Successful treatment of severe monkeypox case with advanced HIV infection using plasma from smallpox-vaccinated healthy population: A case report
Chuming Chen and Weiming Yao contributed equally to this work.
Abstract
Since the first reported case of monkeypox in the UK in May 2022, there has been an upward trend in monkeypox cases and a global outbreak. However, reports of severe cases are relatively limited. In this study, we report a case of severe monkeypox in a patient with HIV. The patient presented with skin lesions that started on his face and around the penis and persisted for several months. Throughout the course of the disease, he received systematic symptomatic supportive treatment, topical remedies, and special care for the rash. He also underwent cidofovir antiviral therapy and smallpox-vaccinated healthy population-derived plasma therapy in succession, with the condition ultimately showing improvement after plasma treatment. After more than 3 months of hospitalization, he fully recovered. To the best of our knowledge, this is the first reported use of smallpox-vaccinated healthy population-derived plasma in the treatment of severe monkeypox cases.
Abbreviations
-
- ART
-
- antiretroviral therapy
-
- CMV
-
- cytomegalovirus
-
- CT
-
- computerized tomography
-
- CT values
-
- cycle threshold value
-
- HIV
-
- human immunodeficiency virus
-
- mpox
-
- monkeypox
1 INTRODUCTION
According to the World Health Organization, as of 30 September 2023, a total of 91,123 cases of monkeypox (mpox), including 157 fatalities, have been reported across 115 countries and territories worldwide [1]. In September 2022, China reported its first imported case of the disease in Chongqing [2]. By June 2023, two indigenous cases were documented in Beijing. From then on, the disease began to emerge in other regions of China [3]. The current outbreak of mpox has a different clinical manifestation from the classic form, with lesions mainly localized in the anal and genital regions and high-risk sexual contact being the primary transmission route [4]. According to a multicenter cross-sectional study conducted in China, individuals living with HIV comprised 56.5% of the mpox population [3]. The overall mortality rate of the outbreak is approximately 1 per 1000. However, among individuals living with HIV, the mortality rate significantly increases to around 30% because of severe immune deficiency [5]. The severe immune depression caused by HIV and co-infections can disrupt and impede immune responses, resulting in continued mpox infection and multiorgan involvement. This explains why severe cases in HIV-positive individuals often exhibit disseminated and necrotizing manifestations instead of following the usual self-limiting course [4]. According to a cohort study from Nigeria, concurrent infection with varicella zoster virus and advanced HIV disease were identified as independent risk factors for severe illness [6]. It is not recommended to delay or withhold antiretroviral therapy (ART) for individuals with both HIV and mpox, despite concerns about immune reconstitution inflammatory syndrome. Instead, ART should be regarded as a crucial aspect of mpox management, especially for patients with advanced HIV [7]. Despite the limited effectiveness data for antiviral drugs such as cidofovir, brincidofovir, and tecovirimat in treating mpox, healthcare providers should still actively consider their use where they are available [5, 8]. We report the first successful treatment of severe monkeypox using plasma from a smallpox-vaccinated healthy population in a patient with HIV.
2 CASE REPORT
2.1 General condition of the patient prior to and upon the last admission
On June 25, 2023, a 29-year-old man presented with symptoms, including fever, peaking at 38.3°C, and muscle soreness. Subsequently, he developed scattered red papules on his face and around his penis, accompanied by itching and pain. As the rash worsened, he sought medical attention at a hospital in Shenzhen. Considering the patient's history of male-to-male sexual behavior, the hospital collected herpes fluid for mpox virus nucleic acid testing, which returned a positive result, confirming the diagnosis of mpox. On July 9, the patient was admitted to a specialized infectious disease hospital for further treatment.
The main clinical manifestations in this patient included facial and penile rashes as well as enlarged lymph nodes in the bilateral neck, axilla, and inguinal regions. During hospitalization, the patient experienced perianal pain, which was determined to be an anal abscess. Concurrently, the patient was diagnosed with HIV infection (HIV ≥ log6 IU/mL). Starting from July 20, the patient received bictegravir/emtricitabine/tenofovir propofol for HIV treatment. On July 22, the patient discharged himself from the hospital, as there was an improvement in the skin lesions. However, he returned on July 30 with right-eye swelling, tearing, and inability to open the eye. At this time, purulent discharge, conjunctival congestion in the right eye, and aggravated skin lesions on the face, head, perineum, anus, penis, and perianal region were observed (Figure 1b). Laboratory test results during this hospitalization showed HIV-RNA quantification of 7.02E+03 IU/mL and a CD4 T lymphocyte count of 103 cells/μL. The White Blood Cell (WBC) count, C-Reactive Protein (CRP), and Procalcitonin (PCT) were measured as 11.21 × 109/L, 55.1 mg/L, and 0.24 ng/mL, respectively (Table 1).
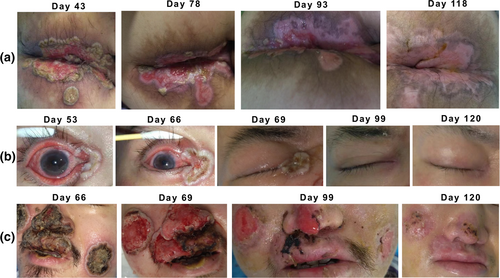
The dynamic changes in skin lesions of the patient throughout the course of the illness. Representative images recorded throughout the course of the illness have been selected carefully and to the best of our knowledge. The dynamic evolution reveals the healing processes for the perianal rash and ulceration (a), in the right eye (b), and in the facial region (c).
| Dates | Jul 10 | Jul 12 | Jul 21 | Jul 31 | Aug 10 | Aug 13 | Aug 17 | Aug 21 | Aug 25 | Aug 27 | Aug 30 | Sep 1 | Sep 2 | Sep 3 | Sep 6 | Sep 13 | Sep 16 | Sep 19 | Oct 1 | Oct 8 | Oct 17 | Oct 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC(10^9/L) | 7.64 | N/A | 4.52 | 11.21 | 5.58 | 1.85 | 2.43 | 2.12 | 2.80 | 3.62 | 1.89 | 36.86 | 29.66 | 14.62 | 5.53 | N/A | N/A | 4.31 | 1.84 | 1.22 | 2.44 | 2.60 |
| N(10^9/L) | 5.49 | N/A | 2.40 | 9.12 | 4.05 | 1.12 | 1.20 | 0.85 | 0.72 | 1.15 | 0.61 | 32.11 | 25.86 | 10.87 | 2.94 | N/A | N/A | 2.46 | 0.83 | 0.42 | 1.15 | 0.79 |
| L(10^9/L) | 1.64 | N/A | 1.45 | 1.31 | 0.91 | 0.43 | 0.70 | 0.78 | 0.69 | 0.45 | 0.67 | 2.34 | 1.67 | 1.65 | 1.21 | N/A | N/A | 0.86 | 0.81 | 0.44 | 0.74 | 1.03 |
| CRP(mg/L) | 14.7 | 47.5 | 19.9 | 55.1 | N/A | 47.8 | N/A | 78.5 | 95.3 | 77.18 | 20.74 | N/A | 15.84 | N/A | N/A | 18.0 | 53.5 | 86.1 | 3.35 | 7.19 | N/A | N/A |
| PCT(ng/mL) | 0.02 | 0.06 | 0.16 | 0.24 | N/A | 0.21 | N/A | 0.52 | N/A | 0.23 | 0.14 | N/A | 0.08 | N/A | N/A | 0.22 | 0.15 | N/A | 0.05 | N/A | N/A | N/A |
| IL-6(ng/mL) | N/A | 43.7 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 89.2 | N/A | N/A | N/A | 27.7 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
- Abbreviations: CRP, C-Reactive Protein; IL-6, Interleukin-6; L, Lymphocyte; N, Neutrophil; N/A: not available due to no examination conducted for this item on that day; PCT, Procalcitonin; WBC, White Blood Cell.
2.2 Treatment process: Empirical systemic and local application of antimicrobial agents, along with symptomatic supports at the early stage
To address the severe skin, mucosal, and soft tissue infections, the medical team initiated empirical intravenous administration of metronidazole and piperacillin–tazobactam. Mupirocin was topically applied for the skin infection. Following consultation with ophthalmologists, ganciclovir ophthalmic gel and levofloxacin eye drops were administered to the right eye. In accordance with surgical consultation, compound huangbai liquid and potassium permanganate sitz bath were provided for the perianal lesions. Regrettably, the patient's skin and mucosal lesions did not exhibit significant improvement, and the perianal erosion worsened. This was confirmed by a perianal ultrasound, which revealed the presence of an abscess. On August 12, the patient developed a fever with a peak temperature of 38.6°C. At that time, the WBC count, CRP, and PCT were measured as 1.85 × 109/L, 47.7 mg/L, and 0.21 ng/mL, respectively (Table 1). Blood culture yielded no positive findings, and there were no notable changes in inflammatory markers. Similarly, secretion cultures did not reveal any specific bacteria or fungal pathogens.
2.3 Treatment process: Mpox antiviral therapy
Polymerase chain reaction testing revealed the presence of mpox virus in samples obtained from pharyngeal swabs, blister fluid, anal swabs, and saliva, suggesting the presence of ongoing viral viremia. In response to this, the medical team commenced compassionate use of intravenous cidofovir on August 14 to August 21. Fortunately, no infusion-related adverse events were observed. However, the patient's fever persisted, reaching a peak temperature of 39.5°C, and the severity of the skin and mucosal lesions worsened.
2.4 Treatment process: Adjusted systemic application of antimicrobial agents
Considering the patient's immunodeficiency, concomitant skin infection, and the possibility of bloodstream infection, fungal infection could not be ruled out. Consequently, the antibiotic regimen was modified to include linezolid and ertapenem to cover both gram-positive cocci and gram-negative bacilli, along with voriconazole for antifungal treatment. A bone marrow puncture was subsequently performed to exclude hematological and neoplastic diseases. The bone marrow smear revealed no evidence of such conditions. Because of the extremely elevated levels of lactate dehydrogenase and ferritin, excisional biopsy of the right inguinal lymph nodes was performed. Lymph node pathology suggested there was reactive proliferation due to HIV-associated Cytomegalovirus (CMV) and Epstein–Barr virus infections (Figure 2). Despite the broad-spectrum antibiotic, antifungal, and antiviral treatments, the patient's fever persisted, and the skin and mucosal lesions on the face, perineum, anus, and penis showed no signs of improvement. On August 26, the patient received an immunoglobulin infusion, which unfortunately resulted in the development of erythematous maculopapular lesions on the face, anterior chest, and proximal upper limbs, indicating the possibility of a drug allergy. To address this, the medical team prescribed dexamethasone for anti-allergic treatment and discontinued all previous anti-infective medications, except fosfomycin, which was continued for treatment.
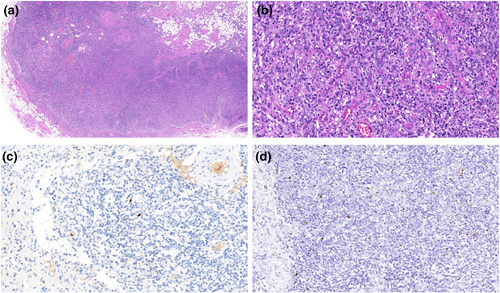
Pathological examination of the right inguinal lymph node biopsy. (a) HE staining pathological findings: Degeneration of the renal tubules, a significant decrease in the number of renal tubules, proliferation of tissue cells, and visible postcapillary venous proliferation. (b) HE staining pathological findings: A significant increase in tissue cells and infiltration of lymphocytes, plasma cells, and immune precursor cells. (c) Immunohistochemical findings: Viral inclusion bodies in brownish-yellow were found within tissue cells and confirmed as Cytomegalovirus infections. (d) In situ hybridization findings: Viral inclusion bodies in brownish-yellow were found within tissue cells and confirmed as Epstein–Barr virus infections.
To further elucidate any potential unidentified pathogens, a high-throughput genetic test for pathogen detection was conducted on the patient's blood sample on August 27. The results revealed a large number of mpox virus sequences, as well as CMV and Leptospiral virus sequences. The skin secretion culture demonstrated the presence of Enterococcus faecium. Therefore, the medical team utilized foscarnet sodium and contezolamide for antimicrobial treatment.
2.5 Treatment process: Plasma therapy
Despite the patient's body temperature gradually returning to normal after September 1, the elimination of the mpox virus remained challenging because of the patient's severely compromised immune status and multiple concurrent infections. Furthermore, there was no discernible improvement in the systemic lesions. Following thorough consultation with the patient and the patient's family, a decision was made to explore compassionate treatment using plasma obtained from a healthy population that had been vaccinated against smallpox. To identify suitable plasma donors, the patient's blood type was determined, and plasma from volunteers born before 1975 who shared the same blood type and had received smallpox vaccination was selected. Subsequently, various plasma samples were evaluated for their inhibitory activity against the mpox virus using a cell infection model. Ultimately, the plasma sample with the highest inhibitory activity was chosen for treatment. A total of 20 plasma samples, each exhibiting high titers of neutralizing antibodies against the smallpox virus, were pooled together, pre-treated, and administered as three separate doses on September 3, September 4, and September 5. Importantly, no adverse events related to the plasma infusion were observed. Following the plasma infusion, subsequent testing revealed an upward trend in mpox virus nucleic acid cycle threshold (CT) values in the patient's blister fluid and anal swab (Figure 3).
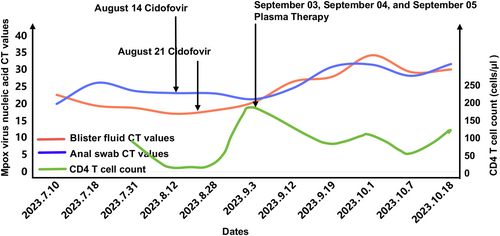
Trends in viral load and CD4 T-cell count change throughout the course of the illness. Throughout the course of the illness, the CT values of monkeypox nucleic acid PCR tests and CD4 T cell counts from patients were collected to showcase the dynamics of both factors during the entire treatment process. By analyzing the dynamic changes in these indicators, the efficacy of the patient's treatment and the progression of the disease was determined. PCR, Polymerase chain reaction.
2.6 Treatment process: Special care for skin lesions
To address the patient's severe and non-healing systemic skin lesions, a comprehensive treatment approach was implemented. Daily dressing changes, potassium permanganate sitz baths, and perianal irrigation were performed. The pressure sore team changed dressings every other day and carefully removed crusts from the face. Topical medications, such as human epidermal growth factor gel, polymyxin B ointment, and compound huangbai liquid, were applied to the skin lesions, while infrared irradiation was utilized to maintain dryness. For the lesion in the right eye, daily conjunctival sac irrigation was performed, and levofloxacin eye drops, sodium hyaluronate eye drops, and ganciclovir ophthalmic gel were administered. Additionally, the patient received a multivitamin supplement and active nutritional support. As a result of this comprehensive treatment approach, the patient's skin rash exhibited significant improvement, with some mpox skin lesions beginning to scab over and the red maculopapular rash subsiding (Figure 1).
On September 16, the patient experienced another episode of fever, although there were no significant clinical symptoms, and the skin rash and ulcers did not worsen. Chest CT revealed ground-glass opacities, suggesting the possibility of Pneumocystis pneumonia infection. Despite the challenge posed by a severe facial rash that covered the entire nasal cavity and hindered bronchoscopy examination, the medical team decided to initiate empirical treatment with sulfamethoxazole–trimethoprim and ganciclovir. Fortunately, the patient's body temperature returned to normal from September 20, and subsequent chest CT showed resolution of the lesions. Moreover, the skin rash on the face and trunk successfully scabbed over without any new rashes emerging. However, the patient continued to experience blurred vision in the right eye, leading to the introduction of sodium hyaluronate eye drops. Ultimately, the patient demonstrated marked improvement and was discharged on October 24.
3 DISCUSSION
The current outbreak is generally mild in severity, with symptoms typically resolving on their own after 2–4 weeks of bed rest and supportive treatment [9, 10]. In this case, the patient had initially mild symptoms that improved after treatment in the fourth week. However, the patient's condition deteriorated significantly by the 5th week, manifesting with ocular involvement, severe skin and mucosal erosions, and necrosis. These severe, newly appearing rashes may have been due to secondary mpox viremia or related to immune reconstitution syndrome after ART initiation. Some studies have suggested that severe mpox lesions may be considered opportunistic infections in late-stage HIV [11], and the appropriate timing of ART initiation needs to be further clarified in such hypothetical scenarios.
The patient presented with an uncertain fever, and possible causes, such as unidentified infections or immune reconstitution inflammatory syndrome, were considered after ruling out blood disorders and tumors. Antibacterial, antifungal, and antiviral therapies were administered for the mpox infection. Despite using cidofovir, there was no significant change in viral load or improvements in fever or inflammatory markers. As the disease progressed and the viral load remained unchanged, empathetic therapy was initiated. After the transfusion of fresh frozen plasma with variola antibodies, subsequent tests showed an increase in mpox virus nucleic acid CT values, suggesting a potential anti-mpox effect of the antibodies. Based on our current understanding, this report is the first documentation of clinicians successfully treating severe mpox using plasma from healthy individuals vaccinated against smallpox. In contrast, other published reports on severe cases indicate that individuals at risk of severe disease, such as immunosuppressed individuals, could potentially benefit from antiviral treatments such as tecovirimat [8]. Therefore, further large-scale clinical research is needed to conduct a systematic evaluation of the efficacy of antiviral drugs such as cidofovir and tecovirimat against monkeypox virus.
In the later stages of hospitalization, the patient's condition significantly improved. Based on our clinical observations, plasma therapy appeared to play a game-changing role in his successful treatment. Additionally, the enhanced immune response from HIV suppression and increased CD4+ T lymphocyte count following ART may have contributed to the positive outcome. Meticulous lesion site management and appropriate anti-infective treatment also played a substantial role in the patient's recovery. Randomized controlled trials are needed to evaluate the safety and efficacy of plasma with variola virus antibodies for treating patients with mpox.
In summary, we present a severe case of mpox with late-stage HIV co-infection. After initiating ART, the patient experienced severe symptoms, including ocular lesions, extensive skin and mucosal ulceration, and necrosis, as well as secondary infections. With a comprehensive treatment involving supportive therapy, antiviral treatment, plasma therapy, topical remedies, and other interventions, the patient's condition significantly improved, leading to successful recovery. Challenges included managing skin and soft tissue infections as well as preventing hospital-acquired infections. To improve outcomes for severe cases, further research and development of effective treatment strategies are necessary.
AUTHOR CONTRIBUTIONS
Clinical management and follow-up: Chuming Chen, Weiming Yao, Siran Huang, Ling Peng, Shiyan Feng, Weibo Wu, and Liuqing Yang. Diagnosis and expert opinions on the patient management: Hongzhou Lu and Fuxiang Wang. Laboratory diagnosis and plasma screening: Yunlan Yi, Zhiqiang Cheng, Yang Yang, and Yan Yang. Funding acquisition: Hongzhou Lu and Fuxiang Wang. Supervision: Hongzhou Lu and Fuxiang Wang. Writing – original draft: Chuming Chen, Weiming Yao, Siran Huang, Ling Peng, and Shiyan Feng. Writing – reviewing and editing: Chuming Chen and Weiming Yao. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We sincerely appreciate the work of all the laboratory physicians, nurses, and related staff involved in the diagnosis and treatment of the patients in our hospital. This work was supported by the National Key Research and Development Program of China (2022YFC2304403, 2022YFC2304404), the National Natural Science Foundation of China (81971915, 82272318), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No. SZGSP011), and Science and Technology Research Projects of Shenzhen (JSGG20220606141001003).
CONFLICT OF INTEREST STATEMENT
Professor Hongzhou Lu is the Editor-in-Chief of the iLABMED. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining authors declare no conflicts of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
ETHICS STATEMENT
This case report presents the clinical information of an individual patient while ensuring the privacy and confidentiality of the patient by not disclosing any identifying details.
INFORMED CONSENT
Informed consent was obtained from the patient for the publication of this case report.
DECLARATION OF GENERATIVE AI AND AI-ASSISTED TECHNOLOGIES IN THE WRITING PROCESS
During the preparation of this work, the authors used Assistant-Poe (Website: https://poe.com/) in order to write clearly, coherently, and concisely in the manuscript. After using this tool, the authors reviewed and edited the content as needed, and they take full responsibility for the content of the publication.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.




