LC/MS analysis of mushrooms provided new insights into dietary management of diabetes mellitus in rats
Abstract
Mushrooms possess antihyperglycemic effect on diabetic individuals due to their nonfibrous and fibrous bioactive compounds. This study aimed to reveal the effect of different types of mushrooms on plasma glucose level and gut microbiota composition in diabetic individuals. The effects of five different mushroom species (Ganoderma lucidum, GLM; Pleurotus ostreatus, POM; Pleurotus citrinopileatus, PCM; Lentinus edodes, LEM; or Hypsizigus marmoreus, HMM) on alloxan-induced diabetic rats were investigated in this study. The results indicated that LEM and HMM treatments showed lower plasma glucose levels. For the microbiota composition, ACE, Chao1, Shannon, and Simpson were significantly affected by PCM and LEM treatments (p < .05), while ACE, Shannon, and Simpson indexes were affected by HMM treatment (p < .01). Simpson index was affected in positive control (C+) and POM groups. All these four indices were lower in GLM treatment (p < .05). Dietary supplementation of mushrooms reduced plasma glucose level directly through mushrooms' bioactive compounds (agmatine, sphingosine, pyridoxine, linolenic, and alanine) and indirectly through stachyose (oligosaccharide) and gut microbiota modulation. In conclusion, LEM and HMM can be used as food additives to improve plasma glucose level and gut microbiome composition in diabetic individuals.
1 INTRODUCTION
Mushrooms possess a necessary dietary ingredient due to their low-calorie value, different bioactive compounds, and rich fiber content. These compounds include vitamins (such as riboflavin and niacin), minerals (such as iron and phosphorus) (Alkin et al., 2021), and fibers (Lu et al., 2020). Moreover, mushrooms differ in their content of phenols (Alkin et al., 2021) and antioxidants (ergothioneine and glutathione) (Beelman et al., 2019). Thus, each mushroom type possesses different content of bioactive compounds, fiber contents, or both (Alkin et al., 2021). Gut microbiota degrades the dietary fibers as a nondigestible carbon sources and change the microbiota composition (Tang et al., 2017). Unfavorable modulations of gut microbiota are associated with multiple chronic diseases including diabetes mellitus (Tang et al., 2017). In particular, mushrooms possess enriched fibers, such as polysaccharides and heteroglucans (Li et al., 2021), these decrease a pathogen proliferation by inducing the growth of probiotic bacteria in gut (Kumari, 2020). For instance, the species of Ganoderm lucidum is a medicinal mushroom which contains various bioactive compounds that operates as antimicrobial agents (Cor et al., 2018). Fruiting bodies and mycelia of G. lucidum contain polysaccharides, such as glycoproteins, (1 → 3), (1 → 6)-a/β-glucans, and water-soluble heteropolysaccharides (Martin & Jiang, 2010). Those polysaccharides have antihypoglycemic effect (Chassaing et al., 2017; Xu et al., 2017). G. lucidum increases anti-inflammatory bacteria (Enterococcus and Dehalobacterium) in mice diabetic individuals (Chen, Liu, et al., 2020; Chen, Xiao, et al., 2020) and decreases the abundance of harmful bacteria, such as Aerococcus, Corynebactrium, Ruminococcus, and Proteus in type-2 mice diabetic groups (Chen, Liu, et al., 2020; Chen, Xiao, et al., 2020). G. lucidum treatment on type-2 diabetes, enhanced SCFA-producing bacterial activity (Chen, Xiao, et al., 2020). In fact, oral administration of extracted polysaccharides from mushrooms (Pleurotus eryngii and Poria cocos) promote SCFA-producing bacterial growth (Li et al., 2021), this improve energy metabolism through affecting the intestinal gluconeogenesis (IGN) and insulin sensitivity simulation (De Vadder et al., 2014). Since IGN releases glucose molecules which can be detected by the glucose sensor in the portal vein. Such signal is transmitted to the brain by the peripheral nervous system regulating glucose metabolism (Delaere et al., 2013). Polysaccharides inhibit digestive enzymes' activity based on the interaction with different sites at enzymes' structure. In addition, polysaccharide's viscoelasticity effect interferences with the enzymes and substrates flow resulting in lipid-lowering effect (Xie et al., 2022).
Most mushroom-related studies have investigated the effect of mushroom fibrous bioactive compounds on individual diabetics. While few studies have investigated the non-fibrous bioactive compounds on diabetic individuals (Cor et al., 2018; Lu et al., 2020). Dubey et al. (2019) indicated that few bioactive compounds of edible mushrooms are identified. In a pilot study, mushroom untargeted molecules' composition was investigated, and analyzed; this revealed the existence of some of bioactive compounds in the tested mushrooms. The literature showed that agmatine has an antihyperglycemic effect through increasing glucose uptake in muscles by simulating insulin secretion (Chang et al., 2010; Malaisse et al., 1989; Naoki & Fujiwara, 2019; Nissim et al., 2006, 2014; Shepherd et al., 2012; Su et al., 2008, 2009). Whereas stachyose decreases the blood glucose level in alloxan-induced diabetic rats (Zhang et al., 2004), in addition to its regulation effect on the intestinal microflora balance (Liu, Jia, et al., 2018; Liu, Wang, et al., 2018). Sphingosine (PHS) activates omega-3 fatty acid receptor (GPR120) resulting in an insulin-sensitizing effect (Rudd et al., 2020). Finally, pyridoxine decreases insulin resistance via scavenging the pathogenic reactive carbonyl species (Haus & Thyfault, 2018). There are epidemiological evidences supporting the non-fibrous bioactive compounds safety use, eight mushrooms have been investigated for their effect on DM, that G. lucidum has showed the highest content of phenolic and flavonoids compounds (Wu & Xu, 2015). But this study did not analyze the other bioactive molecules. Generally, the non-fibrous compounds are naturally existed in human foods, for example, agmatine in fermented foods (Galgano et al., 2012), stachyose (oligosaccharides) as a probiotic in human foods (Yang et al., 2018), sphingosine in dairy products (Possemiers et al., 2005), and pyridoxine is a vitamin B6 (Shaik Mohamed, 2001). This revealed a possible therapeutic potency of mushrooms on diabetic individuals.
Mushrooms are basic food components in Chinese table. Thus, revealing their nutritional value and their therapeutic potency are expected to increase the social awareness about dietary mushroom in addition to their therapeutic effect leading to the improving of public health.
To reveal the effect of those bioactive compounds in mushrooms on a diabetic individual, alloxan injection was used to induce the diabetes type II in rats by damaging pancreatic cells and initiating hyperglycemia (Inalegwu et al., 2021). These animals were subjected to different dietary mushrooms to reveal the effect of bioactive compounds sourced from mushrooms on blood glucose level and intestinal microbial composition as basic indicators for the possible effect.
We hypothesized that dietary mushrooms inclusion may decrease plasma glucose level and modulate microbiota composition directly via their bioactive molecules, and indirectly via fiber content and microflora modulation. This study aimed to reveal the effect of non-fibrous bioactive compounds sourced from mushrooms on blood glucose level and intestinal microbial composition in diabetic rat individuals. These molecules may develop a food additive with an effective and specific health functionality considering food processing conditions and their effect on food quality.
2 MATERIALS AND METHODS
2.1 Experimental ethics and tested animals
This study was conducted under the ethical approval of Animal Welfare and Ethics Committee of the Key Laboratory of Animal Safety Production of Ministry of Education, PR China (no. KT2018011).
Diabetes mellitus is a metabolic disease characterized by hyperglycemia, occurring due to abnormal insulin action or insulin secretion. Alloxan induces diabetes type II by damaging pancreatic cells and initiating hyperglycemia (Inalegwu et al., 2021). Experimental animals Albino male rats (Rattus norvegicus) weighed 160–180 g with age 30 days were obtained from the animal's research center, Jilin Agricultural University, Changchun, China. Alloxan-induced diabetic rats (AIDRs) were used as the diabetic model after acclimation period of 30 days. AIDRs were induced by intraperitoneal injection of Alloxan (150 mg/kg of body weight; Shanghai Sinyu Biotechnology Company) after an overnight fast. Three days after Alloxan injection, rats with a plasma glucose concentration of 11 mmol/L or above and symptoms of polyuria, polyphagia, and polydipsia were considered to have diabetes. The animals were distributed among treatments, seven animals per treatment (Table 1). All experimental animals were weighed weekly using a digital balance (Yeng Heng Electronic Scale Company) within the experimental period (4 weeks).
| Groups | Treatments | Diet (%) | No. of rats (n) |
|---|---|---|---|
| A | C− | 100% Commercial diet | 7 |
| B | C+ | 100% Commercial diet | 5 |
| C | GLM | 75% Commercial diet + 25% GLM | 5 |
| D | POM | 75% Commercial diet + 25% POM | 5 |
| E | PCM | 75% Commercial diet + 25% PCM | 4 |
| F | LEM | 75% Commercial diet + 25% LEM | 5 |
| G | HMM | 75% Commercial diet + 25% HMM | 5 |
- Abbreviations: C−, negative control; C+, positive control; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
2.2 Determination of plasma glucose
Animals were deprived of food and water overnight and a blood glucose meter and test strips (Hangzhou Econ Biotech Company) were used to measure the blood glucose levels.
2.3 Experimental diets and mushrooms' bioactive compounds
The effect of five types of mushrooms (GLM) Ganoderma lucidum mushroom (traditional Chinese medicinal mushroom), (POM) Pleurotus ostreatus mushroom, (PCM) Pleurotus citrinopileatus mushroom, (LEM) Lentinus edodes mushroom, and (HMM) Hypsizigus marmoreus mushrooms were tested in rats that were fed on a commercial diet (Beijing Keao Cooperative Feed Co.) (Table 2). Fresh mushrooms were obtained from the Base Centre of Jilin Agricultural University, Changchun, China. Mushroom fruiting bodies were dried under sunlight for 72 h, and crushed into powder using a laboratory mill. Mushroom powder was mixed with the commercial diet as a daily intake of 8.5 g per individual. The nominated mushrooms' bioactive compounds are shown in Table 3.
| Group | CP (%) | EE (%) | CF (%) | ADF (%) | NDF (%) | Ash (%) | CHO (%) |
|---|---|---|---|---|---|---|---|
| C+ and C− | 19 ± 0.1b | 4 ± 1a | 38 ± 0.2a | 36 ± 0.03b | 46 ± 0.2a | 7.2 ± 0.05a | 68 ± 1ab |
| GLM | 19 ± 1b | 2.4 ± 0.5a | 41 ± 3a | 42 ± 0.1a | 49 ± 0.5a | 4.6 ± 1.07a | 72 ± 1a |
| POM | 24 ± 1a | 4.1 ± 0.7a | 37 ± 0.2a | 42.89 ± 0.3a | 42 ± 3a | 6.3 ± 0.02b | 64 ± 0.4c |
| PCM | 22 ± 3ab | 3.8 ± 0.6a | 38 ± 0.1a | 37 ± 1b | 44 ± 0.3ab | 6.6 ± 0.05a | 64 ± 0.3bc |
| LEM | 21 ± 2ab | 3.8 ± 0.1a | 39 ± 1a | 41 ± 0.5a | 46 ± 0.6a | 6.2 ± 0.06a | 67 ± 2bc |
| HMM | 23 ± 0.9ab | 3.4 ± 0.08a | 37 ± 0.03a | 36 ± 2b | 45 ± 2a | 6.9 ± 0.02a | 65 ± 0.9bc |
| p-Value | .073 | .371 | .295 | .002 | .141 | .010 | .013 |
- Note: Values represented means ± standard deviation. Different letters represent a significant difference in Tukey test at p < .05.
- Abbreviations: ADF, acid detergent fiber; C−, negative control; C+, positive control; CF, crude fiber; CHO, carbohydrate; CP, crude protein; EE, ether extract; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; NDF, neutral detergent fiber; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
| Bioactive compound | C | GLM | POM | PCM | LEM | HMM |
|---|---|---|---|---|---|---|
| Agmatine | 33 | 29 | 73 | 80 | 446 | 829 |
| Stachyose | 40 | 357 | 40 | 73 | 27 | 25 |
| Sphingosine | 31 | 94 | 18 | 23 | 68 | 621 |
| Pyridoxine | 70 | 247 | 32 | 112 | 25 | 21 |
| Linolenic acid | 68 | 104 | 82 | 99 | 145 | 143 |
| Alanine | 34 | 126 | 116 | 59 | 161 | 123 |
- Abbreviations: C, control diet; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
2.4 Liquid chromatography-mass spectrometry (LC–MS)
2.4.1 Sample preparation
Fifty milligrams of mushroom sample was mixed with 1 ml of the mixture (methanol:acetonitrile:water, 2:2:1). The sample was put into a multi-tissue grinder (frequency 60 Hz, 4 min) for tissue fragmentation, and then ultrasonicated for 10 min and then it was kept in the refrigerator for 1 h. the sample was centrifuged at 4°C for 15 min at 10,000 g. Seven hundred microliters of supernatant was put in a vacuum freeze dryer until it evaporated. The solution was resuscitated with 500 μl acetonitrile water (1:1) for 30 s and ultrasonicated for 10 min. The centrifugation was performed at 4°C for 15 min at 10,000 g. A volume of 50 μl of supernatant was put into the injection bottle and detected by LC–MS.
2.4.2 Liquid phase conditions
We used a chromatographic column (Waters ACQUITY UPLC BEH Amide 1.7 μm, 2.1 mm × 100 mm).
2.4.3 Mobile phase
Phase A is ultrapure water containing 25 mM ammonium acetate and 25 mm ammonia, and phase B is acetonitrile. Current Speed 5 ml/min, column temperature 40°C, injection volume 2 μl.
2.4.4 Liquid phase elution gradient
A gradient elution high-performance liquid chromatographic method is described in Table 4.
| Time | A% | B% | Flow rate (ml/min) |
|---|---|---|---|
| 0 | 5 | 95 | 0.5 |
| 0.5 | 5 | 95 | 0.5 |
| 7 | 35 | 65 | 0.5 |
| 8 | 60 | 40 | 0.5 |
| 9 | 60 | 40 | 0.5 |
| 9.1 | 5 | 95 | 0.5 |
| 12 | 5 | 95 | 0.5 |
2.4.5 Mass spectrometry conditions
Temperature of EFI ion source was 65°C. MS voltage was 5500 V (positive ion) and was 4500 V (negative ion). Declustering voltage DP was 6 0 V Ion source gas: gas 1 was 60 psi, gas 2 was 60 psi, and curtain gas (cur) was 30 psi.
2.5 DNA extraction, polymerase chain reaction amplification and high-throughput sequencing
Next-generation sequencing (NGS), including library preparations, was conducted at Genewiz, Inc. using an Illumina MiSeq (Illumina). DNA (30–50 ng) was extracted using TIANGEN DP336 genomic-DNA extraction kits (TIANGEN Biotech [Beijing] Co. Ltd.) and quantified with a Qubit 2.0 Fluorometer (Invitrogen). To generate amplicons (400–450 bp), the MetaVx Library Preparation Kit (Genewiz) was used. For each 40 ng sample of DNA, V3, V4, and V5 hypervariable regions of prokaryotic 16S ribosomal RNA (rRNA) were selected for generating amplicons, following taxonomic analysis. Genewiz has designed a panel of proprietary primers aimed at relatively conserved regions bordering the V3, V4, and V5 hypervariable regions of bacteria and Archaea16S rDNA (if eukaryotic DNA was contaminated, only the V3 and V4 regions were amplified). V3 and V4 regions were amplified using forward primers containing the sequence CCTACGGRRBGCASCAGKVRVGAAT and reverse primers containing the sequence GGACTACNVGGGTWTCTAATCC, the V4 and V5 regions were amplified using forward primers containing the sequence GTGYCAGCMGCCGCGGTAA and reverse primers containing the sequence CTTGTGCGGKCCCCCGYCAATTC. First-round polymerase chain reaction (PCR) products were used as templates for second-round amplicon enrichment PCR. At the same time, indexed adapters were added to the ends of the 16S rDNA amplicons to generate indexed libraries for downstream NGS on the Illumina MiSeq according to Quast et al. (2013).
DNA libraries were validated using an Agilent 2100 Bioanalyzer (Agilent Technologies), quantified by Qubit 2.0 Fluorometer (Invitrogen), multiplexed and loaded onto the Illumina MiSeq as per manufacturer's instructions. NGS was performed using a 2 × 300 paired-end (PE) configuration (Li, Hu, et al., 2017; Li, Wang, et al., 2017). Image analysis was conducted and base calling with the MiSeq Control Software (MCS) embedded in the MiSeq instrument (Yilmaz et al., 2014). The amplicon sequence data were deposited with the National Center for Biotechnology Information (Accession Nos. SRR2579284 and ERS2011824).
2.6 Sequence analysis
Quantitative Insights into Microbial Ecology (QIIME) data analysis software were used to analyze 16S rRNA data (Caporaso et al., 2010). Quality filtering was performed on raw sequences according to Bokulich et al. (2012), as well as on joined sequences. Any sequence that was not <200 bp, with no ambiguous bases and a mean quality score ≥20 was discarded. Forward and reverse reads were joined and assigned to samples based on barcode and truncated by cutting off the barcode and primer sequence. The sequences were compared with the reference database (Ribosomal Database Project [RDP] Gold database) using the UCHIME algorithm (Edgar et al., 2011) to detect chimeric sequences that were removed. Only effective sequences were used in the final analysis. Sequences were grouped into operational taxonomic units (OTUs) and pre-clustered at 97% sequence identity using the clustering program VSEARCH version 1.9.6 (Rognes et al., 2016) against the SILVA 119 database. The RDP classifier was used to assign taxonomic categories to all the OTUs at a confidence threshold of 0.8, according to Crawford et al. (2009). The RDP classifier uses the SILVA 119 database, which predicts taxonomic categories to the species level. Sequences were rarefied prior to calculation of alpha and beta diversity statistics. Alpha diversity indices were calculated in QIIME from rarefied samples using the Shannon index for diversity and the Chao1 index for richness (Chao, 1984; Chao & Lee, 1992). Beta diversity was calculated using weighted and unweighted UniFrac and principal component analysis (Bamberger & Lowe, 1988). An unweighted Pair Group Method with Arithmetic mean (UPGMA) tree from beta diversity distance matrix was built.
2.7 Statistical analysis
Based on the beta diversity distance matrix and on environmental factor data, canonical correspondence analysis (CCA) between RFPs and BCC was integrated by the R-language software application (Team, 2018). All data were analyzed by one-way (mushroom type) analysis of variance (ANOVA) and were performed using SPSS-software, version 11.5 (SPSS, Version 11.5.0; SPSS Inc.). Results were expressed as Mean ± SD. Tukey's contrasts were used to test the significance level for the effects of mushroom types, with p < .05 indicating significant difference.
3 RESULTS
3.1 Feed intake, plasma glucose level, and body weight
There were significant differences in feed intake between PCM and C−, and between POM and C+ (p < .05) (Table 5). All mushroom treatments showed a significant difference compared with C+ except GLM treatments in plasma glucose level, whereas the mushrooms treatments showed a gradually improved plasma glucose level till 45 days of experimentation (p < .05). LEM and HMM showed less significant difference compared with C− in plasma glucose level (p < .05).
| Group | Feed intake (g/day) | Plasma glucose (mmol) | |
|---|---|---|---|
| Before treatment | After treatment | ||
| C− | 37 ± 3bc | 6 ± 0.4b | 5 ± 0.3d |
| C+ | 44 ± 2ab | 26 ± 6a | 23 ± 6a |
| GLM | 43 ± 1ab | 28 ± 5a | 21 ± 4ab |
| POM | 33 ± 12c | 28 ± 4a | 16 ± 3bc |
| PCM | 47 ± 2a | 26 ± 2a | 15 ± 5c |
| LEM | 40 ± 3abc | 24 ± 8a | 11 ± 5c |
| HMM | 43 ± 2ab | 25 ± 6a | 12 ± 2c |
| p-Value | .005 | .001 | .001 |
- Note: Values represented means ± standard deviation. Different letters represent a significant difference in Tukey test at p < .05.
- Abbreviations: C−, negative control; C+, positive control; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
Mushroom treatments showed a significant difference compared with C− in body weight (p < .05) (Table 6). HMM treatment showed a significant difference compared with control (C− and C+) in liver weight (p < .05).
| Treatments | Body weight (g) | |||
|---|---|---|---|---|
| First | Second | Third | Final | |
| C− | 253 ± 5a | 338 ± 22a | 370 ± 21a | 411 ± 31a |
| C+ | 245 ± 4ab | 267 ± 17b | 283 ± 20b | 234 ± 45b |
| GLM | 248 ± 7ab | 2790 ± 31b | 296 ± 47b | 246 ± 74b |
| POM | 240 ± 6b | 266 ± 21b | 268 ± 33b | 220 ± 43b |
| PCM | 250 ± 5a | 266 ± 20b | 284 ± 20b | 232 ± 48b |
| LEM | 247 ± 8ab | 268 ± 11b | 276 ± 22b | 221 ± 42b |
| HMM | 244 ± 5ab | 267 ± 12b | 281 ± 23b | 187 ± 39b |
| p-Value | .047 | .001 | .001 | .001 |
- Note: Values represented means ± standard deviation. Different letters represent a significant difference in Duncan test at p < .05.
- Abbreviations: C−, negative control; C+, positive control; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
3.2 Untargeted molecules analysis by LC/MS
A wide variety of molecules have been identified in diet and mushroom samples, the molecules level was calculated as a percent of the quality control (QC) value, a percent over 100 was nominated as a possible effective molecule. Among them, few molecules have been discussed in this study based on the available literature regarding to the effect on diabetes (Table 7).
| SN | Molecule name | Response (%) | |||||
|---|---|---|---|---|---|---|---|
| Diet | GLM | POM | PCM | LEM | HMM | ||
| 1 | (−)-Riboflavin | 175 | 150 | 6 | 136 | 72 | 69 |
| 2 | 1,3-Diaminopropane | 63 | 258 | 34 | 59 | 3 | 3 |
| 3 | 11-Keto-beta-boswellic acid | 51 | 3 | 30 | 87 | 374 | 360 |
| 4 | 4-Methoxyphenylacetic acid | 198 | 55 | 120 | 126 | 32 | 29 |
| 5 | Acetylcholine | 61 | 63 | 297 | 51 | 16 | 14 |
| 6 | Adenine | 125 | 143 | 150 | 65 | 9 | 9 |
| 7 | Adenosine | 162 | 125 | 61 | 145 | 16 | 16 |
| 8 | Ajmalicine | 156 | 69 | 44 | 119 | 94 | 89 |
| 9 | Allopurinol | 215 | 43 | 35 | 163 | 63 | 60 |
| 10 | Betaine | 80 | 86 | 118 | 96 | 117 | 122 |
| 11 | Choline | 101 | 61 | 198 | 114 | 35 | 31 |
| 12 | Cytidine 5′-diphosphocholine (CDP-choline) | 172 | 7 | 125 | 179 | 3 | 4 |
| 13 | Cytosine | 40 | 331 | 15 | 41 | 62 | 64 |
| 14 | Dehydroascorbic acid (oxidized vitamin C) | 19 | 13 | 157 | 34 | 13 | 13 |
| 15 | Diaveridine | 90 | 233 | 32 | 56 | 430 | 432 |
| 16 | Dimethylglycine | 110 | 43 | 176 | 125 | 39 | 36 |
| 17 | dl-2-Aminoadipic acid | 34 | 29 | 171 | 23 | 25 | 24 |
| 18 | d-Mannitol | 131 | 152 | 142 | 42 | 12 | 10 |
| 19 | Dopamine | 195 | 8 | 120 | 216 | 15 | 15 |
| 20 | d-Ornithine | 117 | 75 | 376 | 90 | 31 | 13 |
| 21 | Edaravone | 142 | 87 | 80 | 150 | 95 | 81 |
| 22 | Eicosapentaenoic acid | 30 | 14 | 7.55 | 18 | 389 | 348 |
| 23 | Ephedrine | 307 | 20 | 95 | 22 | 17 | 13 |
| 24 | gamma-l-Glutamyl-l-valine | 115 | 98 | 56 | 80 | 129 | 113 |
| 25 | Glucosamine | 119 | 175 | 82 | 146 | 28 | 24 |
| 26 | Glutathione disulfide | 208 | 84 | 228 | 174 | 29 | 20 |
| 27 | Gly-Val | 228 | 111 | 43 | 139 | 112 | 36 |
| 28 | Guanosine | 139 | 199 | 53 | 160 | 28 | 24 |
| 29 | Gutathione | 76 | 159 | 73 | 146 | 23 | 31 |
| 30 | Harmane | 120 | 98 | 72 | 112 | 82 | 80 |
| 31 | His-Pro | 247 | 28 | 106 | 149 | 46 | 45 |
| 32 | Inosine | 187 | 77 | 51 | 145 | 65 | 63 |
| 33 | Isopentenyladenosine | 342 | 103 | 56 | 175 | 48 | 51 |
| 34 | Isosorbide | 121 | 160 | 137 | 32 | 15 | 13 |
| 35 | l-Abrine | 141 | 58 | 30 | 305 | 53 | 47 |
| 36 | l-Alanine | 146 | 61 | 154 | 117 | 30 | 29 |
| 37 | Lanosterol | 14 | 1 | 213 | 0.94 | 0.28 | 1 |
| 38 | l-Arginine | 120 | 110 | 98 | 139 | 132 | 109 |
| 39 | l-Asparagine | 162 | 53 | 54 | 143 | 16 | 10 |
| 40 | l-Aspartate | 179 | 29 | 67 | 166 | 75 | 58 |
| 41 | l-Carnitine | 83 | 87 | 112 | 135 | 51 | 47 |
| 42 | l-Citrulline | 317 | 72 | 115 | 35 | 110 | 110 |
| 43 | l-Glutamine | 107 | 157 | 67 | 63 | 38 | 30 |
| 44 | l-Histidine | 334 | 43 | 279 | 168 | 13 | 9 |
| 45 | Linoleic acid | 87 | 104 | 77 | 100 | 127 | 116 |
| 46 | Linoleoyl ethanolamide | 206 | 70 | 151 | 111 | 46 | 53 |
| 47 | l-Methionine | 443 | 19 | 16 | 99 | 11 | 12 |
| 48 | l-Tyrosine | 215 | 20 | 114 | 232 | 15 | 14 |
| 49 | l-Valine | 46 | 13 | 193 | 26 | 28 | 28 |
| 50 | Maltopentaose | 39 | 315 | 31 | 81 | 25 | 17 |
| 51 | Maltotriose | 38 | 301 | 50 | 174 | 19 | 17 |
| 52 | Meperidine | 76 | 20 | 395 | 30 | 14 | 23 |
| 53 | Miltefosine | 95 | 86 | 153 | 185 | 94 | 95 |
| 54 | N,N-dimethylsphingosine | 165 | 100 | 156 | 103 | 276 | 276 |
| 55 | N-acetyl-d-glucosamine | 17 | 534 | 14 | 30 | 15 | 13 |
| 56 | N-acetyl-l-tyrosine | 95 | 55 | 261 | 165 | 90 | 74 |
| 57 | Nalidixic acid | 44 | 170 | 15 | 53 | 215 | 215 |
| 58 | NG,NG-dimethyl-l-arginine (ADMA) | 207 | 81 | 177 | 185 | 39 | 14 |
| 59 | Nicotinamide | 157 | 141 | 75 | 181 | 4 | 4 |
| 60 | Oleic acid | 56 | 78 | 88 | 86 | 135 | 146 |
| 61 | Pantothenate | 63 | 87 | 288 | 138 | 5 | 5 |
| 62 | Phenyllactic acid | 197 | 61 | 112 | 131 | 27 | 25 |
| 63 | Phytosphingosine | 31 | 94 | 18 | 23 | 689 | 621 |
| 64 | p-Phenylenediamine | 95 | 102 | 97 | 99 | 99 | 98 |
| 65 | Pseudouridine | 76 | 204 | 44 | 75 | 46 | 37 |
| 66 | Pyridoxine | 70 | 247 | 32 | 112 | 25 | 21 |
| 67 | Ribitol | 25 | 190 | 21 | 38 | 184 | 163 |
| 68 | Serotonin | 174 | 13 | 4 | 266 | 15 | 16 |
| 69 | S-Methyl-5′-thioadenosine | 381 | 151 | 0.82 | 54 | 0.75 | 0.66 |
| 70 | Stachyose | 40 | 357 | 40 | 73 | 27 | 25 |
| 71 | Tetraethylene glycol | 27 | 36 | 37 | 21 | 13 | 15 |
| 72 | Tetramisole | 96 | 210 | 71 | 739 | 46 | 28 |
| 73 | Thymine | 135 | 71 | 96 | 250 | 71 | 71 |
| 74 | trans-Vaccenic acid | 60 | 99 | 90 | 89 | 151 | 150 |
| 75 | Triethylene glycol | 22.10 | 61 | 36 | 32 | 19 | 17 |
| 76 | Trimethoprim | 79 | 302 | 37 | 150 | 36 | 29 |
| 77 | Tropine | 88 | 114 | 51.31 | 131 | 224 | 193 |
| 78 | Tyramine | 211 | 46 | 119 | 138 | 28 | 22 |
| 79 | Uracil | 137 | 133 | 42 | 136 | 22 | 21 |
| 80 | Uridine | 144 | 124 | 45 | 129 | 24.02 | 22.49 |
| 81 | Xanthosine | 447 | 19 | 15 | 45 | 2.68 | 2.55 |
- Abbreviations: GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
3.3 Bacterial diversity and community structure
3.3.1 DNA sequencing
All colon content samples (n = 36) produced 415,315 original raw sequences. A total of 145,965 high-quality bacterial sequences (average 4054 sequences per sample) were obtained after sequence cleanup (Table 8).
| Group | Phylum | Class | Order | Family | Genus | Species | Unclassified | Total |
|---|---|---|---|---|---|---|---|---|
| C− | 7261 | 7261 | 7260 | 5713 | 2312 | 202 | 7 | 30,016 |
| C+ | 2121 | 2121 | 2121 | 2076 | 1554 | 36 | 2 | 10,031 |
| GLM | 4279 | 4279 | 4278 | 3483 | 1223 | 111 | 4 | 17,657 |
| POM | 4699 | 4699 | 4698 | 4029 | 1519 | 108 | 3 | 19,755 |
| PCM | 5333 | 5333 | 5333 | 4419 | 1754 | 155 | 7 | 22,334 |
| LEM | 5477 | 5477 | 5477 | 4579 | 1867 | 169 | 4 | 23,050 |
| HMM | 5510 | 5510 | 5510 | 4609 | 1844 | 134 | 5 | 23,122 |
| Total | 34,680 | 34,680 | 34,677 | 28,908 | 12,073 | 915 | 32 | 145,965 |
- Note: “Phylum”, “Class”, “Order”, “Family”, “Genus” and “Species” respectively correspond to the number of OTUs that can be classified into doors, classes, orders, families, genera, and species in each sample, and “Unclassified” refers to the number of OTUs that failed to belong to any known taxon.
3.3.2 OTU classification
Similarity among OTUs that were classified as belonging to different phylum, classes, orders, families, genus, and species (Table 8) based on 16S rRNA gene sequences revealed higher abundances for PCM, LEM, and HMM treatments at phylum and genus levels.
3.3.3 Principle component analysis
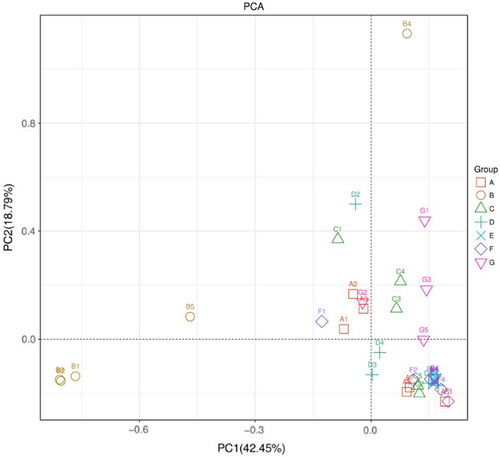
Principle component analysis analysis revealed clear divisions among treatment groups. Group B (C+) was “significantly different” from all other treatments, expressing a clear effect of mushroom treatments (p < .05) (Figure 1). More similarity was observed among A (C−), C (GLM), and G (HMM) compared with D (POM), E (PCM), and F (LEM) while similarity between E (PCM) and F (LEM) was higher.
3.3.4 Alpha diversity
Table 9 showed that indices of ACE, Chao1, Shannon, and Simpson were significantly affected by PCM and LEM treatments (p < .05), while ACE, Shannon and Simpson indexes were affected by HMM treatment (p < .01). Groups C+ and POM affected the Simpson index. All these four indices were lower in GLM treatment (p < .05).
| Treatments | ACE | Chao 1 | Shannon | Simpson |
|---|---|---|---|---|
| C− | 1138 ± 321ab | 1121 ± 330ab | 7.30 ± 0.9a | 0.96 ± 0.03a |
| C+ | 465 ± 117b | 448 ± 112b | 4.60 ± 0.9b | 0.78 ± 0.1b |
| GLM | 1018 ± 351ab | 993 ± 346ab | 6.54 ± 1ab | 0.92 ± 0.08a |
| POM | 1092 ± 627ab | 1042 ± 591ab | 6.59 ± 1ab | 0.93 ± 0.05a |
| PCM | 1598 ± 352a | 1542 ± 349a | 7.75 ± 0.3a | 0.97 ± 0.01a |
| LEM | 1303 ± 474a | 1249 ± 443a | 7.13 ± 0.8a | 0.96 ± 0.02a |
| HMM | 1319 ± 485a | 1239 ± 452ab | 6.82 ± 1a | 0.94 ± 0.03a |
| p-Value | .012 | .012 | .003 | .002 |
- Note: Values represented means ± standard deviation. Different letters represent a significant difference in Duncan test at p < .05. The first column in the table is groups, and the subsequent columns correspond to the calculation results of the diversity index of Chao1, ACE, Shannon, and Simpson and so on for each sample at the same sequencing depth.
- Abbreviations: C−, negative control; C+, positive control; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
3.3.5 Bacterial community composition (BCC)
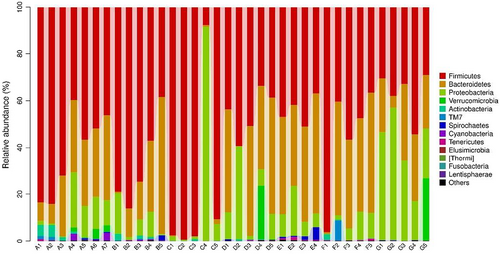
At phylum level, C+ treatment showed higher Firmicutes but lower Bacteroidetes abundances. In contrast, HMM treatment showed lower Firmicutes abundance. C+ treatment PCM treatment showed higher Bacteroidetes abundance. LEM treatment showed lower Proteobacteri and Verrucomicrobi abundances. In contrast, HMM treatment showed higher Proteobacteri and Verrucomicrobi abundances (Table 10 and Figure 2).
| Treatments | Firmicutes | Bacteroidetes | Proteobacteria | Verrucomicrobia |
|---|---|---|---|---|
| C− | 62 ± 17ab | 23 ± 11ab | 9 ± 8a | 1 ± 1a |
| C+ | 78 ± 39a | 0.69 ± 0.8b | 20 ± 39a | 0.019 ± 0.02a |
| GLM | 67 ± 19ab | 23 ± 22ab | 7.31 ± 6a | 0.036 ± 0.05a |
| POM | 45 ± 1b | 35 ± 20a | 14 ± 14a | 4.6 ± 10a |
| PCM | 44 ± 6b | 42 ± 6a | 10 ± 72a | 0.011 ± 0.008a |
| LEM | 55 ± 24ab | 35 ± 2a | 6 ± 5a | 0.0035 ± .007a |
| HMM | 36 ± 1b | 22 ± 1ab | 35 ± 16a | 5 ± 11a |
| p-Value | .05 | .006 | .169 | .577 |
- Note: Values represented means ± standard deviation. Different letters represent a significant difference in Duncan test at p < .05.
- Abbreviations: C−, negative control; C+, positive control; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
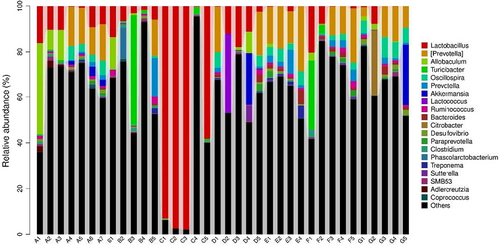
At genus level, Peptostreptococcaceae abundance was higher in C− and GLM treatments. Enterobacteriaceae abundance was higher in C+, POM, and HMM. Allobaculum abundance was high only in C− treatment (Table 11 and Figure 3).
| Genus | Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| C− | C+ | GLM | POM | PCM | LEM | HMM | ||
| Peptostreptococcaceae | 0.15 ± 0.2a | 0.06 ± 0.101a | 0.18 ± 0.2a | 0.03 ± 0.04a | 0.01 ± 0.001a | 0.02 ± 0.03a | 0.06 ± 0.09a | .296 |
| Enterobacteriaceae | 0.01 ± 0.001a | 0.2 ± 0.3a | 0.07 ± 0.06a | 0.07 ± 0.1a | 0.01 ± 0.01a | 0.01 ± 0.005a | 0.15 ± 0.1a | .427 |
| Desulfovibrionaceae | 0.07 ± 0.07a | 0.01 ± 0.001a | 0.01 ± 0.001a | 0.04 ± 0.05a | 0.09 ± 0.07a | 0.05 ± 0.05a | 0.06 ± 0.05a | .117 |
| Ruminococcaceae | 0.04 ± 0.03bc | 0.01 ± 0.001c | 0.03 ± 0.03bc | 0.05 ± 0.03bc | 0.09 ± 0.03a | 0.07 ± 0.05ab | 0.03 ± 0.01bc | .008 |
| Allobaculum | 0.09 ± 0.01a | 0.01 ± 0.001a | 0.03 ± 0.06a | 0.02 ± 0.04a | 0.01 ± 0.007a | 0.01 ± 0.01a | 0.01 ± 0.01a | .376 |
| Turicibacter | 0.01 ± 0.003a | 0.01 ± 0.01a | 0.10 ± 0.02a | 0.01 ± 0.001a | 0.01 ± 0.001a | 0.07 ± 0.1a | 0.01 ± 0.001a | .464 |
| Oscillospira | 0.03 ± 0.02abc | 0.01 ± 0.001c | 0.01 ± 0.003c | 0.02 ± 0.03bc | 0.04 ± 0.015ab | 0.02 ± 0.01bc | 0.06 ± 0.03a | .005 |
| Lachnospiraceae | 0.01 ± 0.01bc | 0.01 ± 0.001c | 0.01 ± 0.01bc | 0.03 ± 0.03abc | 0.05 ± 0.03a | 0.03 ± 0.003ab | 0.02 ± 0.01bc | .032 |
| Prevotella | 0.01 ± 0.009a | 0.01 ± 0.001a | 0.03 ± 0.07a | 0.01 ± 0.009a | 0.04 ± 0.04a | 0.02 ± 0.02a | 0.01 ± 0.001a | .374 |
| Akkermansia | 0.01 ± 0.01a | 0.01 ± 0.001a | 0.01 ± 0.001a | 0.05 ± 0.1a | 0.01 ± 0.001a | 0.01 ± 0.001a | 0.05 ± 0.1a | .577 |
| Clostridiaceae | 0.02 ± 0.02a | 0.02 ± 0.027a | 0.03 ± 0.02a | 0.01 ± 0.002a | 0.00 ± 0.001a | 0.03 ± 0.06a | 0.01 ± 0.01a | .664 |
| Ruminococcus | 0.01 ± 0.005ab | 0.01 ± 0.002b | 0.02 ± 0.01a | 0.01 ± 0.001ab | 0.01 ± 0.003ab | 0.01 ± 0.003ab | 0.01 ± 0.004ab | .219 |
| Bacteroides | 0.01 ± 0.003ab | 0.01 ± 0.001b | 0.01 ± 0.002ab | 0.01 ± 0.01ab | 0.02 ± 0.02a | 0.01 ± 0.008ab | 0.01 ± 0.01ab | .037 |
- Note: Values represented means ± standard deviation. Different letters represent a significant difference in Duncan test at p < .05.
- Abbreviations: C−, negative control; C+, positive control; GLM, Ganoderma lucidum mushroom; HMM, Hypsizigus marmoreus mushroom; LEM, Lentinus edodes mushroom; PCM, Pleurotus citrinopileatus mushroom; POM, Pleurotus ostreatus mushroom.
4 DISCUSSION
Mushrooms have antihyperglycemic effects on diabetic individuals. LEM and HMM treatments showed lower plasma glucose levels. LEM and PCM changed the ACE, Chao1, Shannon, and Simpson microbial indices significantly. Dietary supplementation of mushrooms reduced plasma glucose level directly due to mushrooms' bioactive compounds (agmatine, sphingosine, pyridoxine, linolenic, and alanine) and indirectly through oligosaccharide (stachyose) and gut microbiota modulation. Thus, LEM and HMM can be used as healthy food ingredients to improve gut microbiome composition in diabetic subjects.
Agmatine shows an antihyperglycemic effect through increasing insulin secretion and glucose uptake in muscles, and preventing hepatic gluconeogenesis via β-endorphin secretion enhancement (Su et al., 2009). Since agmatine simulates insulin secretion via inhibiting the ATP-sensitive potassium (KATP) channels in β-islet cells (Chang et al., 2010; Malaisse et al., 1989; Naoki & Fujiwara, 2019; Nissim et al., 2006, 2014; Shepherd et al., 2012; Su et al., 2008). Such inhibited KATP channel elevates the ATP/ADP ratio, leading to K+ accumulation. This cell depolarization simulates a voltage-gated Ca2+ channel activity, resulting in Ca2+ influx and consequent insulin secretion (Velasco et al., 2016). In this study, mushroom treatments (LEM and HMM) showed the lowest blood glucose level, which can be explained by the high content of agmatine in LEM and HMM mushrooms. At the intestinal microbiota level, intestinal bacteria altered insulin secretion via converting arginine to agmatine. Such conversion can be catabolized by bacterial acid-resistant mechanisms, such as Escherichia coli and Enterococcus faecalis (Naoki & Fujiwara, 2019). In our study, LEM treatment showed a high level of Bacteriodetes and Firmicutes phyla, whereas the HMM treatment showed a high level of Proteobacteria. That LEM and HMM mushrooms could manipulate the microbiota composition leading to an increased level of secreted insulin.
Sphingosine (PHS) has a potential therapeutic effect on type II diabetes (Nagasawa et al., 2018). Since PHS activates omega-3 fatty acid receptor (GPR120) mediating potent insulin-sensitizing effects. Such activation promotes incretin GLP-1 secretion, which is notable for having an effects on an anti-metabolic syndrome (Nagasawa et al., 2018). In this study, the HMM mushroom showed the highest level of PHS bioactive compound along with lower blood glucose levels (Rudd et al., 2020). In addition, the HMM mushroom showed the highest level of sphingosine along with lower blood glucose levels.
Pyridoxine decreases insulin resistance via scavenging the pathogenic reactive carbonyl species (Haus & Thyfault, 2018). Such molecule damages insulin protein via covalent modification of some structural amino acids, as well as, via the formation of adducts with phospholipids and DNA (Haus & Thyfault, 2018). In the current study, the GLM mushroom showed the highest level of pyridoxine bioactive compound along with lower blood glucose levels.
Nutritive acids could have a controversial effect on diabetic individuals. For instance, α-linolenic acid is a source for the generated oxylipins. Such molecules are lipid mediators affecting type 1 diabetes (Buckner et al., 2021). In our study, the LEM and HMM mushrooms showed the highest level of linolenic acid along with lower blood glucose levels. However, alanine may induce hyperglycemia in diabetic individuals. Since alanine aminotransferases increased levels are marked in diabetes hepatic cells (Okun et al., 2021). In the current study, the LEM mushroom showed the highest level of alanine along with lower blood glucose levels. The amino acids and fatty acids classes should be considered in the research of diabetic individuals' diet.
In literature, the mushrooms showed different polysaccharides and their effect on diabetes, for example, G. lucidum (polyheterosaccharides); P. ostreatus, (polyheterosaccharides); P. citrinopileatus (acid polysaccharide); L. edodes (glucan, heteropolysaccharides); and H. marmoreus (glycoprotein). Chlorella pyrenoidosa polysaccharides with a low molecular weight (>3000 Da) showed an hypolipidemic effect in rat (Agunloye & Oboh, 2022; Hossain et al., 2021; Jayasuriya et al., 2015; Qiu et al., 2022). Chisandra sphenanthera polysaccharide (191.18 kD) showed antidiabetic effect in rats with type 2 diabetes (Niu et al., 2017). Dendrobium officinale leaf polysaccharides of different molecular weights were orally administered daily at 200 mg/kg/day, this level alleviated type II diabetes in an adult mouse (Fang et al., 2022). Thus, polysaccharides have an effect on diabetes whether they are low or high molecular weights. Stachyose is a non-reducing tetrasaccharide molecule which decreases the blood glucose level in alloxan-induced diabetic rats (Zhang et al., 2004). In addition, stachyose adjusts blood lipid levels in diabetic individuals (Chen et al., 2019). In the current study, the GLM mushroom showed the highest level of stachyose bioactive compound along with lower blood glucose levels. At the intestinal microbiota level, stachyose as a functional oligosaccharide regulates the intestinal microflora balance. Such prebiotic shifts of gut microbiota including Bifidobacterium and Lactobacillus as they are two common genera affecting a host health (Liu, Jia, et al., 2018; Liu, Wang, et al., 2018). In this study, the LEM and HMM mushrooms showed the highest level of Lactobacillus genus along with lower blood glucose levels. LEM and HMM mushrooms could modulate blood glucose levels and intestinal microbiota in diabetic individuals.
Changes in the gut microbiota composition are associated with multiple chronic disease pathologies, such as type 2 diabetes mellitus (Tang et al., 2017). Dietary fiber intake protects against diabetes by lowering dietary glycemic (Anderson et al., 2009). For example, oyster and button mushrooms have hypoglycemic effects, which reduce the fasting blood glucose level (Shehata et al., 2010). G. lucidum extract reduces blood glucose and insulin levels in rats (Hikino et al., 1985). Dietary supplements of I. bartlettii, Bifidobacterium longum, and B. cellulosilyticus in combination with water-soluble viscous fibers improve glucose homeostasis and dyslipidemia. Since gut microbiota affects insulin resistance by decreasing TNF- α level in plasma and improving fasting blood glucose level in mice fed a high-fat diet (Chuang et al., 2012).
Mushroom intake increases lactic acid-producing bacteria (Lactobacillus, Lactococcus and Streptococcus) and SCFA-producing bacteria (Allobaculum, Bifidobacterium and Ruminococcus), which can be explained by the mushroom's fiber content (Takamitsu et al., 2018). Butyrate-producing R. inulinivorans abundance is higher in healthy individuals than in T2D individuals (Tang et al., 2017). F. prausnitzii abundance is low in individuals with T2D (Karlsson et al., 2013; Qin et al., 2012). Insulin resistance is related to B. wadsworthia and C. bolteae abundances (Qin et al., 2012). The species A. muciniphila, B. faecis, B. nordii, B. cellulosilyticus, B. pectinophilus, I. bartlettii, O. splanchnicus, D. longicatena, and R. inulinivorans were negatively associated with insulin resistance or dyslipidemia (Brahe et al., 2015). Bifidobacterium (B. longum) abundance was higher in healthy individuals than in obese individuals and T2D (Karlsson et al., 2013). The more decrease in butyric acid production, the more decrease in C. leptum abundance (Wang et al., 2015). Butyrate has an anti-inflammatory activity that could improve insulin resistance (Brahe et al., 2013). F. prausnitzii affects insulin sensitivity, which may be due to its ability to produce butyrate (Louis & Flint, 2009). In this study, Allobaculum was increased in positive control treatment, whereas the Ruminococcus was increased in GLM treatment.
Regarding the mushroom mix potence, as individual mushrooms showed a significant effect on blood glucose level that mushroom mix could provide more effective role in blood glucose control regarding the gathered bioactive compounds and their possible compatible roles. Future studies are required to investigate the potency of mushroom mix with more diabetes parameters to reveal the underlying mechanism on diabetes-based long-term treatment.
5 CONCLUSIONS
Dietary supplementation with mushrooms reduced the plasma glucose level and modulated gut microbiota in diabetic rats. Mushrooms showed a direct antihyperglycemic effect due to their content of agmatine, stachyose, phytosphingosine, and pyridoxine bioactive compounds. Mixed dietary mushrooms could develop a food ingredient with an effective and specific health functionality for diabetic individuals.
ACKNOWLEDGMENT
This research was funded by the Development of Standard Models for Domestic Animal and Poultry Production of China (2017YFD0502001).
CONFLICT OF INTEREST
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




