Serum proteome-wide identified ATP citrate lyase as a novel informative diagnostic and prognostic biomarker in pediatric sepsis: A pilot study
Abstract
Introduction
ATP citrate lyase (ACLY) is involved in lipid metabolism and inflammatory response in immune cells. However, the serum level of ACLY and its clinical relevance in sepsis is totally unknown.
Methods
We conducted a prospective pilot study in patients with sepsis admitted to pediatric intensive care unit (PICU) from January 2018 to December 2018.
Results
Higher levels of ACLY were detected in sera of pediatric patients with sepsis than that of healthy children. The area under the receiver operating characteristic curve (AUC) of ACLY for diagnosis of sepsis was 0.855 (95% confidence interval [CI]: 0757–0.952), and an AUC of ACLY for predicting PICU mortality was 0.770 (95% CI: 0.626–0.915). ACLY levels ≤21 ng/ml on PICU admission predicted an unfavorable prognosis among patients with sepsis with a sensitivity of 87.5% and a specificity of 67.6%. Moreover, serum ACLY levels were correlated to platelet count, IL-18 levels, and monocyte counts in pediatric patients with sepsis, implying the potential roles of ACLY in immunometabolic regulation in sepsis.
Conclusions
ACLY is firstly identified in sera of patients with sepsis. Serum ACLY level is an additional diagnostic and prognostic biomarker in pediatric patients with sepsis.
Abbreviations
-
- ACLY
-
- ATP citrate lyase
-
- ALT
-
- alanine transaminase
-
- AST
-
- aspartate transaminase
-
- AUC
-
- area under the ROC curve
-
- CRP
-
- C-reactive protein
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- Fib
-
- fibrinogen
-
- IL-6
-
- interleukin-6
-
- IL-10
-
- interleukin-10
-
- IL-17A
-
- interleukin-17A
-
- IL-18
-
- interleukin-18
-
- INR
-
- international normalized ratio
-
- Lac
-
- lactate
-
- LPS
-
- lipopolysaccharide
-
- PCT
-
- procalcitonin
-
- PICU
-
- pediatric intensive care unit
-
- PLT
-
- platelet
-
- PT
-
- prothrombin time
-
- ROC
-
- receiver operating characteristic curve
-
- TNF-α
-
- tumor necrosis factor-α
1 INTRODUCTION
Sepsis is the leading of death in intensive care unit (ICU).1 It accounts for 40% of deaths in children younger than 5 years worldwide.2 Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.3 The immunopathogenesis of sepsis is a very complex process that involves both overactivation and suppression of immune response.4, 5 Lymphopenia is a hallmark of sepsis, and profound apoptosis-induced depletion of lymphocytes including CD4+ and CD8+ T cells, B cells, and natural killer cells demonstrates extensive loss of lymphocytes in spleens, intestines, and other organs in patients who died of sepsis.6-8 Recently, Shankar-Hari et al.9 reported that selective apoptotic depletion of memory B cells contributes to the sepsis-induced immunosuppression. Unfortunately, the evidence about biomarkers for immune status during sepsis is limited, especially in the early stage.
The emergence of the concept of immunometabolism as a major controller of the immune response has raised new hope for re-recognizing the pathogenesis of sepsis and immunomodulatory therapeutic approaches.10 Metabolic reprogramming might reveal to be a novel cornerstone in the treatment of sepsis.11, 12 Reliable biomarkers could greatly help in accurate assessment of the development and evaluation of new treatments.13 The molecules involved in immunometabolism during sepsis could be a potential biomarker for monitoring the immune status, even in predicting the outcome of sepsis.
Up to now, there is no idea about whether the changes of serum protein profiling could reflect the immunometabolic status contributed by activation, secretion, or apoptosis of different immune cells. Our previous study analyzed the changes of serum protein profiling of mice in response to lipopolysaccharide (LPS) stimulation and screened the proteins involved in metabolic molecules.14 The mass spectrometry data have been deposited to the ProteomeXchange Consortium via the PRIDE15 partner repository with the data set identifier PXD014259. By applying LPS administration in mice, serum ATP citrate lyase (ACLY) was firstly identified (Figure S1). The aim of this study was to assess the value of serum ACLY in patients with sepsis.
2 MATERIALS AND METHODS
2.1 Patients
Patients with sepsis who were admitted to the pediatric intensive care unit (PICU) at Shanghai Children's Hospital were screened in this study from January 2018 to December 2018. Pediatric sepsis was diagnosed based on the International Pediatric Sepsis Consensus Conference in 2005.16 The inclusion criteria included (1) aged with 1 month to 18 years old, (2) diagnosed with sepsis when admitted to PICU, (3) PICU stay over 24 h. The exclusion criteria included (1) advanced tumor or life expectancy <1 month, (2) congenital heart disease, and (3) severe primary diseases or heredity metabolic disease. As a control population, we analyzed 30 residual blood samples from healthy children for a physical examination with normal values for blood counts, C-reactive protein (CRP), and liver enzymes.
Patients with sepsis were treated in accordance with the International Pediatric Sepsis Consensus Conference in 200516 and Surviving Sepsis Campaign International Guidelines in 2012.17 The study protocol was approved by the local ethics committee and conducted in accordance with the ethical standards laid down in the Declaration of Helsinki (Ethics Committee of Children's Hospital affiliated to Shanghai Jiao Tong University [approval number: 2018R039-F01]). The informed consent was signed by the patients' parents or relatives.
2.2 Blood sampling and enzyme-linked immunosorbent assay (ELISA)
Blood samples were collected at the time point of patient's admission to PICU. Serum ACLY was determined by ELISA (MultiScience [LIANKE] Biotech Co. Ltd.).
2.3 Observational variables
According to the pre-established case report form, we collected the clinical parameters including age, gender, complications, multiple organ dysfunction syndrome (MODS), mean arterial pressure (MAP), systolic blood pressure (SBP), pediatric risk of mortality III score (PRISM III), support of mechanical ventilator or vasoactive agents. The laboratory indexes include lactate (Lac), routine blood indexes (platelet [PLT], the percentage of lymphocyte, monocytes, and neutrophil) and infectious indexes (CRP, procalcitonin [PCT], Lac, interleukin-8 [IL-8], IL-10, IL-17A, IL-18, γ-interferon [γ-INF], tumor necrosis factor-α [TNF-α], IL-6). The outcome variables including the length of PICU stay and discharged survival status. The laboratory indexes were collected from the first test within 24 h after PICU admission.
2.4 Statistical analyses
Data analyses were performed using STATA 15.0 MP. Continuous variables were summarized as means ± SD for normal distribution data and as median (interquartile range) for abnormal distribution data. Student's t test was used to compare the means of continuous variables and normally distributed data; otherwise, the Mann–Whitney U test was used. The χ2 test was used to compare the categorical data. Multivariate logistic regression analysis was performed to evaluate the association of serum ACLY with PICU mortality, and the correlation between serum ACLY levels and the proportion of immune cells and inflammatory factors were analyzed. Receiver operating characteristic curve (ROC) was generated to assess the diagnostic or prognostic accuracy in pediatric sepsis. A value of p < .05 was considered statistically significant. All p values presented are two-tailed.
3 RESULTS
3.1 Baseline characteristics of patients in this study
To evaluate the potential value of serum ACLY as a biomarker for sepsis, we measured serum ACLY levels at the time point of PICU admission (before therapeutic interventions) in 101 pediatric patients with sepsis and in 30 healthy controls. These patients included 58 males and 43 females with a median age of 19 (5, 60) months. The baseline characteristics of patients enrolled in this study are shown in Table 1. The PICU mortality rate of pediatric sepsis was 10.9% (11/101), and there were significant differences between survivor and non-survivor in aspects of PRISM III score (p = .007), complications with organ dysfunction including respiratory failure (p = .004), shock (p = .001), and liver injury (p = .004), ratio of mechanical ventilator (p < .001) or vasoactive agents supporting (p = .001; Table 1).
| Characteristics | Healthy control (n = 30) | Total (n = 101) | Survivor (n = 90) | Non-survivor (n = 11) | p value |
|---|---|---|---|---|---|
| Age (month) | 96 (48, 144) | 19 (5, 60) | 19 (7, 62) | 5 (2.5, 45) | .357 |
| Gender (male, %) | 12 | 58 | 54 | 4 | .134 |
| Emergency, n (%) | 71 | 66 | 5 | .056 | |
| MAP (mmHg) | 86 (75.7, 97.3) | 86 (74.6, 96.7) | 92 (80, 107) | .208 | |
| SBP (mmHg) | 101 (88, 112) | 100.5 (88, 112) | 102 (92, 115) | .563 | |
| PRISM III | 6 (2, 12) | 6 (2, 10) | 13 (5, 21) | .007 | |
| Complications, n (%) | |||||
| Respiratory failure | 60 (59.4) | 49 (54.4) | 11 (100) | .004 | |
| Shock | 45 (44.6) | 35 (38.9) | 10 (90.9) | .001 | |
| Gastrointestinal disorder | 35 (34.7) | 29 (32.2) | 6 (54.5) | .142 | |
| Liver dysfunction | 11 (10.9) | 7 (7.8) | 4 (36.4) | .004 | |
| Acute kidney injury | 17 (16.8) | 15 (16.7) | 2 (18.2) | .899 | |
| Brain injury | 31 (30.7) | 25 (27.8) | 6 (54.5) | .069 | |
| MODS | 46 (45.5) | 35 (38.9) | 11 (100) | <.001 | |
| Mechanical ventilator, n (%) | 53 (52.5) | 42 (46.7) | 11 (100) | <.001 | |
| Vasoactive agents, n (%) | 54 (53.5) | 43 (47.8) | 11 (100) | .001 | |
| Length of PICU stay, day | 13 (8, 21) | 13 (8, 20) | 17 (4, 29) | .711 |
- Abbreviations: MAP, mean arterial pressure; MODS, multiple organ dysfunction syndrome; PICU, pediatric intensive care unit; PRISM III, pediatric risk of mortality III score; SBP, systolic blood pressure.
3.2 Serum ACLY level is associated with the occurrence and outcome of sepsis
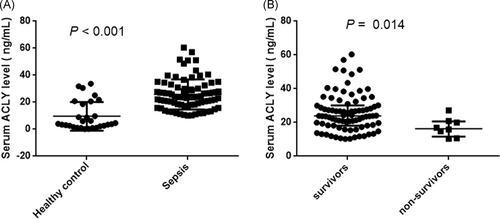
Serum ACLY concentrations on PICU admission were significantly higher in patients who fulfilled sepsis criteria compared with healthy control, and the basic level of serum ACLY in healthy control was very low (Figure 1A). Of note, lower ACLY serum concentration appeared in non-survivors compared with survivors (Figure 1B). The results of subgroup analysis indicated that there was no significant association between serum ACLY levels and organ dysfunction (all p > .05; Figure S2). Moreover, serum ACLY concentrations did not correlate with age or gender, either in controls or in patients (data not shown).
3.3 Serum ACLY levels are closely correlated to PLT count, IL-18 levels, and monocytes count
Besides serum ACLY level, PLT count and the proportion of neutrophils were significantly decreased in non-survivors compared with the survivors (p = .047, p = .009, respectively; Table 2). The proportion of lymphocytes was higher in non-survivors than that in survivors (p = .003; Table 2). Among inflammatory factors, levels of IL-17A and IL-18 were significantly higher on PICU admission in non-survivors compared with survivors (p = .038, p = .027; respectively). The correlation analyses indicated that serum ACLY level was positively correlated to PLT count (p = .006) and the levels of IL-18 (p = .027), as well as negatively correlated to monocytes count (p = .031) after being adjusted by PRISM III score (Table 3).
| Variables | Total (n = 101) | Survivor (n = 90) | Non-survivor (n = 11) | p value |
|---|---|---|---|---|
| PLT (×109/L) | 282 (187, 364) | 292.5 (219, 392) | 172 (146, 302) | .047 |
| Lymphocyte (%) | 21 (10, 31) | 18.5 (10, 29) | 38 (31, 47) | .003 |
| Neutrophil (%) | 72 (58, 83) | 73.8 (60.9, 85) | 56 (42, 60) | .009 |
| Monocytes (%) | 6 (3.4, 9) | 6 (3.4, 9) | 7 (5, 8) | .683 |
| PCT (ng/ml) | 1.22 (0.15, 7.9) | 1.19 (0.13, 7.9) | 1.82 (0.15, 10.13) | .831 |
| CRP (mg/L) | 21 (5, 81) | 20.5 (5, 81) | 23 (5, 150) | .638 |
| Lac (mmol/L) | 1.1 (0.7, 1.9) | 1.1 (0.7, 1.9) | 1.5 (0.9, 2.6) | .367 |
| Lac-72 h (mmol/L) | 2.1 (1.6, 2.6) | 2.0 (1.6, 2.6) | 2.7 (1.7, 3.3) | .108 |
| IL-8 (pg/ml) | 1.68 (0.1, 36.93) | 0.985 (0.1, 13.52) | 47.99 (0.1, 68.96) | .326 |
| IL-10 (pg/ml) | 4.54 (0.1, 19.3) | 3.46 (0.1, 13.95) | 17.82 (9.73, 44.78) | .054 |
| IL-17A (pg/ml) | 0.1 (0.1, 0.75) | 0.1 (0.1, 0.65) | 0.91 (0.23, 4.89) | .038 |
| IL-18 (pg/ml) | 95.15 (31.63, 369.44) | 60.755 (24.09, 356.04) | 479.66 (345.86, 567.02) | .027 |
| γ-INF (pg/ml) | 1.63 (0.29, 5.63) | 1.495 (0.29, 5.36) | 1.99 (0.98, 9.74) | .795 |
| TNF-α (pg/ml) | 0.1 (0.1, 1.5) | 0.1 (0.1, 1.4) | 1.0 (0.1, 31.5) | .273 |
| IL-6 (pg/ml) | 45.0 (0.1, 288) | 40.9 (0.1, 253.2) | 81.5 (48, 14,058.5) | .194 |
- Abbreviations: CRP, C-reactive protein; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; IL-17A, interleukin-17A; IL-18, interleukin-18; Lac, lactate; PLT, platelet; PCT, procalcitonin; TNF-α, tumor necrosis factor-α; γ-INF, γ-interferon.
| Serum ACLY | Coef. | SE | p | 95% CI |
|---|---|---|---|---|
| Model 1 for immune cells | ||||
| PLT | 0.018 | 0.007 | 0.015 | 0.004 to 0.032 |
| Monocytes | −0.664 | 0.285 | 0.022 | −1.231 to −0.096 |
| Lymphocyte | −0.071 | 0.074 | 0.342 | −0.218 to 0.076 |
| Neutrophil | 0.097 | 0.064 | 0.134 | −0.030 to 0.223 |
| Model 2 for inflammatory factors | ||||
| IL-17A | −0.638 | 0.340 | 0.071 | −1.336 to 0.059 |
| IL-18 | 0.004 | 0.002 | 0.030 | 0.0004 to 0.007 |
| Model adjusted by PRISM III | ||||
| PLT | 0.020 | 0.007 | 0.006 | 0.006 to 0.035 |
| Monocytes | −0.624 | 0.284 | 0.031 | −1.189 to −0.059 |
| IL-18 | 0.004 | 0.002 | 0.027 | 0.0005 to 0.007 |
- Abbreviations: ACLY, ATP citrate lyase; CI, confidence interval; IL-17A, interleukin-17A; IL-18, interleukin-18; PLT, platelet; PRISM III, pediatric risk of mortality III score.
3.4 Serum ACLY level is an additional diagnostic and prognostic biomarker in pediatric patients with sepsis
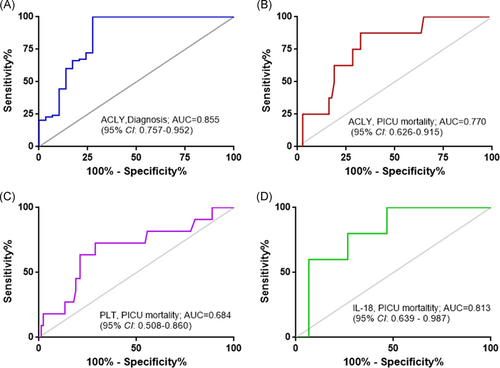
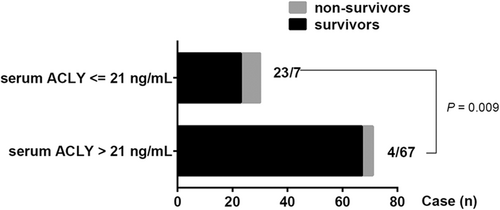
To enclose the potential value of serum ACLY in pediatric sepsis, ROC analysis was performed to assess the power of serum ACLY in assessing the occurrence of sepsis and in predicting the outcome of sepsis. The results showed that serum ACLY levels could be an additional biomarker for sepsis occurrence with a high value of area under ROC curve (AUC) of 0.855 (95% CI: 0.757–0.952; Figure 2A). The cut-off value of serum ACLY level for an additional diagnosis of sepsis was 9.96 ng/ml with a sensitivity of 100% and a specificity of 72.4%. Furthermore, serum ACLY levels displayed high prognostic accuracy for PICU mortality with an AUC of 0.770 (95% CI: 0.626–0.915), and the sensitivity was 87.5% and the specificity was 67.6% at the cut-off value of 21 ng/ml (Figure 2B). In addition, the AUC of PLT count or IL-18 level for predicting PICU mortality was 0.684 (95% CI: 0.508–0.860) or 0.813 (95% CI: 0.639–0.987), respectively (Figure 2C,D). Furthermore, there were seven cases who died in the subgroup of 30 patients with serum ACLY level ≤21 ng/ml, and four cases died in the subgroup of 71 patients with serum ACLY level >21 ng/ml (Figure 3). The PICU mortality was significantly higher in the subgroup of serum ACLY level ≤21 ng/ml compared with the subgroup of serum ACLY level >21 ng/ml (7/30 vs. 4/71; p = .009; Figure 3).
4 DISCUSSION
To the best of our knowledge, it is the first report about serum ACLY in patients with sepsis. serum ACLY level was correlated to PLT count, IL-18 levels, and monocytes count, and serum ACLY level proved to be an additional diagnostic biomarker and a prognostic predictor. These results give a new insight into immunometabolic biomarkers to assess the occurrence and outcome of sepsis.
It is well-known that ACLY is a cytoplasmic enzyme that represents a cross-link between glucose metabolism and fatty acid synthesis/mevalonate pathways.18 Recent studies indicated that ACLY plays a key role in macrophage inflammatory response19 and functional capacity in CD8+ T cells by translating increased systemic acetate concentrations into increased glycolysis.20 Furthermore, increased ACLY expression and enzymatic activity contribute to B-cell differentiation by mediating glucose-dependent de novo lipogenesis in response to LPS signaling.21 To the best of our knowledge, this is the first study to report the existence of ACLY in the serum of mouse responding to LPS, as well as in pediatric patients with sepsis. ACLY plays a remarkable role in metabolic regulation in cancer cell and would be a promising target for cancer therapy.22 There is growing popularity of enclosing the structure and searching the inhibitor of ACLY.23, 24 Recent studies indicated that ACLY plays a key role in macrophage inflammatory response19 and functional capacity in CD8+ T cells by translating increased systemic acetate concentrations into increased glycolysis.20 The apoptosis of lymphocytes and monocytes might result in releasing the Intracellular molecules to circulation, which could contribute to the appearance of ACLY in serum. In our present study, ACLY was almost undetectable in sera of healthy children, and serum ACLY level achieved a higher diagnostic value for pediatric sepsis. So, it is promising that serum ACLY is a powerful additional predictor for sepsis occurrence.
In this study, we demonstrated a prognostic impact of ACLY serum concentrations on PICU admission in pediatric patients with sepsis. This study first demonstrated that serum ACLY level could be a candidate for the prognostic value of sepsis. In particular, patients with lower ACLY serum levels (≤21 ng/ml) had a significantly impaired prognosis compared with patients with higher ACLY levels (>21 ng/ml). Given the important roles of ACLY in mitochondria function and metabolic regulation,25, 26 we could further speculate that ACLY may be implemented into established scoring systems to further improve their sensitivity and specificity, and maybe a drug target to regulate immune response via ACLY-mediating metabolism for sepsis, just like in cancer. However, this assumption is based on a single-center analysis and future prospective studies are needed to test this hypothesis.
As a novel prognostic predictor, the correlations between serum ACLY and other laboratory indexes give some implications for its function in sepsis. PLTs have received increasing attention for their role in the pathophysiology of sepsis via regulating inflammatory and immune responses, clotting cascade, and endothelial injury.27 The relation between PLT parameters and outcome in septic patients has been supported by numerous studies.28, 29 However, there is a limited study about the role of PLT count on PICU admission in sepsis. Thrombocytopenia is defined as a PLT count <150 × 109/L and the severity of thrombocytopenia positively correlates to the severity of inflammation and the hospital mortality in adult patients.30, 31 In our study, PLT count on PICU admission is associated with PICU mortality, and PLT count is also positively correlated with serum ACLY level. The underlying mechanisms of PLT count influencing serum ACLY level need further investigation. Otherwise, we found that serum ACLY levels were correlated with serum IL-8 levels. IL-18 originally named as “interferon-c-inducing factor,” and IL-18 potentiated mortality in both neonatal sepsis and endotoxemia through the induction of IL-17A. In addition, targeting IL-18/IL-1/IL-17A axis may improve outcomes for human neonates with sepsis.32 Consistently, we found the lower levels of ACLY and the higher levels of IL-18 in non-survivors with septic shock than that in survivors. A recent study indicated that elevated plasma IL-18 level was associated with increased mortality in patients with acute respiratory distress syndrome with a hazard of death of 2.3 (95% CI, 1.7–3.1).33 Otherwise, there was no prognostic impact about IL-18 in adult patients with sepsis, and IL-18 was a biomarker better differentiating sepsis and septic shock status than PCT, CRP, and WBC.34 Furthermore, several studies indicated that IL-18 enhances immunosuppressive response by promoting differentiation into monocytic myeloid-derived suppressor cells (MDSCs), by inducing MDSCs migration into the tumor tissue.35-37 Sepsis-induced immunosuppression is the main cause for poor outcome of sepsis contributed by metabolic failure, epigenetic reprogramming, MDSCs, and so forth.38 So, higher levels of IL-18 might contribute to immunosuppression in non-survivors. It is noteworthy that whether the changes of IL-18 results from ACLY-mediated metabolic changes in immune cells in the future. In addition, there was a tendency of increased IL-10 levels in non-survivors compared with survivors. Given that the correlation between serum levels of ACLY and monocytes count, we suspect that apoptosis of monocytes results in releasing ACLY to serum, which needs further investigation in the future.
Several limitations of this study should be considered. First, the sample size of non-survivor in this study was relatively small, and it was a single-center cohort study. Second, the changes of serum levels of ACLY during PICU stay and the correlation of serum ACLY to metabolite levels like citrate or fatty acids were lacking due to the limited blood sampling. Third, how ACLY is released into circulation is still unclear. At present, it is totally unclear which molecular mechanisms are responsible for alterations in serum ACLY levels in sepsis. Moreover, it is still unknown if changes in serum ACLY levels reflect an active secretion to play a functional role as “messengers” or a passive release during cell death. Nevertheless, our findings strongly support future functional and clinical analyses on the role of ACLY in the pathophysiology of sepsis.
In summary, we first identified serum ACLY as a new diagnostic and prognostic biomarker in pediatric patients with sepsis. The relationship between serum ACLY and IL-18 and underlying mechanisms involved in the pathophysiology of sepsis need further study in the future.
ACKNOWLEDGMENTS
This study was supported by the Science and Technology Commission of Shanghai Municipality (18411951000), Natural Science Foundation of Shanghai (19ZR1442500), and Key Developing Subject Program from Shanghai municipal commission of Health and Family Planning (2016ZB0102). Dr. Chunxia Wang is funded by Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant (20171928) and the talent program from Shanghai Jiao Tong University School of Medicine (17XJ11018).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Chunxia Wang and Yucai Zhang conceived and designed the study and wrote the paper. Chunxia Wang, Huijie Miao, Xiaodong Zhu, Yaya Xu, Xi Xiong, Lujing Shao, and Xiaomeng Tang collected and analyzed data. Xiaodong Zhu, Yun Cui, Chunxia Wang and Yucai Zhang contributed to the analysis of tools and discussion.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.