Study of the porous structure of white chocolate by confocal Raman microscopy†
The online version of this article contains colour figures.
Abstract
Confocal Raman microscopy has been shown to be a useful technique for investigation of white chocolate surfaces. The appearance of protrusions and pores, and the distribution of fat, sucrose, and milk powder at and below the surface of white chocolate pralines were investigated using confocal Raman microscopy. Raman horizontal and depth scans showed that the protrusions and pores continue at least 10 µm into the chocolate shell and that some protrusions and channels mainly consist of fat, while some consisted of a fat layer, leaving a hollow space underneath. Further, the pores and their continuing channels consisted of nothing but air. These findings indicate that the protrusions might be connected to channels where we suggest a pressure driven convective flow of liquid fat from within the chocolate matrix that, depending on temperature, moves up to the surface or goes back into the matrix, leaving an empty pore with a shell of fat at the surface, which in some cases collapse and leaves a hollow pore and channel. Therefore, these findings support that the protrusions could be connected to oil migration in chocolate and, thus, further to fat bloom development.
Practical applications: Confocal Raman microscopy can be used to investigate the local distribution of different components in white chocolate. This technique offers the possibility to acquire the local distribution of different components within the sample, with a resolution down to the optical diffraction limit. Further, the analysis can be performed at ambient conditions, without requiring any special sample preparation or marker molecules. The results obtained by using this technique suggest that specific surface imperfections on chocolate could be part of a network of pore structures at and beneath the chocolate surface, which could be related to oil migration and thus, to fat bloom formation.
Abbreviations:
CB, cocoa butter; CLSM, confocal scanning laser microscopy; LV SEM, low vacuum scanning electron microscopy
Introduction
Fat migration in chocolate products, often leading to fat bloom development, is a major issue for the confectionery industry, since it negatively affects both the visual and textural quality of the products. Chocolate that has developed fat bloom is normally characterized by loss of its initial gloss and a whitish haze at the surface. This dulling appearance is generally explained by the scattering of light due to needle-like fat crystals, larger than 5 µm, that are formed at the chocolate surface 1, 2. Previous research has demonstrated that in the presence of these needle-like fat crystals, the cocoa butter TAG can be found in their most stable polymorph, i.e., form β1VI 3-5. Thus, fat bloom on chocolate is often related to this polymorphic form. Still, the development of fat bloom formation is not clearly understood and fat bloom can take various forms, from surface to internal structures, depending on the product and the storage conditions 2, 5-9. Further, it has been shown that the polymorphic transition of form β2V (the desired polymorph in chocolate) to form β1VI may even occur without visual fat bloom development 3.
Fat bloom formation on chocolate products can be related to mainly three factors, i.e., poor tempering (polymorph and crystallization control) during production, storage at high temperatures (>23°C) and/or fluctuating temperature, leading to melting and re-crystallization of cocoa butter (CB), and migration of filling oil into the surrounding chocolate shell in the case of center-filled chocolate products, which promotes crystal growth. The most cited mechanism by which TAG migrates to the surface of filled chocolate has been fat diffusion, where a diffusion equation derived from Fick's second law normally is used to model the migration 10, 11. The diffusive mobility in the mainly crystalline matrix can be expected to depend on the exchange rate at the liquid crystal interfaces as suggested by Lofborg et al. 12. Lately, also capillary flow has been claimed to play a major role 13-15. In a hypothesis paper by Aguilera et al. it is proposed that within the chocolate matrix, formed by an assembly of fat-coated particles, the liquid fraction of CB (which increases with temperature) is likely to move under capillary flow through interparticle passages and connected pores 13. However, the mechanism of fat migration in chocolate pralines is until today not fully understood, and hence the detailed development of fat bloom remains unclear. Some recent studies have focused on the relationship between surface topology and fat migration, where atomic force microscopy, laser scanning microscopy, scanning electron microscopy, and optical profilometry have been used as main techniques 5, 6, 16-23. Some of these studies have reported chocolate surfaces featuring imperfections in form of pores and protrusions, and authors have suggested that these topological features could be related to fat migration and fat bloom development 6, 21, 22. In a study related to this present work, Dahlenborg et al. 6 suggest that there is a pressure driven convective flow leading to oil transportation through the chocolate matrix and to the chocolate surface, and that the oil migrates to the surface or goes back into the chocolate matrix depending on the temperature changes. Further, Loisel et al. have shown the presence of a porous matrix, that was partly filled with liquid CB fractions, in dark chocolate by using mercury porosimetry 24. In this study it was suggested that the porous matrix is a network of cavities closed by multiple walls of fat crystals impregnated with liquid fat. Due to the difference in dilation coefficients between solid and liquid fat and further due to polymorphic evolution, the liquid phase leaves spaces and air fills pores close to the surface or makes empty cavities. During temperature variations the difference in dilation of air and liquid fat could push the liquid fat fraction to the surface and thus contribute to fat bloom formation.
Thus, it is of great relevance to investigate if there could be a connection between this porous matrix and the observed imperfections at chocolate surfaces, and hence, how the different components are distributed at and below the surface of a chocolate product. This can be investigated by using confocal Raman microscopy. This technique has evolved into a fast, direct and non-destructive method applicable in food research 25-31. Confocal Raman microscopy offers the possibility to scan a sample to acquire a Raman spectrum providing chemical information, with a resolution down to the optical diffraction limit, i.e., the resolution limited by the wavelength of the used light. Further, the analysis can be performed at ambient conditions, without requiring any special sample preparation like addition of tracers or dyes, since the endogenic Raman scattering of the chocolate components is employed. Confocal Raman microscopy combines two different techniques, namely confocal microscopy and Raman spectrometry. In confocal microscopy a small pinhole, located in conjunction to the focus of the objective, allows only the light coming from the focal plane to pass into the detector. This focal plane can be moved several micrometers into the sample, which gives the opportunity of gaining 3D information. The possible depth of analysis depends on the properties of the material, in particular light absorption. Further, the Raman spectra allows us to differ between components as the energy of the Raman scattered light is the energy of the incoming light subtracted by the excitation energy, and as the excitation energies depends on the vibration properties of the molecular structures. Performing Raman microscopy, a Raman spectrum is recorded on every image pixel. Thus, when analyzing dedicated peaks of the spectra, a variety of images can be generated using only a single set of data. Post-processing of data enables identification of different components in the sample.
The objective of this study is to evaluate whether the former reported topological imperfections, observed as pores and protrusions at chocolate surfaces, are a part of a network of pore structures beneath and at the chocolate surface. This is done by using confocal Raman microscopy. In addition, low vacuum scanning electron microscopy (LV SEM) is used to identify the presence of topological imperfections. These results can lead to deeper understanding of the chocolate structure and thus, also extend the understanding of migration and thereby fat bloom development.
Materials and methods
Materials
White chocolate pralines with a hazelnut filling were manufactured by Ganache AB (Lilla Edet, Gothenburg, Sweden). The composition of the white chocolate and the hazelnut filling is listed in Table 1. The hazelnut mass consisted of caramelized and mixed hazelnuts, the milk chocolate had a cocoa level of 40% and contained whole milk powder, and the cream in the filling contained 40% fat. The white chocolate was tempered in a tempering unit (LCM 25 Twin), with melting temperature set to 43.0°C and tempering temperature set to 28.5°C. Since considerable fluorescence occurs when analyzing milk- and dark chocolate with confocal Raman microscopy (probably due to cocoa solids), white chocolate was used in this study.
| Components | White chocolate (wt%) | Hazelnut filling (wt%) |
|---|---|---|
| Cocoa butter | 35 | |
| Sugar | 43 | |
| Whole milk powder | 21.5 | |
| Emulsifier and vanilla | 0.5 | |
| Hazelnut mass | 54 | |
| Cream | 27 | |
| Milk chocolate | 19 | |
| Total fat | 41.1 | 36.1 |
| Sugar | 43 | 31.4 |
| Water | <1 | 15 |
Raman reference spectra were recorded for CB, sucrose, whole milk powder and skim milk powder. The CB (ADM Cocoa BV, The Netherlands) was supplied by Buhler AG (Uzwil, Switzerland), the sucrose was supplied by AAK (Aarhus, Denmark), the whole milk powder was supplied by Arla Foods Ingredients (Viby, Denmark), and the skim milk powder was supplied by Semper AB (Sundbyberg, Sweden). The composition of the whole and skim milk powder is presented in Table 2.
| Components | Whole milk powder (wt%) | Skim milk powder (wt%) |
|---|---|---|
| Milk protein | 24–34 | 36 |
| Lactose | 38–42 | 51 |
| Milk fat | 26 | 1 |
Low vacuum scanning electron microscopy
Scanning electron microscopy (SEM) is a technique producing high resolution images of a sample surface. Due to the way in which the image is created, SEM images have a characteristic three-dimensional appearance and are useful for judging the surface structure of a sample. Philips XL30 environmental scanning electron microscope, equipped with a cold stage set to 5°C was used with mixed detectors (biased gaseous detector (GSE) 75% and backscattered electron detector (BSE) 25%), at 15 keV and at low vacuum mode with 0.7–0.9 Torr, which gives an RH of 13% approximately. These parameters were chosen considering that they cause minimal damage of the chocolate surface, and for optimizing the image quality. In this study, analyses of the samples were performed without sample preparation, i.e., without coating the sample surface with a conducting layer. This leads to a sacrifice in magnification and resolution; however, by analyzing without coating the true surface can be monitored. After each analysis the monitored samples were sacrificed due to the potential impact from the microscope such as electron beam, temperature, humidity, and pressure.
Confocal Raman microscopy
The measurements were performed with a WITec alpha300 system (Ulm, Germany) which has a lateral resolution of 300 nm and a vertical resolution of 500 nm, using a 532 nm laser for excitation. The objective was a 50× Nikon objective (NA = 0.75), and the integration time per Raman spectrum was 0.1 s, which gives a sufficient signal-to-noise ratio at the same time as it does not destroy the samples. Further, the microscope was equipped with a Linkam cold stage set to 3°C, preventing the samples from melting under the influence of the laser. Reference spectra were obtained for cocoa butter, sucrose, whole milk powder, and skim milk powder. The scan range for horizontal images was 60 µm × 60 µm with 140 × 140 pixels in xy-direction (nominal resolution 430 nm). For the depth images the scan range varied between 60 µm × 20 µm and 60 µm × 40 µm, with 45 × 140 to 90 × 140 pixels in xz-direction. The penetration depth, i.e., the focal plane, in the xy-scans varied between approximately 6 and 12 µm, depending on the signal strength. A total of 15 horizontal scans and 10 correlating depth scans were performed in this study, which generated representative result images. The data was evaluated using the software program WITec project 2.06 (Ulm, Germany).
Results and discussion
White chocolate pralines containing a hazelnut filling were manufactured with composition and tempering processing securing properties characteristic for high quality chocolate. The surface morphology was characterized using LV SEM 1 week after manufacturing. Result images from LV SEM are displayed in Fig. 1, where the figures show a surface that is partly covered by circular protrusions (P1) and pores (P2). The outer diameter of the protrusions was calculated to a mean of 20 µm, with SD 1.5 µm, and the pore diameter was calculated to a mean of 9.7 µm, with SD 1.2 µm. These dimensions are comparable to the ranges reported in earlier studies 6, 19, 21. The sample surfaces were also monitored by LV SEM from a tilted angle in order to validate that the surface topological features resembling protrusions actually are protrusions. The results of the tilted samples support the interpretation that the surface displays protrusions of various sizes. Similar conclusions have also been reported earlier 6.
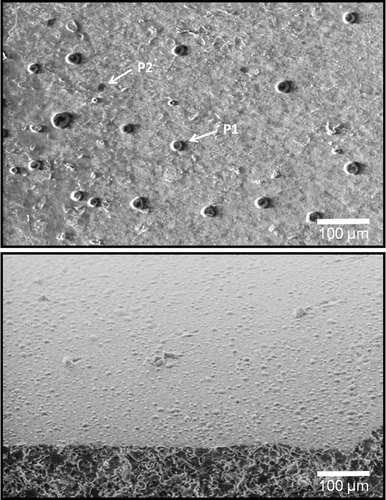
LV SEM images of white chocolate surface, enclosing a hazelnut filling, and a tilted chocolate sample (below), displaying imperfections observed as protrusions (P1) and pores (P2).
Confocal Raman microscopy was applied on the filled white chocolate samples with the objective of correlating the inner chocolate structure with the appearance of the protrusions. Reference spectra have been used to identify different components in the surface layer of the samples. When generating horizontal and depth scans a Raman spectrum is recorded in every image pixel and these spectra are compared to the reference spectra in order to acquire the local distribution of the components. The measured spectra were fitted with a linear combination of the reference spectra. Essentially a least squares fit is made over the whole spectra, i.e., single peaks are not investigated, and images for the different components are overlaid. Thus, an area can consist of several components at the same time even though only one component is shown. Raman reference spectra of cocoa butter (CB), sucrose, whole milk powder, and skim milk powder have been recorded in order to identify the distribution of the components in the surface layer of the samples (Fig. 2). Further, Raman reference spectra of lactose and whey protein were compared to the recorded reference spectra (Fig. 2). Assignments of the characteristic wave numbers to molecular groups are presented in Table 3. These assignments were achieved using the Refs. 30, 32-36. The sucrose spectrum is characteristic with several distinguished peaks due to its crystallinity. A similar feature is visible in the CB spectrum. In the sucrose spectrum the main Raman signals are between 400 and 1550 cm−1. Further, there is a strong hydrocarbon signal with several peaks between 2850 and 3050 cm−1 (ν(CH)) and a broad hydroxyl band (ν(OH)) between approximately 3200 and 3600 cm−1, with a sharp peak at 3550 cm−1. Lactose, as being chemically quite similar, although amorphous, has a similar pattern but with smoother regions of higher peaks. For instance the sharp peaks between 400 and 1550 cm−1 are all wider, the peak between 2830 and 3030 cm−1 is not split and the sharp peak at 3550 cm−1 is missing. Pure protein (whey protein) has a signal pattern that is fairly similar to the saccharides, however with a lower intensity and with a characteristic peak around 1670 cm−1 (the amide I signal (ν(CO)). Further, the broad peak between approximately 3150 and 3600 cm−1 includes amide functionalities (ν(NH)). CB has less peaks below 1000 cm−1 and some more intense peaks between 1000 and 1500 cm−1 as well as a distinct peak at 1664 cm−1 (ν(CC)) and between 2800 and 3060 cm−1 (ν(CH)). The compounded materials display features of several principal components. Skim milk powder mainly consists of milk proteins (36%) lactose (51%) and a lower amount of fat (in this case 1%). The skim milk powder spectrum displays the distinguished protein feature at 1678 cm−1 and the characteristic lactose features at around 400 and 850 cm−1. The whole milk powder spectrum consists of milk proteins (24%), lactose (38%), and fat (26%), providing similar protein and lactose features and a characteristic intensity at 1755 cm−1 that was also observed for CB.
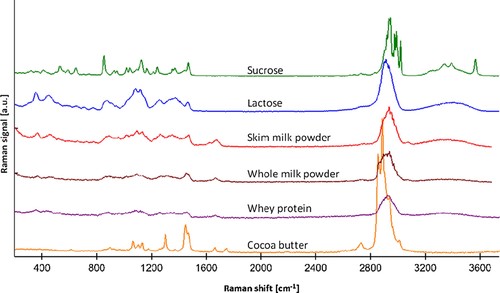
Recorded reference spectra of sucrose, amorphous lactose, skim milk powder, whole milk powder, whey protein, and cocoa butter.
| Sucrose wavenumber (cm−1) | Lactose wavenumber (cm−1) | Skim milk powder wavenumber (cm−1) | Whole milk powder wavenumber (cm−1) | Whey protein wavenumber (cm−1) | Cocoa butter wavenumber (cm−1) | Assignment | Ref. |
|---|---|---|---|---|---|---|---|
| 3200–3600 | 3150–3600 | ν(OH) | 33, 35 | ||||
| 3150–3600 | 3150–3600 | 3150–3600 | ν(NH) | ||||
| 2850–3050 | 2830–3030 | 2800–3050 | 2830–3030 | 2830–3030 | 2800–3060 | ν(CH) | 30, 32-34 |
| 1755 | 1750 | ν(CO) | 30, 32, 34, 35 | ||||
| 1664 | ν(CC) | 30, 34, 35 | |||||
| 1678 | 1668 | 1670 | Amide I/ν(CO) | 30, 32, 35 | |||
| 1467 | 1465 | 1473 | 1455 | 1456 | 1449 | δ(CH2) | 32, 34-36 |
| 1244 | 1355 | 1318 | 1303 | τ(CH2) | 32-34 | ||
| 1266 | 1266 | 1260 | Amide III/δ(NH) | 30, 35 | |||
| 1300–1420 | 1132 | 1132 | δ(COH) | 32, 36 | |||
| 1044 | 990–1180 | 1132 | 1132 | 1055–1145 | ν(CO) | 30, 32 | |
| 854 | 1132 | 1132 | ν(CC) | 32, 33 | |||
| 880 | ν(CNC) |
- ν, stretching mode; δ, bending mode, τ, twisting mode.
It is clear that the differences observed are vague compared to the chemical differences. Hence, the chemical resolution of the methodology is somewhat limited. However, it is also clear that there are possibilities to separate the principal components (milk powder, sucrose, and fat) from each other. Thus, the reference spectra of sucrose, CB, and skim milk powder were used to analyze the chocolate system. The skim milk powder was preferred over the whole milk powder as the butter fat contribution cannot be distinguished from the CB contribution.
Figure 3 represents light microscope images of the sample surfaces, i.e., white chocolate pralines containing a hazelnut filling. The images illustrate surfaces with fine roughness, including protrusions (Fig. 3A and B) and pores (Fig. 3C and D). The overlaid squares display the areas in which horizontal Raman images have been recorded, and the dashed lines indicate where the depth scans have been recorded. Thus, the images in Fig. 3 are connected to the representative Raman horizontal scans and depth scans in Figs. 4, 5. The areas in the result scans in Figs. 4, 5 represent fat rich domains (yellow/light gray), sucrose rich domains (green/gray), milk powder rich domains (red/dark gray) and the black areas indicate that no signal was achieved, i.e., these areas represent air. The dashed lines across the horizontal scans (Figs. 4A1, 4B1, 5C1, and 5D1) show where the depth scans have been recorded (Figs. 4A2, 4B2, 5C2, and 5D2), and the dashed lines in the vertical scans show where the focal plane for the horizontal scans is. Thus, the horizontal scans do not show the upper surface, but approximately 6–12 µm below the average actual surface, in order to avoid effects of surface roughness in the images.
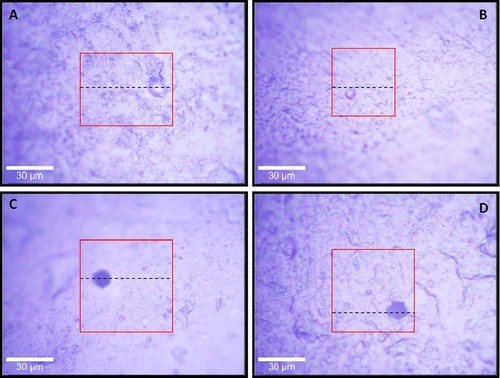
Light microscope images of white chocolate surfaces, enclosing a hazelnut filling. The overlaid squares represent the area over which Raman images have been recorded. (A, B) White chocolate surfaces including protrusions. These microscope images correlate to the horizontal and depth scans in Fig. 4A1 and 4A2, and in 4B1 and 4B2, respectively. (C, D) White chocolate surfaces including pores. These microscope images correlate to the horizontal and depth scans in Fig. 5C1 and 5C2, and in 5D1 and 5D2, respectively.
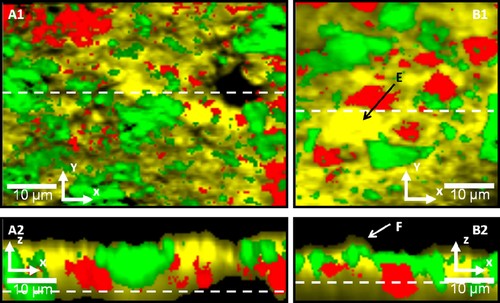
Horizontal scans, xy (A1 and B1), and depth scans, xz (A2 and B2), of white chocolate surfaces, enclosing a hazelnut filling. Yellow/light gray color represents fat rich domains, green/gray color represents sucrose rich domains, red/dark gray color represents milk powder rich domains, and black color represents air. The depth scans are performed over the dashed lines in the horizontal scans, and the position of the focal planes in the horizontal scans are shown with a dashed line in the depth scans. (A1) Scan range 60 µm × 60 µm with 140 × 140 pixels in xy-direction, and a penetration depth (focal plane) of 10 µm. (A2) Scan range 60 µm × 30 µm with 140 × 70 pixels in xz-direction. (B1) Scan range 60 µm × 60 µm with 140 × 140 pixels in xy-direction, and a penetration depth (focal plane) of 7 µm. (B2) Scan range 60 µm × 30 µm with 140 × 70 pixels in xz-direction. Integration time per Raman spectra is 0.1 s. E, F: Indicating a protrusion covered by a region enriched with fat.
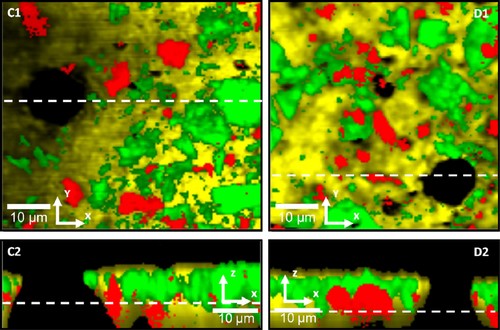
Horizontal scans, xy (C1 and D1), and depth scans, xz (C2 and D2), of white chocolate surfaces, enclosing a hazelnut filling. Yellow/light gray color represents fat rich domains, green/gray color represents sucrose rich domains, red/dark gray color represents milk powder rich domains, and black color represents air. The depth scans are performed over the dashed lines in the horizontal scans, and the position of the focal planes in the horizontal scans are shown with a dashed line in the depth scans. (C1) Scan range 60 µm × 60 µm with 140 × 140 pixels in xy-direction, and a penetration depth (focal plane) of 8 µm. (C2) Scan range 60 µm × 40 µm with 140 × 90 pixels in xz-direction. (D1) Scan range 60 µm × 60 µm with 140 × 140 pixels in xy-direction, and a penetration depth (focal plane) of 9 µm. (D2) Scan range 60 µm × 30 µm with 140 × 70 pixels in xz-direction. Integration time per Raman spectra is 0.1 s.
The images show that the technique allows us to clearly separate between the continuous CB matrix, the sucrose particles, the milk powder particles, and air filled pores. The images show quite well dispersed particles in agreement with the impression from images of milk chocolate that have been generated by using confocal scanning laser microscopy (CLSM) 37. The CLSM provides a somewhat sharper distinction between the particles, but it demands a staining procedure that may cause structural changes such as recrystallization. The confocal Raman images also provide a very clear image of cavities that is more difficult to obtain with CLSM as the sucrose is non-stained in the matrix. It can be noted that the outer layer of the chocolate shell consists primarily of fat, with occasional sucrose particles and milk powder particles appearing close to the surface and sometimes almost penetrating the fat air interface (depth scans in Figs. 4 and 5). The microstructure that appears as a possible protrusion in Fig. 3A is observed as a clear dent in Fig. 4A1. However, from the depth scan in Fig. 4A2 it can be noted that this area is covered by fat, but also by sucrose and milk powder particles, leaving a hollow space underneath. The absence of this overlaying “shell” in the horizontal scan is most probably due to the location of the focal plane (10 µm below the actual chocolate surface). However, the protrusion illustrated in Fig. 3B shows a region enriched with fat in the horizontal image (Fig. 4B1, E). The depth scan connected to this image has been recorded on the border of this area where an indication of a protruding region can be observed (Fig. 4B2, F), and where the uppermost layer consists of fat and sucrose rich domains. Still, further down through the shell the region indicates an area enriched with fat. This area correlates with the focal plane in the horizontal image (7 µm below the chocolate surface). The pores in Fig. 3C and D can be observed as nothing but air in the corresponding horizontal scans (Fig. 5C1 and D1). Further, the depth scans in Fig. 5C2 and D2 show that these air filled pores continue further down through the chocolate shell, at least 10 µm, resembling a channel. In addition, these channels seem to expand a few microns below the surface.
The result images indicate that the protrusions are filled mainly with fat or that they have a fat enriched “shell”, leaving a hollow channel underneath. This can further be connected to the structure of the protrusions that can be observed in the LV SEM images, as seen in Fig. 1. Further, it is shown that the pores are actually voids, consisting of neither fat nor sugar or protein. From the depth scans it seems as if these voids continue into the chocolate as channels, at least 10 µm. In addition, the mean pore size of the protrusions observed in the LV SEM images is estimated to 9.7 µm, with an SD of 1.2 µm, which correlates to the size range of the protrusions, pores, and channels detected in the Raman result images. If these channels continue even further into the chocolate, liquid fat from the filling and the chocolate matrix could be transported through these and up to the surface. The liquid fat might move through the channels, depending on the temperature, crystallize on the edge at the surface and then the liquid fat may return, leaving a hollow protrusion with a fat shell, which in some cases could collapse, leaving a pore at the surface. However, it could be argued that the empty protrusions, pores, and channels might be connected to gas filled bubbles lying under the surface of the chocolate shell. Still, the result images showing protrusions filled with mostly fat does not support this theory. The pressure driven convective flow theory is further supported by earlier studies 6, 21, 22, 24. In a recent study 6 designated protrusions at the surface were followed as a function of time and temperature, and the results showed that some of these protrusions disappear and then reappear, while some stay at the surface while changing their structure. Thus, this was interpreted as a pressure driven convective flow through pores and cracks in two directions, i.e., up to the surface and back into the shell. Smith and Dahlman 21 suggested that bloom growth in pralines is a two-step process, with drops initially forming at the surface and then bloom crystals nucleating and growing from them. In agreement with this study, Sonwai and Rousseau 22 found that cone-like structures might have formed at the surface of milk chocolate by welling and deposition of liquid-state fat pushed from within the matrix onto the surface during contraction. These cones hardened with age, and crystal outcroppings protruded from the cones.
Thus, the findings from this study could provide evidence for the existence of pores in chocolate suggesting that they may play an important role in the development of fat bloom on filled chocolate products.
Conclusions
The objective of this study was to evaluate if the former reported topological imperfections, observed as pores and protrusions at chocolate surfaces, could be a part of a network of pore structures beneath and at the chocolate surface. This was done by investigating the appearance of these imperfections and the distribution of different components within them in white chocolate pralines by using confocal Raman microscopy. This study shows that there are actually protrusions at the chocolate surface (confirmed by LV SEM) having a base mean diameter of 20, with SD 1.5 µm, and an inner pore mean diameter of 9.7, with SD 1.2 µm. Further, Raman horizontal and depth scans indicate that these protrusions continue at least 10 µm in to the chocolate shell. Some of the analyzed protrusions and their continuing channels mainly consisted of fat, while some held a thin shell mainly consisting of fat, leaving a hollow space underneath. The analyzed pores and their continuing channels consisted of nothing but air, as characterized by Raman microscopy. We suggest that the protrusions might be connected to channels where a pressure driven convective flow of fat from within the chocolate matrix that, depending on pressure gradients caused by temperature changes, may be pressed up at the surface or drawn back into the matrix, leaving an empty pore with a shell of fat at the surface, which in some cases collapses and leaves a hollow pore and channel underneath. Thus, the findings from this study support the hypothesis that the pores could be connected to oil migration in filled chocolate products and, thus, further to fat bloom development.
Acknowledgements
This work was funded by EU commission, grant 218423 (ProPraline) under FP7-SME-2007-2, project officer German Valcárcel ([email protected]). The authors thank Ganache AB for providing the chocolate samples, and for valuable input from other partners of the ProPraline project. Further, the grant from Nils and Dorthi Troëdsson Foundation for the combined confocal Raman/AFM equipment is gratefully acknowledged. Finally, the authors thank Adam Feiler, YKI, and Thomas Dieing, WITec, for providing valuable support and helpful information.
The authors have declared no conflict of interest.




