Current Advancements in Serum Protein Biomarkers for Hepatitis B Virus-Associated Hepatocyte Remodeling and Hepatocellular Carcinoma
ABSTRACT
Background
Hepatitis B virus (HBV)-related liver cancer is the third most common cause of cancer-related death globally. Hepatocyte remodeling, also known as hepatocyte transformation and immortalization, and hepatocellular carcinoma (HCC), are brought on by persistent inflammation caused by HBV in the host hepatocytes. One of the main concerns in the perspective of HBV-induced hepatocyte remodeling and liver cancer is accurately identifying cancer stages to maximize early screening and detection. Biological signatures have a significant impact on solving this problem.
Objective
This review article aimed to discuss the novel serum protein biomarkers for HBV-induced hepatocyte remodeling and HCC.
Methods
The information was collected from various peer-reviewed journals through electronic searches utilizing various search engines, including PubMed, Google Scholar, HINARI, and Cochrane Library from 2017 to 2024. Keywords for searches included “serum protein biomarkers in HBV-HCC,” “blood-based biomarkers in HBV-HCC,” and “viral biomarkers for HBV-HCC.”
Results
Recently, novel protein signatures have been discovered for the early diagnosis, treatment, and prognosis of HBV-induced hepatic cell remodeling and HCC from proteomic data sets. We have discussed the recent literature on the clinical utility of the protein signatures for the diagnosis and forecasting of HBV-associated hepatocyte remodeling and HCC, including golgi protein 73 (GP73), glypican-3 (GPC3), midkine (MDK), des-γ-carboxy-prothrombin (DCP), von Willebrand factor (vWF), pentraxin 3 (PTX3), pseudouridine synthases 7 (PUSs 7), squamous cell carcinoma antigen (SCCA), and osteopontin (OPN).
Conclusion
All these protein markers also exhibit the survival of HBV-related HCC patients, the proliferation, migration, antiapoptosis, mitogenesis, transformation, and angiogenesis of HBV-infected hepatocytes.
1 Introduction
Worldwide, hepatocellular carcinoma (HCC) caused by the hepatitis B virus (HBV) accounts for around 90% of instances of primary liver cancer. Liver cancer caused by HBV is the second most common cause of death from cancer [1]. These days, HCC is on the rise and could soon account for one million cases annually [2]. Patients with chronic HBV infection had a nearly 100-fold higher chance of developing HCC, according to prospective cohort studies [3, 4].
HCC may arise from persistent hepatocyte inflammation caused by an HBV infection. Hepatocyte damage and persistent inflammation might result from a prolonged immunological response to HBV infection in the liver [5]. This never-ending cycle of inflammation, regeneration, and cell damage can cause genetic alterations and mutations in the liver cells, which raises the possibility of developing HBV-HCC [6]. The transition from chronic HBV infection to HCC involves an intricate interplay between viral components, host immunological responses, and environmental effects [7].
Hepatocyte remodeling describes the anatomical and functional alterations in liver cells, usually due to liver illness or persistent HBV infection. These alterations may affect hepatocytes’ interactions with the immune system in the instance of HBV-HCC [8]. There is proof that prolonged viral replication and inflammation in chronic hepatitis B infection can change hepatocyte signaling networks and gene expression patterns [9].
In addition, some of the immediate causes of hepatocyte remodeling and HCC are HBV-encoded proteins’ transcriptional activation of differentiation-regulating genes, HBV DNA integration to the hepatocyte's genome, viral mutations, and genomic instability, cellular death, stimulation of signaling networks that cause the liver cell malignancy, and DNA repair [10].
During HBV-related hepatocyte transformations, signals from hepatic stellate cells are coupled with extracellular matrix remodeling and the proliferation of cell types resembling myofibroblasts. HBV-HCC formation is a convoluted process involving connections across multiple genes and stages. Together, several mechanisms that promote cancer accelerate the disease's transition from inflammation to carcinogenesis [11]. There are indications that the HBV virus's altered protein products hasten the development of hepatocyte remodeling and hepatic cancer. Accurate assessment of HBV-induced hepatocyte remodeling and prediction of HCC biomarkers aid in the early identification of HBV-HCC and reduce mortality [12].
Current clinical uses of imaging methods like magnetic resonance imaging (MRI), computed tomography (CT) scans, and ultrasound are principally used for the diagnosis of HBV-HCC [13]. These techniques are useful for identifying liver tumors and determining their location and size. The diagnosis of HCC can also be aided by blood tests that evaluate the levels of particular biomarkers, such as AFP [14]. The early diagnosis and treatment of HCC have greatly improved via these diagnostic techniques. Improving patient outcomes requires prompt intervention and treatment, which they make possible [15].
However, these methods have poor sensitivity, specificity, and accuracy. For instance, the overall sensitivity of MRI, CT, and ultrasound to HCC is only 62%, 48%, and 46%, respectively [16]. Hence, there is a need for increased sensitivity, specificity, and accuracy in the diagnosis of HBV-HCC. Thus, the serum protein biomarkers are being investigated to overcome these problems. Moreover, various types of proteomic signatures have been investigated to indicate HBV-associated hepatocyte remodeling and HCC. This review will summarize recent advances in the role of those signatures in HBV-induced hepatocyte remodeling and HCC.
2 Methods
The information was collected from various peer-reviewed journals through electronic searches utilizing various search engines, including PubMed, Google Scholar, HINARI, and Cochrane Library from 2017 to 2024. Primary studies and meta-analyses were included in this review article. Keywords for searches included “serum protein biomarkers in HBV-HCC,” blood-based biomarkers in HBV-HCC,” and “viral biomarkers for HBV-HCC.” The studies involved were limited to the English language. On the other hand, papers written in languages other than English, papers published before 2017, and review articles were excluded from this review.
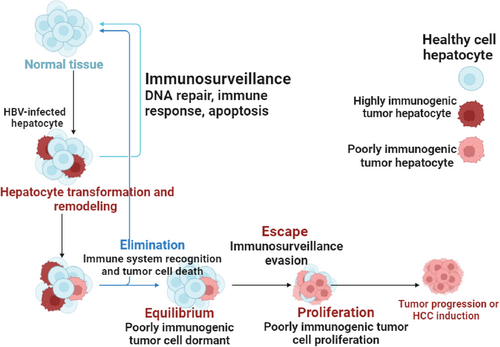
3 Overview of the Immunosurveillance of HBV-HCC and Hepatocyte Remodeling
Immunosurveillance, as it relates to HBV-HCC, is the immune system's tracking and identification of liver cells (hepatocytes) that have become malignant due to HBV infection. The immune system is essential in identifying these aberrant cells and making an effort to get rid of them [17]. On the other hand, chronic HBV infection in HBV-HCC patients may result in immunological suppression or fatigue, thereby undermining the efficacy of immunosurveillance. This may contribute to the initiation and spread of HCC by enabling malignant hepatocytes to avoid immune system recognition and multiply [18].
It is crucial to comprehend the role that immunosurveillance plays in HBV infection to devise methods of boosting immune responses against malignant cells, which could result in better therapies for this kind of liver cancer [19]. Immunosurveillance in HBV-HCC refers to the immune system's capacity to identify and attack hepatocytes that have become malignant due to long-term HBV infection [20]. Moreover, T cells are essential for immunosurveillance processes because they can identify aberrant antigens transported by malignant cells. On the other hand, chronic exposure to inflammatory signals and viral antigens may lead to T-cell depletion or malfunction in the hepatic microenvironment [21]. Immunosurveillance against HBV-infected hepatocytes, which have undergone oncogenic transformation, may be hampered by T-cell malfunction. In the HBV-HCC, apoptosis can eliminate infected hepatocytes, reducing viral replication and preventing the spread of damaged cells. However, HBV has developed strategies to evade apoptosis, which can prolong infection and promote tumor growth [22].
The balance between tumor growth and immune responses is referred to as equilibrium. In the case of HBV-HCC, a state of balance is necessary to regulate viral replication and prevent excessive inflammation that could cause tissue damage. Immune cells such as T cells and natural killer (NK) cells are essential for identifying and eliminating infected hepatocytes [23, 24]. This equilibrium is maintained by a well-regulated immune response, which allows for effective viral control without causing significant damage to hepatocytes. On the other hand, the proliferation of immune cells in response to HBV infection and the subsequent emergence of HCC are significant processes [25].
The expansion of specialized immune cells, particularly cytotoxic T lymphocytes (CTLs), is crucial for targeting and eliminating diseased or malignant hepatocytes. However, unchecked proliferation can lead to liver damage and chronic inflammation, both of which may accelerate the development of HCC [26]. In addition, maintaining genomic stability requires DNA repair mechanisms, especially in hepatocytes that are frequently exposed to HBV infection. Genomic instability and mutations can result from HBV's integration into the host genome. To repair these damages and prevent the accumulation of mutations that may lead to cancer, DNA repair processes such as homologous recombination and nucleotide excision repair are activated [27]. Moreover, tumor-induced changes to the extracellular matrix and architecture of the liver can produce physical barriers that obstruct efficient immune responses to HCC [28] (Figure 1).
4 Overview of the Role of Immune Cells in the Tumor Microenvironment of HBV-HCC and Hepatocyte Remodeling
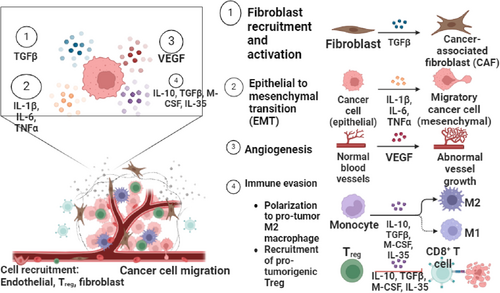
Immune cells are essential for controlling the hepatocyte remodeling and tumor microenvironment during HBV-HCC [29]. Chronic HBV infection can result in a complicated interaction between growth factors, cytokines, and immune cells in the liver, which eventually aids in the development of tumors as well as immune evasion [30]. In particular, vascular endothelial growth factor is in charge of angiogenesis, which is necessary for the growth of HCC. By promoting the development of new blood vessels essential for tumor survival and proliferation, VEGF overexpression in HBV-HCC is associated with increased tumor growth and metastasis [31].
Elevated levels of circulating VEGF have been identified in patients with HCC, and these levels correlate with increased tumor microvessel density and poorer prognosis. Studies have consistently shown that high VEGF levels, both in tissue and serum, are significant predictors of poor overall survival and disease-free survival in HCC patients. Additionally, transforming growth factor-beta (TGF-β) plays a critical role as a regulator of liver fibrosis and immunosuppression. It is also involved in activating fibroblasts and promoting the synthesis of extracellular matrix components, contributing to the progression of liver disease and tumor development [32, 33]. In addition, cell migration and invasion are promoted by the over activation of the TGF-β signaling pathway [34].
Furthermore, pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) can induce angiogenesis and exacerbate hepatocyte damage within the tumor microenvironment [35]. While M-CSF promotes the differentiation of macrophages from monocytes into tissue-resident macrophages involved in tissue repair within HCC tumors, IL-10 suppresses effector T-cell responses in the tumor microenvironment, thereby limiting antitumor immunity [36, 37]. Moreover, how these immune cells interact with stromal elements like fibroblasts shapes the HBV-HCC microenvironment by regulating inflammatory responses and the promotion or suppression of angiogenesis, which in turn affects the disease's progression or regression to a great extent.
Both the immunological response to HBV and the development of HCC are influenced by TNF-α. Its role as a pro-inflammatory cytokine highlights the delicate balance between the potential for liver damage that can lead to cancer. Moreover, the formation and progression of HBV-HCC heavily depend on epithelial-mesenchymal transition, which is triggered by viral proteins such as HBX. These proteins alter cellular signaling pathways and promote a more aggressive tumor phenotype [38]. In addition, the formation and progression of HBV-HCC are significantly influenced by cancer-associated fibroblasts due to their roles in immune evasion, regulation of the tumor microenvironment, stimulation of tumor-initiating cells, and heterogeneity [39].
In the context of HBV-HCC, interleukin-35 (IL-35) plays a critical role in modulating the immune response, particularly through its effects on regulatory T cells (Tregs). Tregs release IL-35, which contributes to immune evasion by promoting T cell exhaustion, including that of CD8+ T cells [40, 41]. As a result, this leads to decreased antitumor immunity. Meanwhile, the balance between M1 and M2 macrophages is important; M1 macrophages are related to pro-inflammatory responses that can inhibit tumor progression, whereas M2 macrophages promote an immunosuppressive environment conducive to tumor development [42, 43]. Furthermore, CD8+ T cells, which are crucial for targeting HBV-infected hepatocytes, often become exhausted due to persistent HBV infection and the presence of Tregs and M2 macrophages [24, 44] (Figure 2).
5 Overview on the Role of Protein Markers in HBV-Related Hepatocyte Remodeling
Protein markers play a multifaceted role in HBV-related hepatocyte remodeling, offering crucial insights into the infection's status, the extent of liver damage, and the risk of progression to more severe liver diseases [45]. Understanding these markers enhances diagnostic strategies, facilitates monitoring of disease progression, and aids in the development of targeted therapies for more effective management of HBV-related liver disease [46]. Through proteomic analysis or other applicable approaches, unique protein markers associated with hepatic remodeling during HBV infection should be identified and verified [47].
The functional roles of these protein indicators are significant, as they are involved in key biological processes such as cellular proliferation, apoptosis (programmed cell death), inflammation, fibrosis (the formation of excess connective tissue), and other mechanisms that contribute to the remodeling of hepatocytes (liver cells) [48]. The effect of these proteins on hepatocyte remodeling can be further elucidated by using molecular biology approaches such as gene silencing or overexpression. Significant evidence of these protein markers’ functional involvement can be obtained by correlating their expression levels or activities with clinical parameters, including liver function tests or disease progression in patients infected with HBV [49].
6 Protein Glycosylation in HBV-HCC
Protein glycosylation has been identified as a critical factor in the development and progression of HCC in the context of HBV infection, exhibiting both tumor-promoting and tumor-suppressing effects. Abnormal glycosylation patterns, such as altered N-glycosylation and O-glycosylation, are frequently observed during HBV-HCC progression and are implicated in the malignant transformation of hepatocytes [50]. These modifications can promote tumor growth by enhancing protumorigenic signaling pathways, facilitating immune evasion, increasing metastatic potential, and contributing to resistance against therapies. For example, dysregulated glycosylation can alter cell-cell interactions and adhesive properties, which are crucial for tumor invasion and metastasis [51].
Conversely, certain glycosylation changes may confer tumor-suppressive effects. For instance, increased α2,6-sialylation has been linked to reduced metastatic capabilities in some HCC cell lines. This duality highlights the importance of the specific context of glycosylation alterations in determining their overall impact on HCC outcomes [52]. Moreover, studies have shown that HBV itself can manipulate glycosylation processes to enhance its replication and immune evasion while contributing to carcinogenesis. For example, N-linked glycosylation in HBV surface proteins has been associated with virion assembly, immune escape, and carcinogenic processes. Understanding the complex dynamics of protein glycosylation not only provides insights into the mechanisms underlying HBV-HCC but also offers potential avenues for developing novel diagnostic markers and therapeutic strategies targeting glycan modifications [53].
Moreover, O-GlcNAcylation stabilizes oncogenic proteins, such as YTHDF2 and SLC35B4, enhancing their activity and driving tumorigenesis in HBV-HCC. Elevated O-GlcNAcylation levels promote the stabilization of mRNA transcripts for cell cycle regulators, contributing to HCC development. For example, O-GlcNAcylation of proteins, such as YTHDF2, has been shown to enhance their stability and oncogenic activity, thereby promoting tumorigenesis in HBV-HCC [54]. Similarly, SLC35B4-mediated O-GlcNAcylation stabilizes c-MYC protein, which is critical for HCC proliferation and migration [55]. Targeting OGT, the enzyme responsible for O-GlcNAcylation, has shown promise as a therapeutic strategy; its inhibition via compounds like OSMI-1 effectively suppresses HBV-HCC progression in preclinical models. Understanding the role of O-GlcNAcylation in HBV-HCC underscores its potential as a target for novel therapeutic interventions [56].
In addition, protein glycosylation often undergoes significant changes as cancer cells proliferate in HBV-HCC. Specifically, the initiation, development, and metastasis of tumors have been shown to be facilitated by alterations in glycan macro-heterogeneity and microheterogeneity in glycoproteins primarily produced by the liver [57].
Furthermore, modifications on the glycosylation pattern can affect how proteins related to the genesis of cancer function and signaling [58]. In addition, changes in cellular glycosylation give cells adaptive advantages during tumor progression [59]. Tumor progression may be impacted by inhibition of enzymes involved in the manufacture of aberrant glycans linked to malignancy [60].
7 Selection Criteria for Appropriate Protein Signatures for HBV-HCC
Several criteria must be carefully considered while choosing protein biomarkers for HBV-HCC to guarantee their efficacy and dependability for therapeutic, prognostic, and diagnostic uses [61]. Selecting protein biomarkers for HBV-HCC requires meeting several important criteria, including specificity, which determines whether HBV-HCC is present; sensitivity, which determines whether early-stage HCC or minimal residual disease after treatment is detected; predictive value for prognosis and treatment response; stability, which links expression levels to relevant clinical parameters like tumor stage and patient survival outcomes; and reproducibility, which can be achieved across different laboratories and experimental settings using standardized protocols and assay kits [62, 63].
When assessing protein markers in HBV-HCC, diagnostic accuracy is crucial, and the area under the curve (AUC) is a useful metric to evaluate overall performance. Cohort sizes in studies assessing protein markers vary often, although bigger cohorts typically yield more accurate diagnostic accuracy estimates [64].
For instance, a recent large-scale cohort of 500 patients with HBV-HCC and 500 healthy controls demonstrated high diagnosis accuracy in a study looking into a protein marker for HBV-HCC. The protein marker showed an important discriminatory ability in separating HCC from nonmalignant conditions with an AUC of 85%, and sensitivity and specificity of ≥ 80%. The detection method employed, the enzyme-linked immunosorbent assay (ELISA), ensures applicability in medical settings. These results demonstrate the potential utility of the protein biomarker as a diagnostic tool for HBV-HCC surveillance and screening initiatives [65].
8 Key Serum Protein Biomarkers for HBV-Associated Hepatocyte Remodeling and HCC
8.1 Alpha-Fetoprotein (AFP)
AFP is one of the most commonly used biomarkers for diagnosing HCC in patients with chronic HBV infection. Moreover, AFP is most commonly recognized as an oncofetal glycoprotein that has been used in conjunction with imaging modalities, such as ultrasound, to improve the diagnosis of HCC [66]. However, its effectiveness is limited by issues related to sensitivity and specificity, which can be influenced by patient characteristics and the varying cut-off values employed for differential diagnosis. Recent studies have identified several limitations related with AFP, including its relatively low sensitivity and high false-positive rate, particularly in cases of HBV-HCC. For example, a study demonstrated that an AFP cutoff of 400 ng/mL yielded a sensitivity of only 51.3% and a specificity of 87.8% for detecting HBV-HCC. Furthermore, combining AFP with other biomarkers, such as des-gamma-carboxyprothrombin, has been shown to enhance diagnostic performance, achieving a combination sensitivity of 55.6% and a specificity of 95.6% [67, 68]. Despite its established role, the effectiveness of AFP is complicated by its elevation in a wide range of liver diseases, highlighting the need for ongoing evaluation of additional biomarkers and combination strategies to improve early detection and management of HCC in patients with chronic HBV infection [69].
8.2 Golgi Protein (GP73)
Transmembrane glycoprotein GP73 is a component of the Golgi apparatus. GP73 is expressed by hepatocytes in HBV-HCC. Hepatic inflammatory activity may be the cause of elevated GP73 levels. The amount of GP73 is elevated in patients with HCC and may be a target for therapy. Its sensitivity and specificity for predicting HBV-HCC are 69% and 75%, respectively, which is far better than AFP's (62% and 25%) detection of early HCC in the same group [70]. The role of GP73 in HCC carcinogenesis is not well discussed, although GP73 has been the subject of numerous research focusing on its use as a marker for early HCC detection [71].
A deeper comprehension of GP73's function in HCC may offer a novel therapeutic target for the disease, considering the low response to the HCC treatments that are now available. According to immunohistochemistry investigations, GP73 protein expression in the liver increased steadily with pathologic progression during persistent HBV infection [72].
In other words, the disease severity of chronic HBV infections was positively linked with serum GP73 concentrations. Since variations in the severity of liver injury are strongly correlated with changes in serum GP73 levels, GP73 may be a valuable novel biomarker for inflammatory liver injury and may be useful for tracking the prognosis of chronic HBV infection. Furthermore, the trend of GP73 fluctuation in chronic liver disease may suggest that serum GP73 monitoring is useful for identifying cirrhosis in populations with chronic HBV infection [73]. GP73 expression is also noticeably increased in HBV-HCC tissues. In vitro, GP73 expression in primary human hepatocytes and hepatoma cells may be induced by ectopic expression of the HBV gene. In hepatoma cells, GP73 encourages HCC. The host's innate immune responses are suppressed in hepatocytes by the ectopic expression of HBV genes, which enhances GP73 expression [74].
8.3 Glypican-3 (GPC3)
A member of the glypicans family, GPC3 attaches to the cell surface using glycosylated phosphatidyl inositol. GPC-3 is recognized as a significant marker for HCC due to its elevated expression in the liver during carcinogenesis and its absence in normal liver tissue [75]. For the identification of HBV-HCC, GPC3 can serve as a marker, demonstrating an AUC of 0.817, a sensitivity of 91.3%, and a specificity of 60.0%. Furthermore, aberrant GPC-3 expression serves as an independent prognostic factor for the survival of patients with HBV-HCC [76].
In other words, GPC-3 is typically overexpressed in malignant tissues while remaining undetectable in healthy liver cells and benign liver lesions. This unique expression pattern enhances its utility as both a diagnostic biomarker and a potential therapeutic target in HCC management. Studies have shown that high levels of GPC-3 correlate with aggressive tumor behavior and poorer patient outcomes, including reduced overall survival and disease-free survival rates. Through several cytokines, the GPC3 protein is closely linked to the body's growth and development. Plasma GPC3 may be a useful marker for identifying people who would benefit from immunotherapies that target GPC3 [77, 78]. Hence, GPC-3 can be targeted for a novel immunotherapy strategy that can specify cell-mediated destruction of neoplastic cells while sparing normal liver tissue [79].
Furthermore, a meta-analysis comparing the diagnostic performance of GPC-3 and AFP revealed significant findings regarding their sensitivity, specificity, and AUC. The pooled sensitivity and specificity were 55% and 58% for GPC-3, 54% and 83% for AFP, and 85% and 79% for the combination of GPC-3 and AFP. The AUC values were 0.7793 for GPC-3, 0.7867 for AFP, and a notably higher 0.9366 for the combined markers. These results indicate that while GPC-3 has comparable sensitivity to AFP, its specificity is lower; however, the combination of GPC-3 and AFP significantly enhances diagnostic accuracy, as demonstrated by the higher AUC for the combined marker compared to either marker alone [80].
Various immunotherapeutic protocols targeting GPC3 have been developed, including the use of humanized anti-GPC3 cytotoxic antibodies, treatment with peptide/DNA vaccines, and immunotoxin therapies [81]. As it may be essential for cell proliferation, metastasis, and colonization as well as mediating oncogenesis and oncogenic signaling pathways, GPC-3 is also a viable target molecule for HBV-related HCC gene therapy [82].
8.4 Midkine (MDK)
Heparin-binding growth factor/cytokine called MDK has several uses and is involved in many physiological processes like viability, cell movement, and other cell processes [83]. Rarely produced by normal tissues, MDK is markedly increased in inflammatory disorders and human malignant tumors, particularly HCC-HBV. Many types of solid tumors, including HCC, exhibit proliferation, migration, antiapoptosis, mitogenesis, transformation, and angiogenesis, and studies have shown that MDK plays a significant role in these processes. The antiapoptotic factor MDK promotes the survival of tumor cells. Additionally, MDK suppresses anoikis to encourage metastasis [84].
Anti-MDK methods may be applied with success in the treatment of HCC, according to some evidence. Additionally, MDK has been suggested as a biomarker in the prognosis and diagnosis of early HCC particularly in the serum negative AFP patients because of the high expression in HCC. The sensitivity, specificity, and AUC of MDK and AFP for identifying HCC were pooled using a random-effects model in the prior systematic review and meta-analysis. The following are the summary values for early-stage HCC detection using MDK and AFP: sensitivity, 83.5 versus 44.4%; specificity, 81.7 versus 84.8%; and AUC, 0.87 versus 0.52. In patients with HCC, this marker may be used as a prognostic indicator [85, 86].
8.5 Des-γ-Carboxy-Prothrombin (DCP)
DCP is increasingly recognized as a critical serum indicator for the diagnosis of HCC-HBV. In addition, DCP levels have been shown to associate with more aggressive tumor behavior and poorer clinical outcomes, making it not only a diagnostic tool but also a prognostic indicator [87]. After curative radiofrequency ablation (RFA), postablation serum levels of DCP serve as a crucial biomarker for predicting survival and recurrence in patients with HBV-HCC. The higher DCP levels following ablation are associated with worse prognosis and an increased likelihood of cancer recurrence. Specifically, individuals with elevated DCP levels after RFA tend to experience poorer overall survival and recurrence-free survival rates compared to those with lower levels [88].
DCP, also known as prothrombin induced by vitamin K absence or antagonist-II (PIVKA-II), is significant in clinical practice. PIVKA-II levels are elevated in HCC patients due to impaired prothrombin carboxylation in cancerous cells caused by a deficiency of vitamin K. This anomaly leads to the accumulation of PIVKA-II in the bloodstream, making it a valuable marker for HCC diagnosis and surveillance. Furthermore, the high levels of PIVKA‑II showed a more aggressive tumor phenotype, and they may also offer new insights into the process underlying the spread of HCC cells and make it easier to create new therapeutic approaches for patients with early HCC [89]. PIVKA-II outperformed AFP in the retrospective cohort study in terms of accuracy for diagnosing overall recurrent HBV-HCC (AUC: 0.883 vs. 0.672; p < 0.0001) [90].
8.6 Von Willebrand Factor (vWF)
VWF is found in platelets and endothelial cells and is essential for hemostasis and tissue damage. A study revealed that VWF's distinct functional structure and capacity could facilitate the metastasis of cancer. An earlier study found that when compared to the HCC group, the VWF plasma concentration was significantly lower in CHB (chronic hepatitis B) and healthy controls [91]. The overexpression of this biomarker was detected from the liver biopsy of patients with CHBV infection [92]. VWF is linked to hepatic spare capacity and HCC and has been linked to angiogenesis and apoptosis. VWF is a biomarker with the potential to be helpful in the diagnosis and prognosis of aggressive HBV infection-related HCC. Hepatic spare capacity and VWF antigen are linked because vWF-Ag is elevated in HCC patients. This pathology is connected to angiogenesis [93]. Disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (DAMTS13) regulates the size and activity of the VWF, and its absence can result in an expansion of the VWF [94].
Previous research suggests that vWF-Ag inhibition therapy should be considered as part of the treatment plan for patients with chronic hepatic inflammation. This approach may assist clinicians in streamlining monitoring methods [95]. Plasma vWF levels and in vitro scores can assess the degree of liver cirrhosis decompensation and the progression of liver disease in patients with HBV infection. They can also predict the development of various complications following liver cirrhosis decompensation and serve as a guide for early intervention strategies [96].
8.7 Pentraxin 3 (PTX3)
The relation between PTX3 and HCC may be affected by its function in the immune response. For instance, interleukin (IL)-1 and tumor necrosis factor (TNF)-α can generate PTX3 expression during HBV-HCC and hepatocyte immortalization [97]. Moreover, PTX3 is involved in the control of biological processes connected to HBV-HCC, including metastasis, angiogenesis, and proliferation. Studies on HCC have revealed that PTX3 may speed up the disease's development. Patients with HCC who have elevated PTX3 expression in tumor tissues have a bad prognosis. Serum PTX3 levels are related to the onset of HCC in HBV infection and are useful in the diagnosis of HCC, even AFP-negative HCC [98-100].
Depending on the study and technique, several diagnostic accuracy metrics such as AUC, sensitivity, and specificity may change. Data from patients with cirrhosis, HCC, and chronic hepatitis caused by HBV were evaluated, as well as data from healthy controls. By using an immunoassay to test the serum PTX3 concentration, it was found that patients with HBV had levels that were significantly greater than those of healthy controls, and patients with HCC had levels that were higher than those of those with chronic hepatitis or cirrhosis [100]. A lower overall survival time was seen in HCC patients with high serum PTX3 levels (PTX3 > 9.25 ng/mL) compared to HCC patients with low serum PTX3 levels (PTX3 9.25 ng/mL). Patients with HCC connected to HBV are prognostically predicted using serum PTX3 levels as a biomarker [101].
Elevated levels of PTX3 represent an independent risk factor for HBV-HCC. In patients with HCC, PTX3 levels appear to correlate with tumor differentiation. The significance of serum PTX3 levels lies in their ability to effectively differentiate HCC from chronic hepatitis, cirrhosis, and chronic HBV infection without HCC. Moreover, measuring PTX3 levels could provide substantial diagnostic value for HBV-HCC, particularly in cases that are AFP-negative and at early stages of the HCC [100]. With an AUC of 0.948, the combination of AFP and PTX3 enhanced the capacity to distinguish between early HCC and chronic HBV infection. In preclinical models and certain cancers, PTX3 is an extrinsic oncosuppressor by controlling complement-driven macrophage-mediated tumor growth and adjusting cancer-related inflammation [98, 100].
8.8 Pseudouridine Synthases (PUSs)
PUSs are emerging as a novel therapeutic target for HBV-HCC. Specifically, PUSs act as a glioma growth regulator that can be targeted, with higher expression levels associated with poorer patient survival outcomes. Furthermore, treatment with PUS7 inhibitors has been shown to slow tumor growth and extend the lifespan of mice with tumors [102]. Studies have shown that many PUS genes, such as DKC1, PUS1, and PUS7, are significantly upregulated in HBV-HCC tissues and are related with poor patient prognosis. These enzymes are involved in posttranscriptional RNA modifications, influencing key cellular processes such as metabolism, cell cycle regulation, and immune response. Elevated PUS expression has been identified as a potential diagnostic indicator for HBV-HCC and is implicated in disease progression [102, 103]. Additionally, their role in critical metabolic pathways highlights the potential of targeting PUSs as a new therapeutic approach for HBV-HCC treatment [103].
8.9 Squamous Cell Carcinoma Antigen (SCCA) and Antibodies Against SCCA/SERPINB3
Recent study has revealed that systemic concentrations of squamous cell carcinoma antigen-immunoglobulin M (SCCA-IgM) increase over time and may indicate the development of cirrhosis in individuals with chronic hepatitis. SCCA has been shown to prevent apoptosis in cancer cells in HBV-HCC, and its measurement has proven valuable in identifying individuals with HCC. Elevated SCCA-IgM levels can effectively distinguish HCC from chronic hepatitis, cirrhosis, and chronic HBV infection without HCC, highlighting its potential role in monitoring disease progression and guiding clinical management [104].
The ability of AFP to diagnose HCC is enhanced by SCCA by up to 90%. There is strong proof that SCCA activity rises gradually throughout the development of liver cancer, from chronic hepatitis to dysplastic tumors to HCC. SERPINB3, also known as squamous cell carcinoma antigen-1 (SCCA1), is a serine protease inhibitor that plays a dual role in cellular processes. While it protects cells from reactive oxygen species, its persistent expression can contribute to the development of HBV-HCC. SERPINB3 promotes oncogenesis by inhibiting apoptosis, facilitating the transition from epithelial to mesenchymal phenotypes, reducing desmosomal junctions, and enhancing cell proliferation and invasion. This multifaceted involvement in cancer progression underscores its potential as a therapeutic target, particularly in HBV-HCC, where its elevated levels may indicate disease severity and poor prognosis [105]. The most significant specific response for human SerpinB3 is demonstrated by anti-P#5 antibodies, which are generated by targeting the reactive helix of SerpinB3 [106].
HepG2 cells inducing SerpinB3 were used to test the biological efficacy of each immunoglobulin formulation. Anti-P#5 antibody decreased the spread of cells by 75% and multiplication by 12%, whereas the other immunoglobulin formulations produced negligible outcomes [107]. These results suggest that SerpinB3's responsive region is crucial for the intrusion properties this serpin induces, and it may develop into a potential druggable domain. SB3, which is generated by damaged or inflamed hepatic cells may function as an inflammatory mediator in HBV-HCC, accelerating the course of the illness. A single serum SCCA-IgM assay aids in identifying hepatic cirrhosis patients who are more likely to have HCC and die from it [108].
8.10 Osteopontin (OPN)
OPN is a highly modified integrin-binding glycoprotein found abundantly in various cells and tissues. It plays a significant role in cancer metastasis, cellular metabolism, tissue healing, and carcinogenesis. In particular, tumor organs such as the liver have been shown to overexpress OPN [109]. Moreover, increased circulating OPN concentrations in HBV-HCC have been linked to intrahepatic metastasis and the early stages of tumor development. OPN is frequently used as a biomarker to predict the progression of HCC, particularly in its initial stages. Its elevated levels are related with poor prognosis among individuals with HCC, suggesting that OPN may play a significant role in tumor marker-driven cancer progression [110].
OPN levels in the HBsAg-positive HCC population are significantly higher than those in HBsAg-negative individuals with other malignancies and in the unaffected control group. The absence of OPN is related with increased overall survival, reduced chronic inflammation, and a lower incidence of poorly differentiated tumors in HCC. Furthermore, OPN outperformed AFP in terms of diagnostic sensitivity, specificity, and overall accuracy for HCC patients compared to the cirrhotic group (97%, 70%, and 84% at the 90 ng/mL cut-off value for OPN vs. 90%, 63%, and 77% at the 5.5 ng/mL cut-off value for AFP) [111, 112] (Table 1). The major protein signatures with their respective diagnostic accuracy are summarized in Table 1 below.
| Proteomic signatures | Detection techniques | Patient groups (HBV-HCC stages) | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC | Cut-off values (ng/mL) | Ref. |
|---|---|---|---|---|---|---|---|---|
| GP73 | ELISA | Early stage | 76.3 | 80.1 | 79.6 | 0.78 | > 5 | [71] |
| GP73 + AFP | ELISA | All stages | 89.2 | 85.2 | 96 | 0.82 | 6.5 | [74] |
| GP73 + DKK-1 | ELISA | Advanced stage | 97.4 | 93.1 | 94.5 | 0.86 | 7.4 | [71] |
| GPC3 | IHC | Recurrence stage | 55 | 58 | 56.6 | 0.46 | 6.1 | [80] |
| GPC3 + AFP | Western blot analysis | Recurrence stage | 85 | 79 | 82.2 | 0.94 | 6.8 | [80] |
| MDK | IHC | Early stage | 93 | 85 | 89.3 | 0.95 | > 5 | [86] |
| DCP | RIA | Recurrence stage | 59.9–71 | 89.4–93 | 74.4–82.2 | 0.76–0.91 | 8.1 | [87, 88, 113] |
| vWF | Protein microarrays | Early stage | 79.5 | 92.3 | 88.6–90.9 | 0.721–0.964 | 6.3 | [93, 95, 96] |
| PTX3 | Western blot analysis | Intermediate stage | 85.1 | 89.3 | 87.3 | 0.93 | 9.2 | [100] |
| PTX3 + AFP | IHC | Early stage | 89.7 | 87.4 | 88.4 | 0.96 | 9.1 | [101] |
| SCCA | IHC | Cirrhotic patients | 59 | 76 | 67.3 | 0.78–0.80 | 6.7 | [108] |
| ESERPINB3/SCCA-IGM | ELISA | Intermediate stage | 85 | 92 | 89 | 0.88 | > 5 | [114] |
| SCCA-IGM + AFP | CLIA | Intermediate stage | 88 | 94 | 91.3 | 0.90 | > 5 | [115] |
| OPN | CLIA | Early-stage | 97 | 70 | 84 | 0.86 | > 5 | [116] |
- Abbreviations: AUC, area under the curve; CLIA, chemiluminescent immunoassay; DKK-1, dickkopf-1; ELISA, enzyme linked immunosorbent assay; IHC, Immunohistochemistry; ng/mL, nanograms per milliliter; Ref, reference; RIA, radioimmunoassay.
9 Limitations of the Present Review
Selection bias could affect this review and cause the evidence to be presented in an unbalanced way. It also does not have a methodical strategy for finding and combining evidence, which leaves gaps in the literature's coverage and raises the possibility of missing important studies. Furthermore, there may be significant variations in the breadth and quality of the included research, which could result in inconsistent findings from the review. Furthermore, by focusing only on published literature and ignoring unpublished or gray literature, this review may be more vulnerable to publication bias due to its lack of quantitative data analysis. Due to the absence of a consistent technique for data synthesis, the conclusions drawn from this research may not necessarily apply to other populations. Subsequently, there is a greater chance that subjective interpretation will affect the conclusions made from the information because it mostly relies on qualitative interpretation rather than just quantitative analysis.
10 Conclusions and Future Directions
Even with the increasing understanding of the pathogenesis of HBV-HCC, HBV-induced hepatocyte remodeling and HCC remain challenging to identify and treat in the early stages. Evaluation is still needed for newly developed protein signatures (indicators) specific to liver malignancies that ensure better patient identification and longevity. Our review summarized the types and clinical applications of protein signatures for the prompt identification and diagnosis of HBV-induced hepatocyte transformations and hepatocarcinogenesis. On the other hand, these signatures served as diagnostic, therapeutic, and prognostic targets for HBV-caused hepatocyte remodeling and HCC.
Future developments in proteomic biomarkers for HBV-HCC are probably going to concentrate on enhancing these biomarkers’ sensitivity, specificity, and accuracy to facilitate tailored treatment plans and aid in early diagnosis, prognosis, and treatment response monitoring. In general, greater sensitivity and specificity, standardization/validation efforts, integration with other omics data types, noninvasive detection techniques, personalized medicine approaches, and tracking disease progression/treatment response are likely to be the main focuses of serum protein biomarkers for HBV-HCC in the future. These developments could lead to better patient outcomes as well as more effective resource allocation within the healthcare system.
Author Contributions
Adane Adugna: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, writing – original draft, writing – review and editing. Gashaw Azanaw Amare: conceptualization, methodology, software, supervision, validation, visualization, writing – original draft, writing – review and editing. Mohammed Jemal: conceptualization, data curation, formal analysis, supervision, validation, visualization, writing – original draft, writing – review and editing.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data presented in this manuscript have been available on the hand of corresponding author for reasonable request.




