Isomerization of anacardic acid: A possible route to the synthesis of an unsaturated benzolactone and a kairomone
Abstract
Crystalline unsaturated lactone, 8-hydroxy-3-tridecyl-1H-isochromen-1-one (6) has been synthesized by isomerization of anacardic acid having heterogeneous alkyl side chains (a mixture of mono-, di-, and tri-unsaturated anacardic acid) (1). Hydrogenation of 8-hydroxy-3-tridecyl-1H-isochromen-1-one produced a saturated lactone, 8-hydroxy-3-tridecyl-3,4-dihydroisochromen-1-one (7). Isomerization of monoene anacardic acid resulted in a crystalline isoanacardic acid, (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid (8) as a major product. This was then metathesized with 2-butene to give 3-prop-1-enylphenol (10). Both isomerization reactions used a 1,2-bis(ditertiarybutylphosphinomethyl)benzene modified palladium catalyst. The two products, 8-hydroxy-3-tridecyl-1H-isochromen-1-one and (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid have been crystallographically characterized.
Practical applications: Unsaturated lactones are structural elements often found in natural products, which have medicinal value. Benzolactones derived from anacardic acid reported in this work have some structural similarity with lactones such as massoia lactone having medicinal value. Therefore with this idea in mind, the unsaturated benzolactones reported in this work will be tested for their anti pathogenic activity. 3-Propylphenol is used in combination with racemic 1-octen-3-ol and p-cresol to prepare a kairomone for tsetse fly traps. Results from this work describe the suitability of anacardic acid for synthesizing 3-propylphenol. The fact that 3-propylphenol can be synthesized from anacardic acid, a component of cashew nut shell liquid is of particular interest since most of the areas affected with tsetse flies are suitable for growing cashew plants. This means the raw materials (CNSL) for synthesis of 3-propylphenol will be obtained from within the same region where the kairomone is to be applied, although we appreciate that specialized facilities would be required for the types of transformation described.
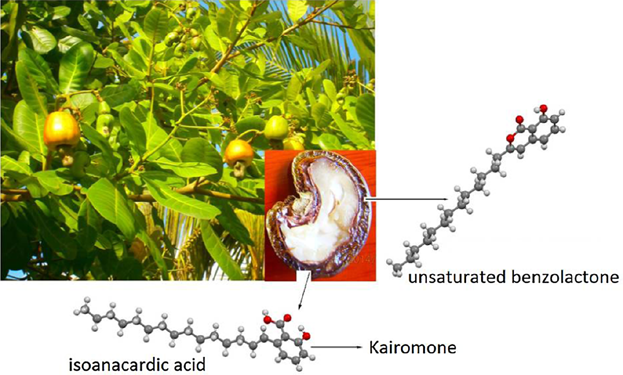
Unsaturated benzolactone and a desired isomer of anacardic acid for synthesis of kairomone have been synthesized by isomerization of heterogeneous and monoene anacardic acid, respectively.
Abbreviations
-
- CNSL
-
- cashew nut shell liquid
-
- dba
-
- dibenzyldeneacetone
-
- DTBPMB
-
- bis(ditertiarybutylphosphinomethyl)benzene
1 Introduction
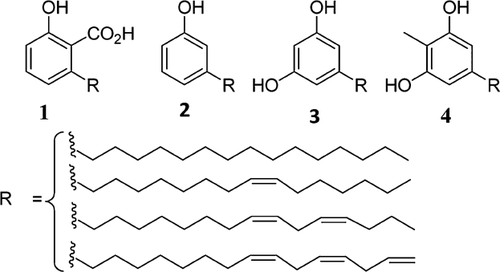
Synthesis of useful chemicals from renewable resources, which are not in competition with food production is of particular importance in the current efforts to replace non-renewable resources. One example of a potential renewable resource, which is attracting the attention of researchers in the synthesis of useful chemicals is cashew nut shell liquid (CNSL). CNSL is a by-product of cashew processing factories consisting of anacardic acid (1), cardanol (2), cardol (3), and methylcardol (4) (Fig. 1) 1. The structures of these components present a multitude of possibilities for converting them into diverse chemicals. For instance, the presence of double bonds in their side chains and the phenol group allow techniques such as metathesis 2, 3, polymerization 4, 5, ozonolysis 6, and isomerization 7 to be employed to transform them into more useful chemicals. The side alkyl chains of these components are heterogeneous in the sense that they are mixtures of one, two, and three double bonds. Cardanol can be selectively converted to the monoene component by using ruthenium trichloride as a transfer hydrogenation catalyst and propan-2-ol as the reducing agent 8. Isomerization of the double bond of cardanol from its C-8 natural position in the chain toward the aromatic ring makes cardanol a potential precursor for synthesis of 3-propylphenol, a kairomone component, albeit in low yield 7. Synthesis of 3-propylphenol on a kilogram scale is achieved by reacting 3-hydroxybenzaldehyde with ethylmagnesium bromide in tetrahydrofuran (THF) using toluene as co-solvent. However, this reaction produces salt wastes from the Grignard reagent 9. In this paper, we report the synthesis of monoene anacardic acid and its isomerization in an attempt to improve the yield of the desired isomer for synthesis of 3-propylphenol, which can potentially also be further elaborated by cross metathesis to cinnamic acids following the precedent for 4-propenylphenols 10. We employ the isomerization catalytic system of Pd2(dba)3, DTBPMB (bis(ditertiarybutylphosphinomethyl)benzene), and methane sulfonic acid7, 11-14 to isomerize the double bond of monoene anacardic acid from deep in the chain (C-8) toward the aromatic ring. During the course of the reaction, the double bond isomerizes backwards and forwards along the chain 15, 16.
In our initial studies on the isomerization of cardanol 7, we anticipated that the double bond would be trapped in the desired 1 position by conjugation with the aromatic ring. In fact, the maximum fraction of this conjugated isomer was only about 40% with the remaining 60% being a mixture of all other double bond positional isomers along the chain. In this paper, we show that, using anacardic acid in place of cardanol, the proportion of the conjugated isomer increases to 67%.
3-Propylphenol is one the constituent phenols of cow and buffalo urine. Other components are: 4-methylphenol, 3-methylphenol, 4-ethylphenol, 3-ethylphenol, 4-n-propylphenol, and 2-methoxyphenol 17. Because of their ability to attract tsetse flies, these phenolic components of cattle and buffalo urine have been used together with traps and insecticides to control tsetse flies in an effort to fight against trypanosomiasis 17.
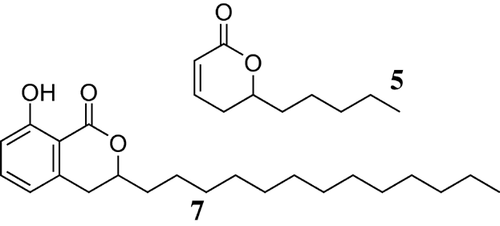
We also report the synthesis of unsaturated and saturated lactones from isomerization of a mixture of monoene, diene, and triene anacardic acid using the same catalytic system of Pd2(dba)3, DTBPMB, and methane sulfonic acid. Unsaturated lactones are structural elements often found in natural products, which have medicinal value. One example of an unsaturated lactone is massoia lactone (Fig. 2), which has been long been used as a constituent of native medicines 18, 19. Massoia lactone and analogs are known to possess good antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, and Esherichia coli. They have also been reported as potential anticancer and anti-inflammatory agents 18.
2 Materials and methods
2.1 Materials and instruments
All reagents and solvents were purchased from Sigma–Aldrich and used as received. Silica for chromatography was Davisil Grade 62. The cashew nuts were collected from Tanga, Tanzania and shelled. CNSL was obtained from the shells by the solvent extraction method 20. All reactions were carried out using standard Schlenk lines and glove box techniques with nitrogen gas for maintaining an air free environment. GC–MS analyses were carried out using a Hewlett-Packard 6890 series gas chromatograph instrument equipped with a FID for quantitative analysis and a Hewlett-Packard 5973 series mass selective detector fitted with hp1 film for mass spectral identification of products. The temperature program used was 50°C (4 min), 20°C/min to 130°C (2 min), 20°C/min to 220°C (15.5 min). Helium was used as the carrier gas with initial flow of 1 mL/min. 1H and 13C NMR spectra were recorded at 298 K on a Bruker 300 MHz or 400 and 75 MHz or 100 MHz spectrometers for 1H NMR and 13C NMR, respectively. Samples for NMR analysis were dissolved in deuterated solvents, the peaks of which were used to reference the spectra to tetramethylsilane at δ 0 ppm. The IR spectra were recorded on Perkin Elmer Spectrum GX FT-IR spectrometer using KBr discs.
2.2 Extraction of CNSL and isolation of anacardic acid
Fresh and dry cashew nuts were scrub washed, sun dried, and shelled to obtain clean shells. The shells (2000 g) were soaked in petroleum ether (5000 mL) for 4 days. The resulting solution was concentrated at 40°C under reduced pressure using a rotary evaporator to afford a brownish oil, CNSL. The recovered solvent was used to re-extract the oil to ensure maximum yield (362 g, 18%). Isolation of anacardic acid followed the procedure reported by Paramashivappa et al. 1 starting with 50 g of CNSL to yield 29 g (58%) of anacardic acid. 1H NMR (400 Hz; CDCl3): δ 11.06 (s, 1H, COOH), 7.40 (t, J = 8.0, 1H, ArH), 6.92 (d, J = 8.8, 1H, ArH), 6.82 (d, J = 8.0, 1H, ArH), 5.94–5.77 (m, 0.4H, CHCH), 5.52–5.31 (m, 3.2H, CHCH), 5.14–4.97 (m, 0.8H, CHCH), 3.03 (t, J = 7.6, 2H, ArCH2), 2.90–2.75 (m, 2H, CH2), 2.17–1.95 (m, 3.4H, CCCH2), 1.74–1.56 (m, 2H, ArCCH2), 1.49–1.24 (m, 14H, alkyl chain), 1.0–0.86 (m, 2.1H, terminal CH3/CH2). 13C NMR (100 Hz; CDCl3): δ 14.7 (CH3), 23.2, 26.0, 27.6, 30.0, 32.4, 37.1 (CH2), 115.2 (ArCCOOH), 116.4 (ArCH), 127.5, 128.2, 128.6, 129.8, 130.4 (CH), 136.1 (ArCH), 148.4 (ArCR), 164.13 (ArCOH), 176.91 (ArCOOH). IR: 914.4 cm−1 (m), 1233.3 cm−1 (s), 1447.4 (m), 1644.7 cm−1 (s), 2924 cm−1 (m), 3016.4 cm−1 (m), 3430 cm−1 (s, b).
2.3 Unsaturated benzolactone, 8-hydroxy-3-tridecyl-1H-isochromen-1-one (6)
Anacardic acid (2 g, monoene 52.6%, diene 18.3%, triene 28.9%, and saturated 1.1%) was dissolved in methanol (2 mL) followed by addition of toluene (10 mL). The Schlenk tube containing the solution was connected to a Schlenk line and left under nitrogen for 10 min. In the glove box, Pd2(dba)3 (0.28 g, 0.00030 mol) and DTBPMB (1.23 g, 0.0026 mol) were put into an autoclave charged with a magnetic stirrer. The autoclave was connected to the Schlenk line and degassed three times. By means of a 20 mL syringe, the whole solution of anacardic acid was transferred into the autoclave followed by addition of methane sulfonic acid (0.2 mL). The isomerization reaction was left to proceed under nitrogen atmosphere at 80°C for 96 h. Separation of the products was done by column chromatography using hexane/ethyl acetate (90/10) to separate 8-hydroxy-3-tridecyl-1H-isochromen-1-one (6, 0.3 g, 15%) followed by hexane/ethyl acetate (80/20) to separate (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid, 8 contaminated with other double bond positional isomers (0.2 g, 10%; 67% (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid, 33% other positional isomers. This corresponds to a yield of (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid of 6.7%) (Fig. 6). Crystals of 6 suitable for structure determination were obtained by layering a dichloromethane solution with methanol and slow diffusion. Found C 73.49, H 9.14%; C22H32O3 · 0.2CH2Cl2 requires C 73.76, H 9.03%. 1H NMR (400 Hz; CDCl3): δ 0.91 (t, J = 6.7, 3H, CH3), 1.22–1.44 (m, 20H, CH2), 1.68–1.79 (m, 2H, CH2), 2.54 (t, J = 7.8, 2H, CH2), 6.29 (s, 1H, CH), 6.84 (d, J = 7.9, 1H, ArH), 6.95 (d, J = 8.7, 1H,ArH), 7.58 (t, J = 7.8, 1H, ArH), 11.06 (s, 1H, ArOH). 13C NMR (100 Hz; CDCl3): δ 14.5 (CH3), 23.2, 27.4, 29.9, 32.7, 33.8 (CH2), 104.4 (CH), 114.8 (ArCH, ArCCOOH), 116.1 (ArCH), 137.8 (ArCH), 138.7 (ArCR), 158.0 (ArCOOH), 161.9 (CCO), 167.4 (ArCOH). IR: 725 (m), 1146.9 (s), 1233.5 (s), 1470.3 (m), 1574.3 (m), 1649.3 (s), 1689.8 (s), 2856.4 (m), 2920 (s), 3445 (s, b). (E)-2-Hydroxy-6-(pentadec-1-enyl)benzoic acid; Found C 76.09, H 10.01%; C22H34O3 requires C 76.26, H 9.89% 1H NMR (400 Hz; CDCl3): δ 0.81 (t, J = 6.6, 3H, CH3), 1.11–1.48 (m, 22H, CH2, alkyl chain), 2.17 (q, J = 7.1, 2H, CH2), 5.89–6.0 (m, 1H, CCH), 6.84 (d, J = 8.1, 1H, ArH), 6.9 (d, J = 7.6, 1H, ArH), 7.01, 6.96 (s, 2H, ArCCHC), 7.33 (t, J = 8.1, 1H, ArH), 10.93 (s, 1H, COOH). 13C NMR (100 Hz; CDCl3): δ 14.1 (CH3), 22.7, 29.5, 31.3, 33.1, 36.1 (CH2), 110.4 (ArCCOOH), 116.1 (ArCH), 120.6 (ArCH), 123.1 (ArCHCH), 130.3 (CCH), 135.8 (ArCH), 143.3 (ArC), 163.4 (ArCOH), 176.1 (ArCCOOH).
2.4 Saturated benzolactone, 8-hydroxy-3-tridecyl-3,4-dihydroisochromen-1-one (7)
8-Hydroxy-3-tridecyl-1H-isochromen-1-one (0.3 g) was dissolved in ethyl acetate (10 mL). The solution was transferred into the autoclave charged with a magnetic stirrer followed by addition of Pd-C (0.03 g). The autoclave was pressurized with hydrogen to 30 bar. The reaction was left to proceed at 80°C for 12 h. The product was filtered using a sintered funnel with the aid of celite powder to remove the catalyst. Ethyl acetate was removed under vacuum to yield a white solid, saturated benzolactone (0.291 g, 97%). Found C 76.17, H 9.84%; C22O34 requires C 76.26, H 9.89%. 1H NMR (400 Hz; CDCl2): δ 0.91 (t, J = 6.6, 3H, CH3), 1.3 (m, 22H, CH2, alkyl chain), 1.69–1.81, 1.85–1.97 (m, CH2), 2.96 (2H, ArCCH2R), 4.54–4.65 (m, 1H, RCO(H)R), 6.71 (d, J = 7.5, 1H, ArH), 6.91 (d, J = 8.4, 1H, ArH), 7.43 (t, J = 8.4, 1H, ArH), 11.06 (s, 1H, ArOH). 13C NMR (100 Hz; CDCl3): δ 14.5 (CH3), 23.1, 25.2, 29.9, 32.3, 33.4, 35.2 (CH2), 80.16 (CH, RCO(H)R), 116.49 (ArCH), 118.37 (ArCH), 136.48 (ArCH), 139.9 (ArCR). IR: 738.9 cm−1 (m), 1035 cm−1 (m), 1127.6 cm−1 (m), 1245.5 cm−1 (s), 1467.1 cm−1 (m), 1585 cm−1 (w), 1660 cm−1 (s), 2854 cm−1 (m), 2923.5 cm−1 (s), 2958.1 cm−1 (m), 3441.6 cm−1 (b).
2.5 Monoene anacardic acid
The procedure follows that developed by Perdriau et al. 8 for cardanol. Anacardic acid (2.5 g, monoene 52.6%, diene 18.3%, triene 28.9%, and saturated 1.1%) was dissolved in propan-2-ol (20 mL) in a 100 mL round bottomed flask to which ruthenium trichloride (0.075 g, 0.36 mmol) was added. The reaction mixture was refluxed for 18 h. Propan-2-ol was removed from the product under reduced pressure using the rotary evaporator. The concentrated product was eluted through a silica column using dichloromethane to remove the catalyst. The final appearance of monoene anacardic acid was a grayish semi solid (1.7 g, 68%). The gray color presumably arises from Ru residues, which we have not been able to remove, but which do not appear to affect the subsequent reactions. Found C 76.12, H 9.80%; C22O34 requires C 76.26, H 9.89%. 1H NMR (400 Hz; CDCl2): δ 0.91 (t, J = 6.9, 3H, CH3), 1.21–1.48 (m, 15H, CH2, alkyl chain), 1.58–1.70 (m, 2H, ArCCCH2), 1.94–2.1 (m, 4H, CH2CC), 3.02 (t, J = 7.7, 2H, ArCCH2), 5.33–5.50 (m, 2H, CHCH), 6.81 (d, J = 7.7, 1H, ArH), 6.91 (d, J = 8.6, 1H, ArH), 7.40 (t, J = 8.2, 1H, ArH), 11.04 (s, 1.4H, COOH). For this compound, as for anacardic acid itself, the signal at δ 11.04 consists of a reasonably sharp singlet with a very broad resonance underneath it. There is no resonance observed in the position expected for the phenolic OH proton (ca. 5 ppm). We assume that the phenolic proton is hydrogen bonded to the carbonyl O atom of the carboxylic acid, as seen in the crystal structure, moving the resonance to δ 11.4 ppm. The integration for this signal is still low, however, corresponding to ca. 1.5 protons. We note that the phenolic OH protons of the lactones, 6 and 7 also resonate near δ 11 ppm. 13C NMR (100 Hz; CDCl3): δ 14.1 (CH3), 22.7, 27.2, 29.5, 32.0, 36.5 (CH2), 110.6 (ArCCOOH), 116 (ArCH), 122.6 (ArCH), 130.1 (CC), 135.6 (ArCH), 148.0 (ArCR), 163.7 (ArCOH), 176.6 (ArCCOOH). IR: 725.2 cm−1 (m), 825.1 cm−1(m), 910.9 cm−1(m), 970.3 cm−1 (m), 1254.1 cm−1 (s), 1452.1 cm−1 (s), 1663.3 cm−1 (s), 2862.2 (s), 2937.3 (s), 3000.3 (s, b).
2.6 (E)-2-Hydroxy-6-(pentadec-1-enyl)benzoic acid (8)
The synthesis of (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid from monoene anacardic acid was as for the synthesis of unsaturated benzolactone (Section 2.3). The column chromatography separation employed ethyl acetate/hexane (20/80) as the eluting system from which a white solid (0.8 g, 40%) comprising of the desired (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid (67%) and other double bond positional isomers (33%) was obtained. The product was recrystallized from warm hexane in a freezer. Crystals of 8 suitable for structure determination were obtained by layering a methanol solution with petroleum ether and slow diffusion. Found C 76.09, H 10.01%; C22H34O3 requires C 76.26, H 9.89%. 1H NMR (400 Hz; CDCl3): δ 0.81 (t, J = 6.6, 3H, CH3), 1.11–1.48 (m, 22H, CH2, alkyl chain), 2.17 (q, J = 7.1, 2H, CH2), 5.89–6.0 (m, 1H, CCH), 6.84 (d, J = 8.1, 1H, ArH), 6.9 (d, J = 7.6, 1H, ArH), 7.01, 6.96 (s, 2H, ArCCHC), 7.33 (t, J = 8.1, 1H, ArH), 10.93 (s, 1H, COOH). 13C NMR (100 Hz; CDCl3): δ 14.1 (CH3), 22.7, 29.5, 31.3, 33.1, 36.1 (CH2), 110.4 (ArCCOOH), 116.1 (ArCH), 120.6 (ArCH), 123.1 (ArCHCH), 130.3 (CCH), 135.8 (ArCH), 143.3 (ArC), 163.4 (ArCOH), 176.1 (ArCCOOH).
2.7 Cis-2-butene metathesis of (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid
(E)-2-Hydroxy-6-(pentadec-1-enyl)benzoic acid (0.6 g, 1.73 mmol) was dissolved in dichloromethane (5 mL) in a Schlenk tube where it was bubbled with nitrogen for 10 min. Hoveyda-Grubbs 2nd generation catalyst, [1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(o-isopropoxyphenylmethylene)ruthenium) (0.01 g, 0.016 mmol) was measured and put into the autoclave charged with a magnetic stirrer. (E)-2-Hydroxy-6-(prop-1-enyl)benzoic acid solution was transferred by means of a syringe to the autoclave under nitrogen followed by addition of cis-2-butene (6 mL). The reaction was left under stirring at RT for 24 h. Analysis by GC–MS of the product mixture showed poor conversion of (E)-2-hydroxy-6-(prop-1-enyl)benzoic acid.
2.8 Decarboxylation of (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid
Isoanacardic acid, (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid (0.5 g) was placed in a 10 mL Schlenk tube. It was heated at 190°C in a graphite bath for 3 h to produce a brownish oil, (E)-3-(pentadec-1-enyl)phenol(isocardanol) (0.43 g, 98%; isocardanol 66%, other double bond positional isomers 32%). 1H NMR (400 Hz; CDCl3): δ 0.91 (t, J = 6.8, 3.8H, CH3), 1.16–1.42 (m, 24H, CH2), 1.46–1.73 (m, 2H, CH2), 1.90–2.11 (m, 1.5H, CH2), 2.17–2.28 (q, J = 7.0, 0.6H, CH2), 2.57 (t, J = 8.4, 0.7H, CH2), 5.31–5.5 (m, 0.7H, CH2), 6.17–6.40 (m, 0.5H, CH), 6.69 (s,1H, ArH), 6.78 (d, J = 8.7, 0.5H, ArH), 6.86 (s, 0.5H), 6.93 (d, J = 8.0, 0.5H, CH), 7.17 (t, J = 6.5, 1H, ArH). 13C NMR (100 Hz; CDCl3): δ 14.7 (CH3), 23.2, 29.9, 31.7, 32.4, 33.3, 36.6, (CH2), 112.9 (ArCH), 115.7 (ArCH), 119.4 (ArCH), 121.4 (ArCHCH), 129.7 (CHCH), 132.2 (ArCH), 156.2 (ArCOH).
2.9 Synthesis of 3-prop-1-enylphenol
Monounsaturated anacardic acid (2 g) was isomerized as in Section 2.6, above. After the 96 h reaction time, the solution was filtered through celite to remove the catalyst and evaporated to dryness. The oil was then treated as in Section 2.8 to effect decarboxylation. The (E)-3-(pentadec-1-enyl)phenol so formed was dissolved in dichloromethane (5 mL) in a Schlenk tube where it was bubbled with nitrogen for 10 min. Hoveyda-Grubbs 2nd generation catalyst, [1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(o-isopropoxyphenylmethylene)ruthenium) (0.02 g, 0.032 mmol) was measured and put into the autoclave charged with a magnetic stirrer. The (E)-3-(pentadec-1-enyl)phenol solution was transferred by means of a syringe to the autoclave under nitrogen followed by addition of cis-2-butene (6 mL). The reaction was left stirring at RT for 24 h. The product mixture was analyzed by GC–MS (Fig. 9). Separation of the products was by column chromatography using hexane/ethyl acetate (90/10) to isolate the desired product (though still contaminated with about 15% other chain length products); (E)-3-(prop-1-enyl)phenol (137 mg, 18%) 1H NMR (400 Hz; CDCl3): δ 1.79 (d, J = 6.6, 2.5H, CH3), 6.07–6.30 (m, 1.6H, CH), 6.53–6.63 (m, 1.5H, ArH, CH), 6.73 (s, 0.9H, ArH), 6.82 (d, J = 7.8, 0.9H, ArH), 7.07 (t, J = 7.8, 1H, ArH). 13C NMR (100 Hz; CDCl3): δ 19.0 (CH3), 112.9 (ArCH), 114.4 (ArCH), 119.0 (ArCH), 126.7 (CH), 130.2 (ArCH), 131.1 (ArCR), 156.4 (ArCOH).
2.10 X-ray crystallography
XRD data for compounds 6 and 8 were collected at 173 K by using either a Rigaku MM-007HF High intensity microfocus RA generator/confocal optics (Cu Kα radiation, λ = 1.5406 Å) and Rigaku XtaLAB P100 system (6), or a Rigaku MM-007 High brilliance RA generator/confocal optics (Cu Kα radiation, λ = 1.5406 Å) and Saturn92 CCD system (8). Intensity data were collected using ω and ϕ steps (6) or ω steps (8) accumulating area detector images spanning at least a hemisphere of reciprocal space. All data were corrected for Lorentz polarization effects. A multiscan absorption correction was applied by using CrystalClear 21. Structures were solved by direct methods (SHELXD-2013 22 or SIR2004) 23 and refined by full-matrix least-squares against F2 (SHELXL-2013) 22. Non-hydrogen atoms were refined anisotropically, and carbon-bound hydrogen atoms were refined using a riding model. In 8, hydrogen atoms bound to oxygen were located from the difference Fourier map and refined isotropically subject to a distance restraint. In 6, the hydrogen atom bound to oxygen could not be located from the difference Fourier map, so was placed in calculated position and refined using a riding model. All calculations were performed using the CrystalStructure 24 interface. Crystallographic data for CCDC 1009987–1009988 can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Double bond isomerization of anacardic acid
We have previously shown 7 that the maximum yield of the conjugated isomer of cardanol, (E)-3-(pentadec-1-enyl)phenol, that can be obtained by isomerization of cardanol is 40% and we believe this to be the thermodynamic limit because the stabilization by conjugation is insufficient. This belief has been confirmed by de Vries and co-workers using monounsaturated cardanol approaching the equilibrium from the isomer with the double bond predominantly in the 8 position and from the isomer with the conjugated double bond made by a Wittig reaction 25.
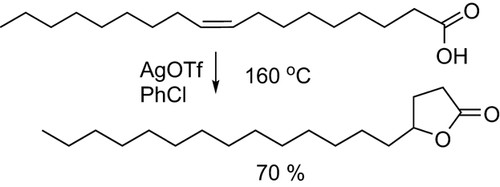
Inspired by the work of Goossen et al. 26, who showed that the double bond of oleic acid can be trapped in a lactone in the presence of silver triflate as an isomerization catalyst (Fig. 3), we reasoned that this type of trapping might also be possible for anacardic acid. Subsequent ring opening and decarboxylation would then lead to (E)-3-(pentadec-1-enyl)phenol, which we desired for further metathesis reactions.
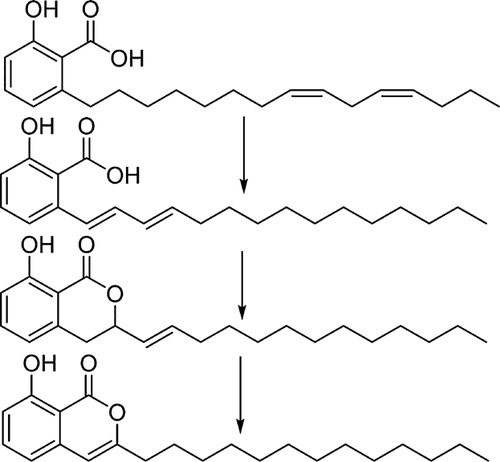
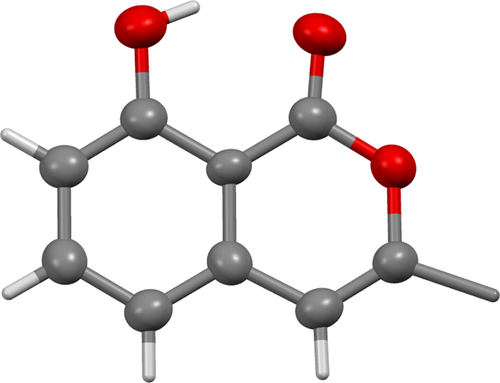
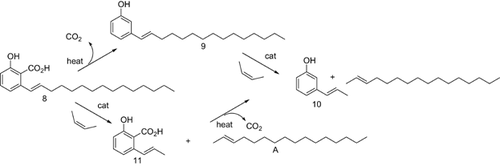
Two major products could be isolated from the Pd/BDTBPMB/H+1 catalyzed double bond isomerization of anacardic acid possessing a mixture of mono-, di-, and tri-unsaturation in the C15 side chain. One of the products isolated was 8-hydroxy-3-tridecyl-1H-isochromen-1-one (6, Figs. 4-6). The compound was white, crystalline and insoluble in methanol with a molecular mass of 344 g/mol, which is the same as the molecular mass of diene anacardic acid. The NMR spectra and crystal structure revealed that the compound was 6. Formation of unsaturated lactones by palladium-catalyzed cyclization of alkenoic acids is not a new concept and has been reported to proceed with good yields 28.
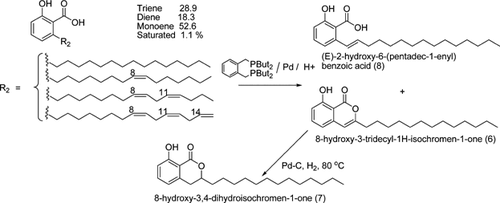
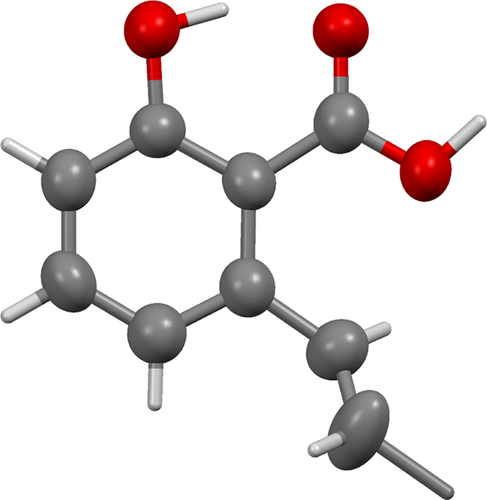
The other isolated product (10%) from this reaction is a white crystalline solid, which NMR studies suggest consists of double bond positional isomers of anacardic acid. The main component is isoanacardic acid, (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid (8, 67%) with other positional isomers making up 33%. We propose that these products are formed from monounsaturated anacardic acid by double bond isomerization. As indicated above, the maximum amount of (E)-3-(pentadec-1-enyl)phenol (9) among the monounsaturated isomers that we have been able to obtain by isomerization of cardanol is 40%.
However, starting from anacardic acid, the conjugated isomerized product makes up 67% of the mixture of isomers, which are isolated in 10% yield from this reaction. We assume that this is because the carboxylic acid in the ortho position relative to the chain increases the conjugation length and hence the stabilization of the conjugated isomer. Somewhat surprisingly, no saturated lactone, 8-hydroxy-3-tridecyl-3,4-dihydroisochromen-1-one (7), the product expected from trapping the conjugated monounsaturated isomer by lactonization, could be detected in the product from isomerization of anacardic acid containing mixed mono-, di-, and tri-unsaturated sidechains.
According to other workers 23, 2-vinylbenzoic acid forms isocoumarin (unsaturated lactone) in the presence of palladium (II) salts via an intramolecular carboxylate palladium complex, followed by elimination of HPdCl. 2-Vinylbenzoic acid has a double bond and carboxylic group at the same relative positions as in isoanacardic acid, 8, but the reaction of 2-vinylbenzoic acid was carried out under oxidative conditions.
Di-unsaturated anacardic acid is the most probable component in the starting mixture to lead to the formation of the unsaturated lactone, 6. The low yield of the lactone (15%) based on anacardic acid would also be consistent with its coming from the diene, which represents only 18.3% of the total anacardic acid. However, we were unable to detect any products, which obviously arise from tri-unsaturated anacardic acid (28.9% of the starting material) by GCMS analysis of the crude reaction product. This together with the observation that dibenzylideneacetone (dba) from the palladium catalyst precursor appears as dibenzylacetone with the double bonds of dba saturated, suggests that some transfer hydrogenation is occurring in this system, perhaps converting the tri-unsaturated chains of the starting material into chains with lower degrees of unsaturation. We do not see any increase in the fully saturated anacardic acid at the end of the reaction, so we propose that the triene is susceptible to hydrogenation, while the diene undergoes isomerizing lactonization and the monoene undergoes isomerization. The source of hydrogen is unknown, but could possibly be methanol in a transfer hydrogenation reaction.
A possible reason for the ability of diene anacardic acid to form unsaturated lactone is that, the two double bonds isomerize together within the alkyl chain (Fig. 5). When they are in conjugation with the aromatic ring and the carboxyl group, the second double bond will reduce the electron density at C2 of the chain, making it a better electrophile and favoring lactone formation. The second double bond must then isomerize further to form the lactone with unsaturation in the ring. Hydrogenation of 8-hydroxy-3-tridecyl-1H-isochromen-1-one to form saturated lactone (7) was easily achieved using Pd/C. The X-ray structure of 6 is shown in Fig. 6.
3.2 Isomerization of monoene anacardic acid
Realizing that higher yields of the desired iso-anacardic acid, 8 might be available from monounsaturated anacardic acid than from cardanol 7, we investigated the isomerization of monounsaturated anacardic acid, obtained by transfer hydrogenation of anacardic acid using iPrOH catalyzed by RuCl3. H2O, a system that had proved effective for cardanol 8 and also proved effective for anacardic acid.
As expected the isomerization of monoene anacardic acid led smoothly to a mixture of positional isomers of 3-pentadecenylphenol with the desired isomer (8) making up 67% of the product. After chromatography, the product can be crystallized, apparently still as a mixture of isomers, but crystals of 8 suitable for X-ray analysis (see Fig. 7) could be selected from the batch.
3.3 Synthesis of 3-propenylphenol (10)
Two possible routes are available for converting isoanacardic acid, (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid, 8, into 3-propylphenol (Fig. 8). One route is metathesis of 8 with cis-2-butene followed by decarboxylation and hydrogenation reactions. The other route is by decarboxylation of 8 to form isocardanol, (E)-3-(pentadec-1-enyl)phenol (9) followed by metathesis of isocardanol with cis-2-butene and hydrogenation to form 3-propylphenol. We attempted both approaches but found that the but-2-enolysis of 8 was very poor compared with that of 9.
3.4 Decarboxylation of (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid
The fact that the metathesis reaction of isoanacardic acid (8) with 2-butene is poor compared with isocardanol (9) makes decarboxylation of 8 an important step. The decarboxylation process proceeded smoothly on heating 8 to 190°C under N2 for 3 h. Interestingly, the heating had no effect on the ratio of the conjugated isomer to other double bond positional isomers. In our case, the sample for decarboxylation had the desired isomer (67%) and other isomers (33%). After decarboxylation reaction, these ratios remained the same.
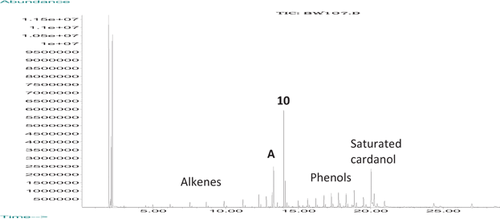
3.5 But-2-enolysis of isocardanol
In our previous study 7, the two main problems with forming 3-prop-1-enylphenol and hence 3-propylphenol from cardanol were the low percentage of isocardanol, 9, after the isomerization reaction and the self-metathesis of 3-propenylphenol, 10, during butenolysis of 9. The overall yield of 10 from isomerized cardanol was only 4.4%. Having in hand isomerized cardanol with a higher proportion of the conjugated isomer, 9 (67% compared with 40%), we also adopted the suggestion 7 that a larger amount of 2-butene would suppress the self-metathesis of (E)-3-(prop-1-enyl)phenol. GCMS analysis of the crude reaction mixture clearly shows that (E)-3-(prop-1-enyl)phenol and 2-hexadecene, dominate over the other products (Fig. 9 and Supporting Information Fig. S11). Isolation by column chromatography afforded (E)-3-(prop-1-enyl)phenol (137 mg, 18%) contaminated with ca 15% of longer chain isomers). The overall yield starting from CNSL is 10.4%.
3.6 X-ray structures of 6 and 8
Both the structures of 6 and 8 adopt an extended conformation of their tridecyl chains, although in 6 this chain lies close to the plane of the isochromene-1-one ring system, whereas in 8 the pentadec-1-enyl chain is twisted further from the plane of the 2-hydroxybenzoic acid. Both compounds pack to give regions of the crystal comprising solely aliphatic or solely aromatic groups, but how they do so differs. Compound 6 is arranged in a strictly head-to-tail manner, giving wide aliphatic regions separated by narrow aromatic regions. In contrast, compound 8 packs with the pentadec-1-enyl chains interdigitating, and also with the 2-hydroxybenzoic acid groups overlapping. These packing motifs arise from the very different sets of weak interactions that occur in each compound. The predominant interaction in compound 6 is an intermolecular CH π interaction that links adjacent isochromene-1-one rings, supported by weak CH
π interaction that links adjacent isochromene-1-one rings, supported by weak CH O hydrogen bonds. The only OH
O hydrogen bonds. The only OH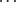 O hydrogen bonds occurring in compound 6 are intramolecular, between the alcohol and carbonyl. In compound 8, in contrast, the primary interaction is the formation of hydrogen-bonded benzoic acid dimers. No π-interactions occur; both CH
O hydrogen bonds occurring in compound 6 are intramolecular, between the alcohol and carbonyl. In compound 8, in contrast, the primary interaction is the formation of hydrogen-bonded benzoic acid dimers. No π-interactions occur; both CH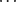 O and OH
O and OH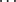 O hydrogen bonds are intramolecular.
O hydrogen bonds are intramolecular.
4 Conclusions
The isomerization of natural anacardic acid obtained from CNSL produces two main isolable fractions, the unsaturated lactone, 8-hydroxy-3-tridecyl-1H-isochromen-1-one (6), which is assumed to arise from anacardic acid molecules containing two double bonds in the chain, and isomerized monounsaturated anacardic acid, with the isomer containing the double bond conjugated to the aromatic ring, (E)-2-hydroxy-6-(pentadec-1-enyl)benzoic acid, 8, being favored (67, 33% other double bond positional isomers). Compound 6 could be hydrogenated to the saturated lactone, but attempts to ring open the lactone to give 8 were unsuccessful.
Since the conjugated isomer was more favored in the isomerization of anacardic acid than of cardanol, monounsaturated anacardic acid was prepared by transfer hydrogenation of natural anacardic acid and isomerized to give a moderate yield of a mixture of isomers containing predominantly 8 (67%). This product was decarboxylated with no change in the isomer ratio and the 3-pentadecenylphenol, 9 so formed metathesized with 2-butene to give 3-propenylphenol, a precursor to 3-propylphenol, a tse-tse fly attractant.
Although this route starting from anacardic acid is superior to the route we have previously described using cardanol, because the presence of the carboxylic acid group increases the proportion of the isomer with the double bond conjugated to the aromatic ring, it still provides the desired 3-propenylphenol in low yield (18% based on the starting amount of anacardic acid; 10.4% based on the starting amount of CNSL). We are currently investigating isomerizing metathesis as a preferred route to 3-propenylphenol 29.
We thank the Royal Society Leverhulme Africa Program for funding this project. We also thank the University of Dar es Salaam (UDSM-DUCE) for granting study leave.
The authors have declared no conflict of interest.




