An efficient and expeditious synthesis of phytostanyl esters in a solvent-free system
Abstract
Esterification of natural phytostanols with various fatty acids by using Lewis acid-surfactant combined catalyst was investigated. For synthesis of phytostanol esters of saturated fatty acids, cuprum dodecyl sulfate [Cu(DS)2] was the most desirable catalyst due to its high selectivity, reusability, activity, and less corrosivity, whereas stanol selectivity with other catalysts, such as ZnCl2 and tungstophosphoric acid. The substrate molar ratio of 1.2:1 (lauric acid/phytostanols) was the optimal. For synthesis of phytostanol esters of unsaturated fatty acids, cerium dodecyl sulfate [Ce(DS)3] was better than [Cu(DS)2] which was based on the oxidation of the unsaturated fatty acids during the reaction. The chemical structure of the sitostanyl stearate, sitostanyl oleate, and sitostanyl linoleate were confirmed by FTIR, MS, and NMR, respectively. As a result, the [Cu(DS)2] and [Ce(DS)3] were screened to synthesize phytostanyl esters of fatty acids for commercial production.
Practical applications: Phytostanols are important for human health and nutrition. Unfortunately, due to the poor solubility of free stanols (unesterified) in fats and oils, there is a demand for a good way to improve the solubility or bioavailability of phytostanols, such as esterification of phytostanols with fatty acids. This study aims at finding an efficient and expeditious synthesis of phytostanyl esters. At the same time, environmental impact and the oxidation of the unsaturated fatty acids during the reaction should be considered.
Abbreviations:
[Ag(DS)], silver dodecyl sulfate; AV, anisidine value; [Ce(DS)3], cerium dodecyl sulfate; CHD, coronary heart disease; [Cu(DS)2], cuprum dodecyl sulfate; [Fe(DS)3], ferrum dodecyl sulfate; SL, sitostanyl linoleate; SO, sitostanyl oleate; SS, sitostanyl stearate; TC, cholesterol concentration; [Zn(DS)2], zincum dodecyl sulfate
Introduction
Plant sterols and stanols, which are mainly present in nuts, vegetable oils, seeds, cereals, and beans, are structurally related to cholesterol, but are characterized by an extra ethyl (β-sitosterol) or methyl group (campesterol) in the side chain 1. They are added to special margarines that are commercially available as functional foods with the ability to reduce both total and LDL cholesterol levels 2, 3. The significance of phytosterols and phytostanols in food industry has grown dramatically in recent years. Their presence in adequate amounts (2–3 g/day) in human diet is known to substantially reduce cholesterol absorption by the digestive tract thus lowing the serum total cholesterol concentration (TC) as a result 4. Although both phytosterols and phytostanols present the cholesterol-lowering properties, the superior ability of phytostanols to inhibit cholesterol absorption is known. The difference is also attributed to small structural differences: the longer the side chain attached to the sterol molecule and the fewer double bonds there are, the less sterol/stanol is adsorbed 5.
Despite their potential attractiveness, the usefulness of phytostanols has been greatly limited by their higher melting point, lower solubility in both water and oil phases, and their chalky taste. As a food additive to be used broadly, phytostanols should be conveniently incorporated into food, without the adverse organoleptic effects. For this reason, the esterification of phytostanols is a good way to improve the solubility or bioavailability of phytostanols 6. Recently, phytostanyl esters have been found to be effective in lowering plasma TC by inhibiting the absorption of cholesterol from the small intestine 7. As the free stanols (unesterified) are poorly soluble in fats and oils, their solubility improvement for incorporation into foods could be a result of phytostanols esterification with fatty acids. On the other hand, phytostanyl esters can be used in cosmetics as emulsifiers (significant solubility in water) or as anti-inflammatory and antioxidant compounds 8. Esterification or transesterification of phytostanols with fatty acids can increase their lipid solubility and thus facilitates the incorporation into a variety of foods. As it is reported that fatty acids in esters are more stable than free fatty acids in respect to the combination with phytostanols. Plant sterol/stanol esters have generally been recognized as safe by Food and Drug Administration (FDA) since September 2000 9. FDA also has authorized a claim that foods containing plant sterol/stanol esters may reduce the risk of coronary heart disease (CHD). The Scientific Committee on Foods of the European Union concluded that phytosterol/phytostanol esters margarine were safe for human use 3.
In recent years, the phytostanyl esters can be prepared by enzyme technologies or chemical esterifications. Enzyme technologies are still limited by high costs of lipases, lower productivity, and longer reaction time (as long as 24 h). In addition, enzymatic synthesis of phytostanyl esters in supercritical carbon dioxide was studied by King et al. 10, but it is still far from meeting industrial demands. The chemical synthesis is still the main method for commercial production of phytostanyl esters.
Traditional chemical method generally requires longer time under high temperature conditions, and easily produces side effects during the reaction, and it is difficult to separate from the products and lead to excessive waste. The typical operation is a transesterification reaction of phytostanols and edible oil methyl esters, catalyzed by sodium methylate. However, the need to dispose strong corrosive sodium methylate could potentially pollute the environment. For example, acid and alkali as a catalyst, which itself has a very strong corrosivity, acid catalysts may favor the dehydration of plant sterols or stanols to olefins and alkali saponification help ester hydrolysis; reaction temperature is generally above 150°C, fatty acids especially PUFA prone to oxidation and decomposition reactions. It was also suggested by Pouilloux et al. 11 and Valange et al. 12 that a solid base catalyst for the synthesis of phytostanol esters under 240°C is inappropriate for the synthesis of PUFA due to easy oxidative decomposition under high temperatures.
Currently, the most attentions are attached to food additives preparation through green chemistry technology. To avoid organic solvents for its environmentally unfriendly, solvent-free technique is undoubtedly an important tool 13. In recent years, there are a lot of researches about the application of surfactants in influencing, regulating, and controlling the chemical reaction process, in particular, research about the micellar catalysis which is formed by Lewis acid-surfactant combined catalyst and surfactants being used in organic reactions is very active 14-17. For example, high yielding preparation of structurally different thiiranes, β-amino alcohols, and bis(β-amino) alcohols from the reaction of epoxides with thiourea and amines in the presence of catalytic amounts of aluminum tris (dodecyl sulfate) trihydrate Al(DS)3 · 3H2O as a Lewis acid-surfactant-combined catalyst at RT in water has been described by Firouzabadi et al. 18. Weng et al. 19 reported a Lewis acid-surfactant-combined copper bis(dodecyl sulfate) [Cu(DS)2] catalyst served as an efficient and reusable catalyst for the thioacetalization and transthioacetalization of carbonyl compounds and O,O-acetals in water at RT. Ghesti et al. 20 also reported the application of cerium(III) trisdodecylsulfate trihydrate (Ce(OSO3C12H25)3 · 3H2O) as a Lewis acid-surfactant-combined catalyst for the production of alkyl esters by solvent-free transesterification and esterification reactions.
Therefore, in order to accelerate the development of Lewis acid-surfactant-combined catalyst in the field of organic reactions and to build a green chemical synthesis of phytostanyl esters, various catalysts (including chloride salts, Lewis acid-surfactant combined catalysts, etc.) were screened for the synthesis of phytostanyl esters in this study. The prepared phytostanyl esters were isolated by column chromatography, and the identification of the produced sitostany esters were conducted using FTIR, MS, NMR, and the befitting conditions using dodecyl sulfate in solvent-free media were explored.
Materials and methods
Materials
Lauric acid, myristic acid, palmitic acid, stearic acid, eicosanoic acid, oleic acid, linoleic acid, FeCl3 · 6H2O, CuCl2 · 2H2O, CeCl3 · 7H2O, ZnCl2, SnCl2 · 2H2O, AgNO3, NaHSO4, sodium dodecyl sulfate (SDS), glacial acetic acid, n-hexane, ethyl acetate, and petroleum ether were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, P. R. China). Phytosterols were a generous gift from Jiangsu Spring Fruit Biological Products Co., Ltd. (Taixing, P. R. China). The purity of plant sterols was >97% (43% β-sitosterol, 28% campesterol, 23% stigmasterol, and 3% brassicasterol).
Preparation of phytostanols
Phytostanols were synthesized according to previous study 6 by catalytic hydrogenation of phytosterols under hydrogen pressure of 2 Mpa, using Pd/C as catalyst. The reaction conditions were isopropanol as solvent, m(Pd)/m(phytosterols) of 0.2%, reaction temperature 65°C, and reaction time 5 h. The hydrogenation rate of phytosterols was 98.2% by determinating of iodine value (AOCS Official Method Da 15-48, 1997). The purity of plant stanols was >96% (65% sitostanol and 31% campestanol).
Preparation of Lewis acid-surfactant combined catalysts
Preparation of cerium dodecyl sulfate ([Ce(DS)3]). Aliquots of SDS (0.15 mol/L) were added to the CeCl3 solution (0.16 mol/L) at 70°C at the volume ratio of 3:1. Followed by the formation of white precipitates immediately, the mixture was stirred tempestuously at 70°C for 30 min. The white precipitate was filtered, washed with cold distilled water, and dried in a desiccator with anhydrous CaCl2 (treated at 100°C for 24 h) 20.
Cuprum dodecyl sulfate [Cu(DS)2], ferrum dodecyl sulfate [Fe(DS)3], zincum dodecyl sulfate ([Zn(DS)2]), and silver dodecyl sulfate ([Ag(DS)]) were prepared according to the above method with minor modification. Additionally, CuCl2, FeCl3, ZnCl2, and AgNO3 was used instead of CeCl3 in the preparation of above samples and the volume ratios of SDS to chloride salts solution were 2:1, 3:1, 2:1, and 1:1, respectively.
Esterification method
A phytostanol ester mixture was prepared in a reaction tube. Phytostanols and fatty acids were dried in vacuum oven overnight prior to reaction. The esterification was carried out as follows: a mixture of catalyst and fatty acid in a certain molar ratio was added to the reaction tube. Then it was heated to the desired temperature under nitrogen, the nitrogen flow rate was controlled at 1.5 mL/min and the phytostanols was not added until the fatty acid melt. The reaction was continued for a certain time and the conversion rate of the reaction was monitored with TLC. The reaction scheme was shown in Fig. 1.
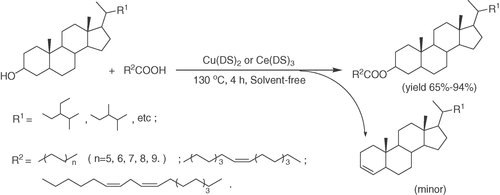
Esterification schematic.
Repeated use of catalyst
When the reaction was finished, the catalyst was separated by heat filtration, eluted by n-hexane, hot air dried, weighed, and reused in a next batch for esterification. Five cycles of the esterification reaction were conducted. The conversions were measured after each run. The catalyst reuse trial of five run reactions was carried out in triplicate.
Purification of phytostanol esters by column chromatography
In a typical example, the reaction mixture resulting from chemical esterification in situ of plant stanols with fatty acids was filtered with a silica gel column (100–200 mesh, 12 mm × 600 mm) and then eluted with cyclohexane: ethyl acetate (19:1 v/v). The flow rate was 40 mL/h, and the eluent, 1 tube/8min, was collected and then detected by TLC. The fractions containing the desired products were collected by rotary evaporator.
Qualitative analysis by TLC, FTIR, MS, and NMR
Samples were taken from the reaction mixtures, catalyst was separated by centrifugation, and the development was carried out in cyclohexane/ethyl acetate by TLC on a 0.25-mm layer of silica gel G254 (Merck), using cyclohexane/ethyl acetate (19:1 v/v). Spots were located by iodine staining for 1 h. Alternatively, the TLC plates were sprayed with sulfuric acid/ethanol (1:19 v/v), heated in oven and kept at 110°C for 20 min. Rf values of different substrates and products were: 0.01–0.03 (fatty acid), 0.08–0.11 (phytostanols), 0.75–0.80 (phytostanyl esters), and 0.9–0.95 (by-products), respectively.
Isolated sitostany ester was analyzed by FTIR, MS and NMR. FTIR measurements were performed on a Nicolet Nexus470 FTIR spectrophotometer with attenuated total reflectance (ATR), Number of Scans: 32, Resolution: 4 cm−1. A mass spectrum was obtained by MS (Waters Maldi Synapt Q-TOF, USA) with positive ESI mode. 1H and 13C NMR spectra were measured in CDCl3 as solvent using tetramethylsilane (TMS) as the internal standard with a Bruker AMX-400 (400 MHz) NMR spectrometer (Bruker Analytische Messtechnik Gmbh, Rheinstetten, Germany) operating at 400 and 100 MHz for 1H and 13C, respectively. The results are as follows
Sitostanyl laurate
The condensation products sitostanyl laurate were analyzed by FTIR, MS, 1H NMR, and 13C NMR. Assignment of hydrogen and carbon resonances in NMR can get from our previous publication 6.
Sitostanyl stearate (SS)
The maximum absorption of the IR spectrum of SS was observed in Fig. 2 at the following positions: 1739 cm−1 (CO), 1464 and 1177 cm−1 (CO). The absorbance of hydroxyl group was observed in plant stanols and stearic acid, but not in the synthesized product. The absorbance of carbonyl group in the product shifted to a higher wave numbers by 39 cm−1, indicating the formation of ester bond. MS m/z 705.3 (M++Na).
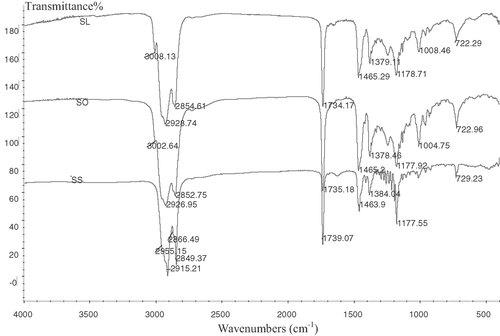
The FTIR spectrum of SS, SO, and SL.
1H and 13C NMR spectral data of SS were as follows: 1H NMR (400 MHz, CDCl3): δ = 0.58 (3H, s), 0.73–0.84 (20H, m), 0.91–0.97 (4H, m), 1.01–1.12 (6H, m), 1.18–1.26 (37H, m), 1.39–1.43 (2H, m), 1.46–1.67 (8H, m), 1.71–1.75 (2H, m), 1.87–1.91 (1H, m), 2.18 (2H, t, J = 7.6 Hz), 4.58–4.66 (1H, m, 3′H). 13C NMR (100 MHz, CDCl3): δ = 11.98 (CH3), 12.07 (CH3), 12.23 (CH3), 14.12 (CH3), 18.73 (CH3), 19.04 (CH3), 19.82 (CH3), 21.21 (CH2), 22.70 (CH2), 23.07 (CH2), 24.22 (CH2), 25.11 (CH2), 26.10 (CH2), 27.54 (CH2), 28.27 (CH2), 28.63 (CH2), 29.12 (CH), 29.16 (CH2), 29.26 (CH2), 29.38 (CH2), 29.46 (CH2), 29.60 (CH2), 29.66 (CH2), 29.67 (CH2), 29.72 (4 × CH2), 30.91 (CH2), 31.94 (CH2), 32.01 (CH2), 33.93 (CH2), 34.09 (CH2), 34.79 (CH2), 35.48 (quaternary C), 35.50 (CH), 36.18 (CH), 36.78 (CH2), 39.99 (CH2), 42.60 (quaternary C), 44.68 (CH), 45.85 (CH), 54.24 (CH), 56.18 (CH), 56.43 (CH), 73.44 (3CH), 173.46 (1′CO).
Sitostanyl oleate (SO)
Unlike the maximum absorption of the IR spectrum for SS, SO was observed in Fig. 2 at the following positions: 1735 cm−1 (CO), 1464 and 1178 cm−1 (CO), 3003 cm−1 (CH), and 1652 cm−1 (CC). The absorbance of hydroxyl group was also not observed in the synthesized product. The maximum absorption of the IR spectrum for SO at 3003 and 1652 cm−1 showed the presence of double bond in the production. MS m/z 703.3 (M++Na).
1H and 13C NMR spectral data of SO were as follows: 1H NMR (400 MHz, CDCl3): δ = 0.66 (3H, s), 0.76–0.97 (21H, m), 0.98–1.08 (4H, m), 1.08–1.23 (6H, m), 1.23–1.41 (29H, m), 1.44–1.54 (2H, m), 1.54–1.91 (10H, m), 1.96–2.09 (4H, m), 2.27 (2H, t, J = 7.6 Hz), 4.66–4.77 (1H, m, 3H), 5.32–5.42 (2H, m). 13C NMR (100 MHz, CDCl3): δ = 11.98 (CH3), 12.07 (CH3), 12.23 (CH3), 14.12 (CH3), 18.73 (CH3), 19.04 (CH3), 19.82 (CH3), 21.21 (CH2), 22.70 (CH2), 23.07 (CH2), 24.22 (CH2), 25.09 (CH2), 26.10 (CH2), 27.20 (CH2), 27.22 (CH2), 27.54 (CH2), 28.27 (CH2), 28.63 (CH2), 29.10 (CH), 29.16 (CH2), 29.34 (CH2), 29.36 (CH2), 29.54 (CH2), 29.60 (CH2), 29.70 (CH2), 29.78 (CH2), 30.90 (CH2), 31.92 (CH2), 32.01 (CH2), 33.93 (CH2), 34.09 (CH2), 34.79 (CH2), 35.48 (quaternary C), 35.50 (CH), 36.18 (CH), 36.78 (CH2), 39.99 (CH2), 42.60 (quaternary C), 44.68 (CH), 45.85 (CH), 54.25 (CH), 56.18 (CH), 56.43 (CH), 73.44 (3CH), 129.76 (CC), 129.97 (CC), 173.39 (1CO).
Sitostanyl linoleate (SL)
With slightly different from the maximum IR spectrum absorption of SO, SL was observed in Fig. 2 at the following positions: 1734 cm−1 (CO), 1465 and 1179 cm−1 (CO), 3008 cm−1 (CH), and 1652 cm−1 (CC). MS m/z 701.3 (M++Na).
1H and 13C NMR spectral data of SL were as follows: 1H NMR (400 MHz, CDCl3): δ = 0.66 (3H, s), 0.82–0.94 (20H, m), 0.98–1.07 (4H, m), 1.08–1.21 (6H, m), 1.23–1.40 (26H, m), 1.44–1.74 (10H, m), 2.02–2.10 (4H, m), 2.24–2.30 (2H, t, J = 7.6 Hz), 2.76–2.81 (2H, m), 4.66–4.76 (1H, m, 3H), 5.30–5.44 (4H). 13C NMR (100 MHz, CDCl3): δ = 11.97 (CH3), 12.06 (CH3), 12.22 (CH3), 14.07 (CH3), 18.73 (CH3), 19.04 (CH3), 19.81 (CH3), 21.21 (CH2), 22.58 (CH2), 23.07 (CH2), 24.22 (CH2), 25.07 (CH2), 25.62 (CH2), 26.09 (CH2), 27.19 (CH2), 27.20 (CH2), 27.53 (CH2), 28.27 (CH2), 28.63 (CH2), 29.09 (CH), 29.11 (CH), 29.16 (CH2), 29.35 (CH2), 29.59 (CH2), 30.88 (CH2), 31.53 (CH2), 32.00 (CH2), 33.92 (CH2), 34.09 (CH2), 34.75 (CH2), 35.47 (CH2), 35.49 (quaternary C), 36.17 (CH), 36.78 (CH), 39.99 (CH2), 42.59 (quaternary C), 44.67 (CH), 45.84 (CH), 54.24 (CH), 56.18 (CH), 56.43 (CH), 73.43 (3CH), 127.91 (CC),128.02 (CC), 130.04 (CC), 130.18 (CC), 173.37 (1′CO).
HPLC quantitative analysis
The peak area (%) of the produced phytostanyl esters was calculated as the esterification rate and the peak area (%) of unreacted phytostanols was calculated as the conversion of phytostanol by HPLC with symmetry-C18 column (5 µm, 4.6 mm × 150 mm, Waters, USA) and evaporative light scattering detector (ELSD) 2420 (Waters). The column temperature was 35°C and the ELSD was operated at 42°C with N2 as a nebulizing gas at a pressure of 172.2 kPa. The mobile phase was a mixture of methanol, isopropanol, and n-hexane (8:1:1 by volume), and the flow rate was 1.0 mL/min. The calibration curve was prepared using the purified phytostanyl esters (≥98.5%).
Measurement of anisidine value (AV)
AV was expressed as the carbonyl content of oils or fats, and was determined by the British Standard method BS 684-2.24. A solution of reaction mixture (2% w/v) was prepared with n-hexane as solvent. Absorbance (A1) was measured at 350 nm in a spectrophotometer, against a blank of n-hexane. An aliquot (5 mL) of the solution of reaction mixture or 5 mL of n-hexane (as blank) was transferred to each of two 10 mL test tubes and 1 mL anisidine solution (0.25% g/v glacial acetic acid) was added to each. The test tubes were shaken and allowed to stand for 10 min. Absorbance (A2) was measured at 350 nm, against n-hexane containing p-anisidine. AV was calculated using the formula AV = 25 (1.2 A2 − A1)/m, m standing for sample weight 21.
Results and discussion
Influence of catalysts
The effects of different catalysts on the esterification of plant stanols and fatty acids were studied. Different catalysts were firstly selected based on the previous reports about the synthesis of phytosteryl esters 22-25. Furthermore, chloride salts and Lewis acid-surfactant combined catalysts were also chosen to explore the activity on the synthesis of phytostanyl esters due to their potential activity on some esterification reactions. In this study, 12 catalysts, mainly including chloride salts, Lewis acid-surfactant combined catalysts, heteropolyacid, and p-toluenesulfonic acid, had been initially screened as the reaction catalysts using the batch method. The results are shown in Table 1.
| Entry | Catalysts | Conversion of phytostanolsa) (%) | Selectivity (%) | Yieldb) (%) | |
|---|---|---|---|---|---|
| Phytostanyl estersc) | Side productsd) | ||||
| 1 | CeCl3 | 22.2 | 59.0 | 41.0 | 13.1 |
| 2 | FeCl3 | 69.4 | 76.6 | 23.4 | 53.1 |
| 3 | ZnCl2 | 48.8 | 69.6 | 30.4 | 34.0 |
| 4 | CuCl2 | 23.4 | 52.3 | 47.7 | 12.3 |
| 5 | SnCl2 | 32.6 | 41.0 | 59.0 | 13.3 |
| 6 | p-Toluenesulfonic acid | 40.1 | 74.1 | 25.9 | 29.7 |
| 7 | [Ce(DS)3] | 92.4 | 88.2 | 11.8 | 81.6 |
| 8 | [Ag(DS)] | 78.3 | 84.7 | 15.3 | 66.3 |
| 9 | [Fe(DS)3] | 79.3 | 87.4 | 12.6 | 69.3 |
| 10 | [Zn(DS)2] | 56.8 | 92.5 | 7.5 | 52.5 |
| 11 | [Cu(DS)2] | 98.1 | 91.9 | 8.1 | 90.2 |
| 12 | Tungstophosphoric acid | 67.8 | 67.5 | 32.5 | 45.8 |
- Conditions: catalyst 5% (the molar percentage of phytostanols); reaction temperature, 120°C; substrate molar ratio (lauric acid vs. phytostanols), 1.2:1; reaction time, 4 h; 500 rpm.
- a) Base on the peak area(%) of unreacted phytostanols.
- b) Base on the peak area(%) of produced phytostanyl esters.
- c) c = b/a.
- d) d = 1 − c.
As shown in Fig. 1, the main products of the reaction were the phytostanyl esters, and there were also some other side-products, such as olefin which was the product of the dehydration of the phytostanols (seen in Fig. 1). It could be supported by Meng et al. 26 research concluded that the high temperature may favor the dehydration of sterols to dienes. In addition, the major phytosterol oxides (the epimers of 7-hydroxysterols, the epimers of 5,6-epoxysterols, and 7-ketosterols) during thermo-oxidation were identified by their elution order and mass spectrometric data 27. In the same way, the dehydration of little stanols which is similar in structure to the sterols might be favored under high temperature. Therefore, the selectivity of the catalyst as well as the yield of phytostanyl esters should be considered.
It was obvious that the used catalysts had different activities for the same esterification reaction from Table 1. Under the same condition, chloride salts and tungstophosphoric acid seemed to give lower conversion than Lewis acid-surfactant combined catalysts. For example, the esterification degree achieved is 81.6% employing DS-Ce as catalyst, while only 13.1 and 34.0% esterification degree were obtained with CeCl3 · 7H2O and ZnCl2, respectively. As shown in Table 1, among the Lewis acid-surfactant combined catalysts, [Cu(DS)2] showed the highest esterification and very low conversion of side-products. The high selectivity of Lewis acid-surfactant combined catalysts might be related to the micellar catalysis and catalytic mechanism. Dodecyl sulfates belonged to anionic surfactant. For hydrophobic substrates, such as phytostanols, the equilibrium position between the substrates and the products (esters) would lie at the ester side, which was contributed to the fact that water molecules generated during the reaction would be removed from the droplets due to the hydrophobic nature of their interior 28. In addition, through hydrophobic interactions, there would be more chances for the collision of the hydroxyl of carboxylic acids with the hydroxyl of phytostanols. As a result, the dehydration reactions would efficiently proceed.
From the above-obtained results, it was believed that the concentration of catalyst played a key role in the esterification reaction due to its strong influence on the reaction rate. Therefore, the influence of the catalyst concentration should be investigated and the conversion of plant stanol at different initial catalyst concentrations was displayed in Fig. 3.
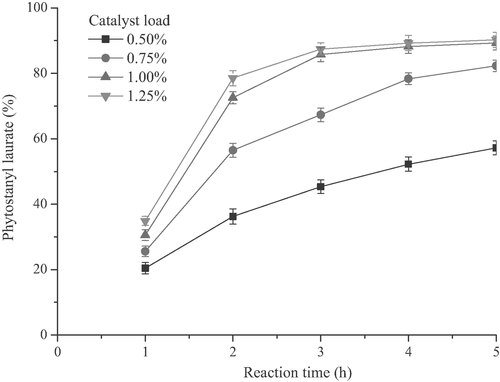
Influence of different concentrations of [Cu(DS)2] (the molar percentage of phytostanols) on the yield of phytostanyl laurate. Reaction conditions: substrate molar ratio (lauric acid vs. phytostanols) 1.2:1, 120°C, 4 h, 500 rpm.
The influence of the [Cu(DS)2] load in the range of 0.5–1.25% (the molar rate of catalyst to phytostanols) was evaluated with a 1:1.2 phytostanols/lauric acid molar ratio (Fig. 3). It was observed that the higher the [Cu(DS)2] load concentration, the better the phytostanyl laurate formation was. Furthermore, not surprisingly, it was also shown that when the concentration of catalyst was over 1.0%, the reaction rate increased at the initial reaction time until leveled off nearly after 4 h. However, this experiment was useful to determine a good combination between esterification rate and economical interest of the reaction, the latter point was directly influenced by the recycling of catalyst from the reaction and heating time. Herein, although the use of minimal amount of [Cu(DS)2] such as 0.5% would be economically attractive, it did not result in any satisfactory production of phytostanyl laurate due to its low esterification rate after 4 h (52.3%). Increasing the catalyst load appeared to be more satisfactory. At 0.75% catalyst concentration the reaction kinetic was faster but still only reached 78.3% of phytostanyl laurate formation after 4 h. When further increasing the catalyst load to 1.0%, the formation of the phytostanyl laurate was much faster and resulted in a 85.8% conversion after 4 h. It was also found that there was no significant difference in esterification rate between catalyst load of 1.0 and 1.25%, especially when prolonging the reaction time to 3 h. On the other hand, the color of reaction productions would be deepen with increasing of the [Cu(DS)2] load.
Influence of molar ratio of fatty acids to phytostanols
The influence of molar ratio of fatty acid to phytostanols on the conversion of phytostanols was evaluated in the esterification of plant stanols with lauric acid in the solvent-free system using [Cu(DS)2]. As expected, although equimolar ratio of both substrates can appear as ideal in terms of economical aspect of the process and further purification of the end products, such ratio was not advantageous for phytostanyl laurate synthesis. Indeed, with equivalent molar amounts of phytostanols and lauric acid, no displacement of the equilibrium is possible and as mentioned phytostanyl laurate formation only reached 80% after 4 h. Increasing the molar ratio of lauric acid to phytostanol led to the increase in the extent of esterification and the highest conversion was achieved at the molar ratio of 1.6:1. This behavior is in agreement with the reports in literature for heterogeneous catalysts 26. Esterification of phytostanols was an equilibrium reaction, and the use of higher molar ratios was necessary to shift the reaction equilibrium to the products. However, the decrease of conversion was observed above the ratio of 1.6:1, indicating that the excessive use of lauric acid could not improve the efficiency of the esterification reaction. Noted that from 1.2:1 to 1.6:1 only led to a slight increase, therefore, considering the huge cost in the downstream isolation process, the high yield and efficiency, the optimum substrate molar ratio should ensure that the esterification reaction was performed with high yield and efficiency, the optimum substrate molar ratio was selected at a molar ratio (lauric acid vs. phytostanol) of 1.2:1 under the temperature of 130°C.
Influence of the various fatty acid chain length
The synthesis of alternative esters formed between various fatty acids and phytostanol was also evaluated. The results from the preliminary esterification reactions conducted in the batch mode were then applied to study the reaction of phytostanols and fatty acids with various chain lengths. Reaction temperature of 130°C and the molar ratio of 1.2: 1 (fatty acids vs. phytostanols) were used for these reactions. The catalyst [Cu(DS)2] load of 1.0% was selected for all the reported synthesis reactions. The results were presented in Fig. 4 for the even-carbon number fatty acids from C12 to C22. It was found that reaction rate decreased with increasing carbon chain length of fatty acids. This phenomenon may be related to steric effect. As the size of the alkyl chain in the fatty acid increased in size, its steric effect increased.
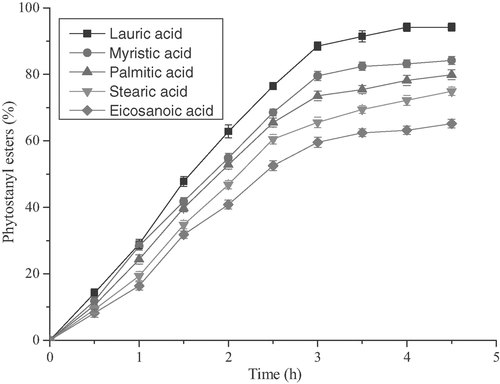
Influence of the various fatty acids chain length. Reaction conditions: DS-Cu 1.0% (the molar percentage of phytostanols), 130°C, 4 h, 500 rpm.
Synthesis of phytostanyl esters of unsaturated fatty acids
Because there was little oxygen in the nitrogen used in the synthesis of phytostanyl esters of unsaturated fatty acid esters, different from the synthesis of phytostanyl esters of saturated fatty acid, the oxidation of unsaturated fatty acid should be considered and detected. The influence of different Lewis acid-surfactant combined catalysts on the oxidation of unsaturated fatty acid was investigated. The AV of reaction system was detected according to the condition in Section 2.9 to reflect the oxidation degree. The oxidation of the unsaturated fatty acid was not only influenced by high temperature, but also by the transition metal ions. As shown in Fig. 5, under the same condition, [Cu(DS)2] and [Ce(DS)3] seemed to give higher conversion than others. Although [Zn(DS)2] gave lower conversation, its effects on the oxidation of unsaturated fatty acid was slight, which was opposite to [Cu(DS)2]. This difference might be related to the activity of metal ion. Considering the relationship between esterification rate and the extent of oxidation, [Ce(DS)3] was a better choice for the synthesis of phytostanyl esters of unsaturated fatty acids. The microabsorption of the IR spectrum for SL and SO (Fig. 2) at 966 cm−1 also showed low trans fatty acid content in the production. Influence of the reaction temperature on the synthesis of the phytostanol oleate was shown in Fig. 6. Over the range of 120–170°C, the catalyst activity increased with the increasing reaction temperature. It was considered as a result of the increase of the activation energy with increasing temperature. However, the AV of reaction system also increased with the increasing reaction temperature. The reason could be explained that PUFA were prone to oxidation and isomerization under the high temperature 28. The conversion decreased when the temperature exceeded 170°C, it could be attributed to the loss of phytostanols and oxidation of oleic acid at high temperature. As shown in Fig. 6, for synthesis of phytostanyl oleate, when the reactions were conducted at 170°C, the highest conversion of 80.5% was observed.
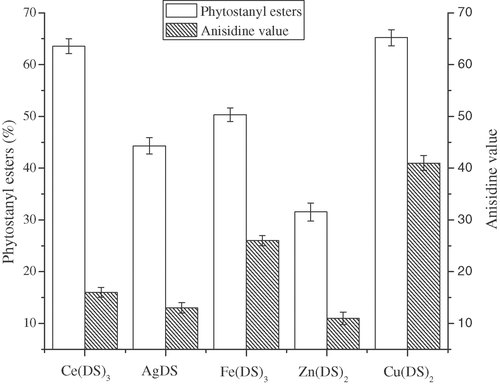
Influence of varying dodecyl sulfate catalysts on esterification. Reaction conditions: catalyst load 1.0% (the molar percentage of phytostanols), substrate molar ratio (oleic acid vs. phytostanols) 1.2:1, 130°C, 4 h, 500 rpm.
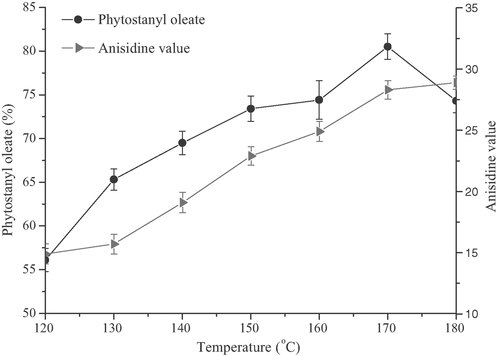
Influence of the reaction temperature on the synthesis of the phytostanol oleate. Reaction conditions: catalyst [Ce(DS)3] load 1.0% (the molar percentage of phytostanols), substrate molar ratio (oleic acid vs. phytostanols) 1.2:1, 4 h, 500 rpm.
Repeated use of catalyst
The esterification reactions were performed with lauric acid to plant stanol ratio of 1.2:1 and [Cu(DS)2] load of 1.0% at 130°C for 4 h for each run. After the end of reaction, the catalyst was separated by filtration and reused in a next batch of esterification. Five cycles of this reaction were conducted. The conversion rate of the reaction was measured after each run. The synthesis ratio was then measured and plotted in Fig. 7.
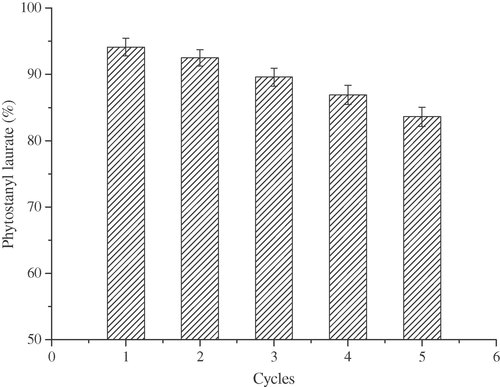
Reuse of catalyst. Reaction conditions: catalyst [Cu(DS)2] load 1.0% (the molar percentage of phytostanols), substrate molar ratio (lauric acid vs. phytostanols) 1.2:1, 130°C, 4 h, 500 rpm.
Figure 7 showed that the phytostanyl ester synthesis ratio after three cycles was still remained above the high level of 90%. Even after five consecutive applications, 83% of the original catalytic activity remained. It was observed that the catalyst did not significantly lose their activity after five cycles repetition. The reason caused the reduction of phytostanyl ester synthesis ratio might be the lost of a part of catalyst. For example, in the process of separating the reaction solution, a part of the catalyst might dissolve in the generated water. This part of the catalyst was lost after filtration and, thereby the esterification rate had a reduction. Of course, further optimization would be required for a technical application of the process.
Conclusions
In summary, a batch method was developed for testing the feasibility of conducting esterification reactions catalyzed by Lewis acid-surfactant combined catalysts, with phytostanols and fatty acids as the starting substrates. The results showed that [Cu(DS)2] and [Ce(DS)3] were active for the esterification of saturated fatty acids and unsaturated fatty acids with plant stanols, respectively. It should be noted that a relatively low reaction temperature and a shorter reaction time of the dodecyl sulfate-catalyzed esterification of phytostanols would be required. The synthesis of alternative esters formed by various fatty acids and phytostanols was also evaluated. The present study demonstrated that [Cu(DS)2] and [Ce(DS)3] could be promising catalysts in the process of commercial synthesis of phytostanyl esters from fatty acids and phytostanols. From the viewpoint of industrial economics and environmental friendliness, and compared with the high temperature-assisted enzymatic method, it might be more practical for large-scale industrial application.
Acknowledgements
This study was financially supported by the NSFC (31071600), Natural Science Foundation of Jiangsu Province (BK2011155), Funding of Jiangsu Innovation Program for Graduate Education (CXZZ11-0485), and Fundamental Research Funds for the Central Universities (JUDCF10054).
The authors have declared no conflict of interest.




