-β-d-glucan in diagnosis of invasive fungal infections
Abstract
Background
The association between 1,3-β-d-glucan (BDG) levels and infections caused by Pseudomonas aeruginosa or Streptococcus pneumoniae, and the stability of BDG under different storage conditions are unclear.
Methods
Strains of Pseudomonas aeruginosa and S. pneumoniae were grown in medium and human serum. The BDG concentrations in culture supernatants were measured. The specificity and stability of BDG were also evaluated.
Results
P. aeruginosa produced high levels of BDG in Luria–Bertani medium (>4 × 104 pg/mL) and human serum (527.0 pg/mL), whereas S. pneumoniae produced low levels of BDG in THY medium (175.6 pg/mL) and human serum (78.3 pg/mL). The BDG produced by these two bacteria was specifically degraded by 1,3-β-d-glucanase. BDG was degraded when stored at different temperatures, decreasing by 22.5% and 9.3% at −20°C and −70°C, respectively, for 63 days; by 30.7% at 4°C for 12 days; and by 12.6% and 22.0% at 37°C for 6 and 12 h.
Conclusion
BDG false-positivity must be considered in patients with bacteremia caused by P. aeruginosa when diagnosing invasive fungal infection. Human serum samples for the BDG test in medical facilities should be tested as soon as possible or stored at low temperatures before testing.
Abbreviations
-
- BDG
-
- 1,3-β-d-glucan
-
- IFI
-
- invasive fungal infection.
1 INTRODUCTION
1,3-β-d-Glucan (BDG) is a cell-wall component of almost all fungi, with the exception of Zygomycetes. Its presence in the bloodstream during invasive fungal infections (IFIs) makes it an early and rapid serological biomarker of invasive fungal diseases, including invasive aspergillosis and invasive candidiasis. The detection of BDG has been widely used for this purpose in the clinic and is included as a microbiological criterion in the definitions of IFIs by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group since 2008 [1]. Although the BDG test shows good sensitivity, good specificity, and high negative predictive values as well as reproducible and acceptable diagnostic performances [2], it can also produce a high frequency of false-positive results. These can be caused by the administration of BDG-containing medical products (such as antibiotics, immunoglobulins, albumins, filtered blood agents, fungus-derived agents, gauzes), bacterial bloodstream infections, or intestinal bacterial translocations [3].
False-positive BDG results are reportedly associated with bacteremia [4-6], although such results have also been observed for which bacteremia is not the cause [7-9]. These discrepancies may be attributable to complicated episodes during the treatment of patients. The presence of a potential IFI or treatment with a BDG-containing medical product can also bias BDG test results in patients with bacteremia. Alcaligenes faecalis, Pseudomonas aeruginosa, and Streptococcus pneumoniae have previously been shown to produce BDG in human serum in vitro [6]. However, this association has not been comprehensively investigated. The stability of BDG at different temperatures is also unclear. In this study, the association between elevated BDG levels and infections caused by P. aeruginosa or S. pneumoniae and the stability of BDG under different storage conditions were investigated.
2 MATERIALS AND METHODS
2.1 Strains and media
P. aeruginosa standard strain PA01 was kindly provided by Dr. Weihui Wu from Nankai University (Tianjin, China). S. pneumoniae (ATCC 49619) and Staphylococcus aureus (ATCC 43300) are stored in our laboratory. Three clinical P. aeruginosa isolates (PA14, PA23, and PA26) were isolated from our medical center and identified with Vitek® 2 system (bioMérieux, Marcy l’Etoile, France) and DNA sequencing. The strains of P. aeruginosa and S. aureus were cultured in Luria–Bertani (LB) medium. The strain of S. pneumoniae was cultured in Todd–Hewitt medium supplemented with 10% fetal bovine serum (FBS).
2.2 Growth curves
P. aeruginosa strain PA01 (or S. pneumoniae strain ATCC 49619) was grown in 3 mL of LB (or THY plus 10% FBS) medium overnight at 37°C with shaking (200 rpm). The overnight cultures were washed twice with the corresponding medium, used to inoculate (1:100) fresh medium, and their growth continued under the same conditions. Bacterial growth was measured as the optical density at a wavelength of 600 nm (OD600) at intervals of 1 h for 12 h. The growth curves (OD600 against time) were plotted with GraphPad Prism version 8.0.1 (GraphPad Software, La Jolla, CA, USA).
2.3 Detection of BDG
BDG was detected with a fungal 1,3-β-d-glucan detection kit (Zhanjiang A & C Biological LTD, Zhanjiang, China), which is widely used by a large number of medical facilities in China. Briefly, the mixtures of samples and reagents were incubated at 37°C for 75 min, and then measured with a Kinetic tube reader (Zhanjiang A&C Biological LTD, Zhanjiang, China). Culture medium and the BDG standard were used as the negative and positive controls, respectively. Each sample was measured twice. The BDG concentrations were determined as the mean optical density of duplicate readings and compared with the corresponding standard curve of predetermined concentrations ranging from 10 pg/mL to 500 pg/mL. The samples were serially diluted and retested if the concentrations were higher than 500 pg/mL. A test was considered valid when the sample was tested twice and the two values differed by < 15%. According to the instructions for the kit, BDG concentrations <100.5 pg/mL were considered negative. The intra-run and inter-run precisions of this kit were ≤10% and ≤12%, respectively.
2.4 BDG production in medium and human serum
P. aeruginosa (PA01, PA14, PA23, and PA26) and S. pneumoniae strain ATCC 49619 were grown in LB and THY plus 10% FBS, respectively, as described above for growth curves. The BDG concentrations in the PA01 culture supernatant were measured at 0, 1, 3, 6, 9, and 12 h, and those in the culture supernatants of three clinical P. aeruginosa isolates (PA14, PA23, and PA26) were measured at 6 h. The BDG concentrations in the S. pneumoniae ATCC 49619 culture supernatant at 0, 6, and 12 h were also measured.
Aliquots (3 mL) of overnight cultures of P. aeruginosa PA01 and S. pneumoniae ATCC 49619 were pelleted, washed three times with pooled human sera with BDG concentrations <30 pg/mL, and then suspended in 3 mL of the same human serum. The same human serum was then inoculated with the bacterial suspensions in a ratio of 100:1. The bacteria were grown overnight (12 h) and the BDG concentrations in the supernatants were measured as described above.
2.5 Enzyme assay
A stock solution of 5 mg/mL 1,3-β-d-glucanase (Sigma, USA) in 50 mM sodium acetate (pH 5.0) was prepared and stored at −20°C. Mixtures of 1,3-β-d-glucanase (0.5 mg/mL), BDG (bacterial culture supernatants), and 50 mM sodium acetate (pH 5.0) were incubated at 37°C for 1 h, and the BDG concentrations were measured as described above. Mixtures without enzyme were used as the negative controls.
2.6 Stability of BDG at different temperatures
Aliquots of fresh BDG-positive human sera were incubated at −70°C (63 days), −20°C (63 days), 4°C (12 and 24 days), or 37°C (6, 12, and 24 h). The BDG concentrations were then measured as described above.
2.7 Data analysis
The concentrations in the two groups were compared with a summary t test in SPSS version 22.0 (SPSS, Chicago, IL, USA). p < 0.05 was considered significant.
3 RESULTS
3.1 High levels of BDG produced by P. aeruginosa, but not S. pneumoniae
The BDG concentrations in each medium used in this study (LB medium, THY medium plus 10% FBS, and human serum) were 108.0 pg/mL, 165.1 pg/mL, and 22.6 pg/mL, respectively. To eliminate the contribution of the BDG in the medium to the results, all BDG test results were presented as raw concentrations minus the corresponding BDG concentration of the medium alone.
The BDG concentrations of P. aeruginosa PA01 in LB medium increased over time during the incubation phase and the exponential phase of growth, and the maximal levels (>4 × 104 pg/mL) were achieved in the plateau phase, which showed characteristics similar to its growth curve (Figure 1A). Three clinical P. aeruginosa isolates (PA14, PA23, and PA26) also produced high BDG concentrations of >8.0 × 103 pg/mL at 6 h (Figure 1B), consistent with that of PA01.
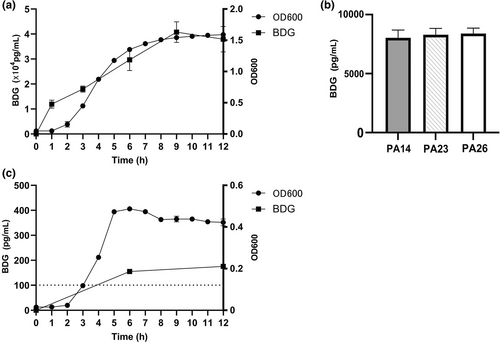
BDG production by Pseudomonas aeruginosa and Streptococcus pneumoniae. (a) BDG production and growth curve of P. aeruginosa PA01. (b) BDG production by clinical P. aeruginosa isolates PA14, PA23, and PA26. (c) BDG production and growth curve of S. pneumoniae ATCC 49619. Line with solid circles: growth curve; line with solid squares: BDG production; dashed line: positivity cut-off value of 100.5 pg/mL suggested by the BDG detection kit used in this study.
The concentrations of BDG produced by S. pneumoniae ATCC 49619 in THY plus 10% FBS were 155.3 pg/mL at 6 h and 175.6 pg/mL at 12 h (Figure 1C), which were low compared with those of P. aeruginosa.
The concentration of BDG produced by S. aureus ATCC 43300 in the LB medium was 5.1 pg/mL.
3.2 Production of BDG in human serum
To mimic bacterial growth during infections, the human serum was inoculated with P. aeruginosa PA01 or S. pneumoniae ATCC 49619, which were grown overnight (12 h). The BDG concentrations in the supernatants from the overnight PA01 serum cultures were 527.0 pg/mL, significantly higher than the cut-off value (100.5 pg/mL), whereas S. pneumoniae ATCC 49619 produced an extremely low concentration (78.3 pg/mL) of BDG in human serum.
3.3 BDG was degraded by 1,3-β-d-glucanase
To demonstrate the specificity of the BDG produced by P. aeruginosa and S. pneumoniae, the BDG-containing supernatants from the bacterial cultures were degraded with 1,3-β-d-glucanase. After incubation at 37°C for 1 h, the BDG concentration decreased dramatically from 601.9 pg/mL to 69.9 pg/mL in PA01 and from 208.6 pg/mL to 54.0 pg/mL in S. pneumoniae ATCC 49619 (p < 0.05; Figure 2).
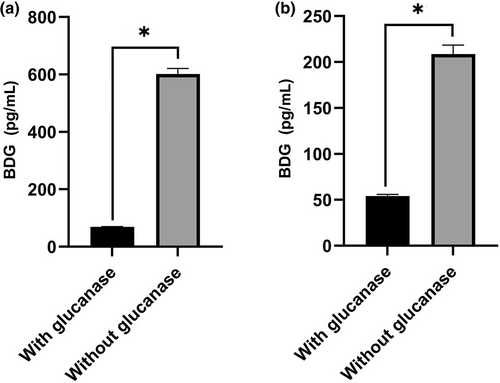
Specificity of BDG produced by Pseudomonas aeruginosa and Streptococcus pneumoniae. BDG produced by P. aeruginosa PA01 (a) or S. pneumoniae ATCC 49619 (b) was digested with 1,3-β-D-glucanase. Asterisk indicates statistical significance (p < 0.05).
3.4 Degradation rates of BDG increased with increasing temperature
To investigate the thermostability of BDG, BDG-positive human sera were stored at different temperatures (−70, −20, 4, and 37°C). After storage for 63 days, the concentrations of BDG stored at −20 and −70°C decreased by 22.5% and 9.3%, respectively (Figures 3A and 3B). When stored at 4°C for 12 days, the BDG concentration decreased by 30.7% (Figure 3C). When stored at a higher temperature (37°C), BDG was degraded more rapidly, decreasing by 12.6% at 6 h and 22.0% at 12 h (Figure 3D). Interestingly, no changes in the BDG concentrations were observed at 4°C during the interval from 12 to 24 days or at 37°C during the interval from 12 to 24 h (Figures 3C and 3D).
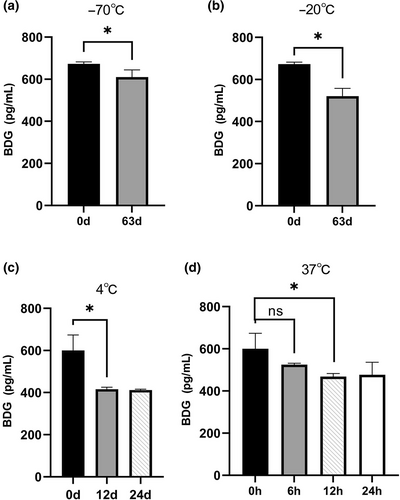
Stability of BDG under different storage conditions. (a) At −70°C for 63 days; (b) at −20°C for 63 days; (c) at 4°C for 12 and 24 days; (d) at 37°C for 6 h, 12 h, and 24 h. Asterisks indicate statistical significance (p < 0.05). “ns” indicates not statistically significant (p > 0.05).
4 DISCUSSION
The validity of testing human serum for BDG has been assessed in several studies [10-12]. The heterogeneity of those results may be attributable to different study designs, types of patients, methods of screening, criteria used to define a positive test, and the definitions of IFI. Because numerous factors are reportedly associated with elevated BDG levels, false-positivity must be considered when interpreting positive BDG test results. As demonstrated in this study, P. aeruginosa produces extremely high levels of BDG (>4 × 104 pg/mL) in LB medium and high levels (527.0 pg/mL) in human serum, and the BDG concentration increases over time in LB medium. The elevated BDG levels during the growth of P. aeruginosa provide clear evidence that patients with P. aeruginosa bacteremia can cause positive BDG tests. Based on the results in this study, negative BDG test results in patients with P. aeruginosa bacteremia [7, 8, 13] might arise from samples collected in the early stage of infection, which is characterized by low bacterial loads in the blood. A similar phenomenon was previously observed in patients with P. aeruginosa bacteremia, who showed increased BDG together with a major biomarker of bacterial infection (procalcitonin) [14]. Therefore, the BDG test should be used with caution to diagnose IFI in patients with P. aeruginosa bacteremia, especially those with sepsis or a high bacterial load in the blood.
Compared with P. aeruginosa, S. pneumoniae produce low levels of BDG in THY medium and human serum. The BDG concentration in human serum was even lower than the cut-off value (100.5 pg/mL). S. pneumoniae does not significantly increase the BDG concentration in blood during infection; therefore, it may not contribute to a false-positive diagnosis of IFI in patients with bacteremia caused by this bacterium.
The stability of BDG at different temperatures is rarely investigated. A previous study indicated the instability of BDG at 21°C, with concentrations decreasing by 1.14% per day [15]. The present study provides further evidence of the instability of BDG at various temperatures, and has shown that concentrations decrease by 0.15% per day at −70°C, 0.36% per day at −20°C, 2.56% per day at 4°C, and 2.10% per hour at 37°C, suggesting that higher temperatures increase the degradation rate of BDG. If 15% is set as the acceptable percentage of BDG degradation, which is the cut-off value of the test kits used in this study, the maximal storage times at −70, −20, 4, and 37°C are 101 days, 42 days, 6 days, and 7 h, respectively. It is also noteworthy that the BDG degradation rate decreases with time, and BDG reaches a relatively stable concentration in a later phase of storage (Figures 3C and 3D) [15], which might be attributable to the chemical equilibrium of the BDG degradation reaction. Therefore, fresh BDG-containing human serum samples should be tested as soon as possible or be stored under proper conditions, as described above.
This study had one limitation. Although P. aeruginosa and S. pneumoniae both produced BDG in their culture media, no blood samples from patients with bacteremia caused by these two pathogens were used to confirm the conclusion drawn. This is because a number of factors can influence BDG test results. First, antibiotic treatments, the use of surgical materials (such as medical dressings and gauzes), and unidentified fungal infections, among other factors, can increase the BDG levels in patients. Second, in this study, we show that the BDG concentration increases as the bacterial load increases; therefore, low bacterial loads in the blood may produce negative BDG test results. This may have caused the contradictory results published previously [7-9]. Therefore, the culture of P. aeruginosa and S. pneumoniae in human serum was used here to mimic bloodstream infections and to exclude the possible influences of all potential confounding factors, although it was not a perfect experiment.
5 CONCLUSION
False-positivity must be considered when interpreting positive BDG test results in patients with P. aeruginosa bacteremia. On the contrary, S. pneumoniae poses a low risk of BDG false-positivity in patients with bacteremia caused by this bacterium. Given the instability of BDG, human serum samples for BDG testing in medical facilities should be tested as soon as possible or stored at low temperatures beforehand.
AUTHOR CONTRIBUTIONS
Zilan Wei performed the measurements and collected and analyzed data; Jie Xu performed the measurements; Fang Yuang, Wendong Fang, and Jiahui Wu collected the samples; Youliang Wang designed and supervised the study; Shuiping Chen designed and supervised the study and wrote the manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data pertaining to this study are available from the corresponding authors upon request.




