Lipase-catalyzed transesterification to remove saturated MAG from biodiesel
Abstract
Saturated MAG (SMG) are known to be present in FAME intended to be used as biodiesel. These SMG can strongly affect the properties of biofuels such as the cloud point (CP), and they have been implicated in engine failure due to filter plugging. It is shown here that lipase G from Penicillium camemberti can be efficiently used for the transesterification of SMG to fatty acid methyl ester and glycerol even in the presence of the bulk biodiesel. Thus, in samples of commercial biodiesel to which glycerol monostearate (GMS) and glycerol monopalmitate (GMP) had been added, their levels were enzymatically reduced from 2% (w/v) to 0.22% (w/v) for GMP and 0.14% (w/v) for GMS as confirmed by GC-MS analysis.
Practical applications: SMG present in biodiesel can have a pronounced negative effect on the CP, and/or filterability and in-field performance of the fuel. The lipase-catalyzed transesterification shown in this paper enables a significant reduction in the amount of SMG, leading to superior biodiesel quality.
Introduction
As the demand for alternative, renewable fuels with reduced greenhouse gas emissions and enhanced air quality has increased, and engine and fuel systems became ever more demanding, the quality and properties of biodiesel (FAME) have become more significant. Despite the generally successful use of biodiesel as an alternative diesel fuel for over a decade, instances still occur where the fuel does not perform as expected. In some cases low temperature is a contributing factor to this poor performance.
Particulate matter in biodiesel can clog fuel filters and cause engine failure due to fuel starvation. The offending material can be solid particulate contaminants that enter the fuel during production, distribution or storage. Solids can also form from the fuel itself, for example, if low temperatures cause some of the fuel components to solidify. To avoid such problems, existing standards for biodiesel such as EN 14213 (the EU standard for biodiesel for uses in heating oil) 1 and ASTM D6751 (the ASTM standard for pure biodiesel intended for use as a blend stock) 2 call for the conduct of cold filter plugging point or cloud point (CP) assays, respectively, to avoid use of fuels prone to form solids under expected operating temperatures. Nonetheless, filter-plugging events have been reported. In some cases, these have even occurred following fuel storage and/or engine operation at temperatures above the CP of the biodiesel.
MAG are the final intermediates in the transesterification of TAG to produce biodiesel. Their rate of transesterification to produce a fatty acid methyl ester and glycerol is substantially lower than the corresponding transesterification rates of tri- and di-acylglycerols 3. As such, they are the most likely contaminating unreacted acylglycerol species in biodiesel. The average MAG content of US biodiesel has been estimated to be approximately 0.5 wt% 4.
It has come to be appreciated that MAG play a crucial role in particulate formation in biodiesels. Along with sterol glucosides, fatty acid soaps, and water, MAG have been shown to impact solids formation and filter plugging tendencies in biodiesel 5. MAG containing saturated fatty acids (saturated MAG, SMG), particularly play a role in such phenomena because of their high melting points: 77°C for glycerolmonopalmitate and 81.5°C for glycerolmonostearate 6. It has been observed that the addition of SMG to a biodiesel can raise its final melting temperature by as much as 25°C 7. Material obtained from plugged vehicle fuel filters obtained in the winter of 2005–2006 in the state of Minnesota (USA) consisted of a preponderance of SMG 5. SMG have been associated with fuel filter plugging during low temperature operational studies of heavy-duty trucks and in the plugging of fuel dispenser filters 8, 9.
Recently, it has been shown that under relatively rapid cooling rates which occur in standard methods for determining CP in biodiesel, SMG will initially crystallize in “alpha” forms, which display low melting points and relatively small crystal sizes 10. These small crystals are able to pass unimpeded through typical fuel filters and do not interfere with engine operation. Over time, and even under conditions of slow warming, these can convert to larger, higher melting point “beta” form crystals that persist at temperatures above the CP of the fuel 10. These can especially contribute to filter plugging, and are thus implicated in engine operability issues occurring above the CP of the fuel.
Due to these problems there is interest in the development of suitable methods for the removal of MAG, especially SMG, from biodiesel. One approach, chilling followed by filtration (e.g., “winterization”), is employed by some biodiesel manufacturers. However, this method can suffer from unavoidable entrapment and loss of the desirable biodiesel product, and generates a solid byproduct in need of disposal or use. Because the methyl esters of saturated fatty acids have considerably lower melting points (palmitate: 28–31°C, stearate: 37–39°C 11) than the corresponding monacylglycerols, another approach employed by industry is to extend the final transesterification or conduct an additional transesterification reaction in order to increase the conversion of SMG to FAME. This can be achieved using the alkaline catalysis transesterification technology currently well established in the biodiesel industry. The ability of CO2 to increase the rate of this reaction has been described 12.
An enzymatic transesterification would be an ideal solution compared to its chemical counterpart as no harmful chemicals need to be used and processing with immobilized enzymes is a rather straightforward route. Enzymatic transesterification may offer a lower cost approach than winterization. However, a lipase needs to be identified which preferentially acts on the small residual amounts of MAG (<2%) rather than the bulk FAME (>98%) present in biodiesel. We have therefore chosen a lipase from Penicillium camemberti (lipase G) as this has been reported to exhibit a unique preference for MAG 13, 14 and is also known to be stable and active in the presence of organic solvents 15, which is an important feature since FAME has solvent like properties. We report here the results of an investigation of the ability of this lipase to reduce the levels of SMGs in biodiesel by transesterification.
Materials and methods
Chemicals and enzymes
Biodiesel was purchased from a local gas station in Greifswald, Germany. The fatty acid composition of its methyl esters, determined by GC and reference to known standard FAME (below) was C16:0: 4.4%; C18:0:1.5%; C18:1:65.2%; C18:2:19.6%; C18:3:8.2%; C22:1:1.1%. This is consistent with production of this biodiesel from rapeseed oil, the prevalent feedstock for European biodiesel 16. Glycerol monostearate (GMS) and lipase G (Penicillium camembertii lipase dry powder, ≥50,000 U/g) were purchased from Sigma–Aldrich, Steinheim, Germany. Glycerol monopalmitate (GMP) was synthesized in our laboratory as described below. Palmitic acid chloride (>98% purity) was obtained from Fluka (Buchs, Switzerland).
Synthesis of GMP
Seventy milliliters dry tetrahydrofuran (THF) and 20 mmol glycerol (1.5 mL) were added to a dry round bottom flask. Twenty mmol triethylamine (2.78 mL) was added dropwise through a septum followed by stirring for 10 min. Then 17.8 mmol (4.9 g or 5.45 mL) palmitic acid chloride, pre-dissolved in 10 mL THF, was added dropwise and the reaction continued for 24 h at RT. The reaction mixture was then filtered, solvents were evaporated in vacuo and the product was recrystallized from petroleum ether. The product GMP obtained in 66.4% yield (3.91 g) was characterized by 1H, 13C-NMR and GC-MS and matched literature data 17. Unless stated otherwise, all reagents were obtained from common commercial suppliers.
GC-MS analysis of the MAGs
Simultaneous analysis of GMP, GMS, and the corresponding methyl esters was not possible using the available FAME GC column (see below). Hence the MAGs were analyzed by GC-MS. Both GMP and GMS were derivatized using pyridine, hexamethyldisilazane (HMDS) and trimethylsilylchloride (TMSCl) as reported in literature 18. The trimethylsilyl (TMS) derivatized samples were analyzed with a Shimadzu Gas Chromatograph MS-QP2010S GC-MS device, equipped with a BPX column (5% phenyl polysilphenylene siloxane/95% methyl polysilphenylene siloxane, 25 m × 0.22 mm × 0.25 µm) with the temperature program: 50°C for 1 min, then increased at 15°C/min to 320°C and held for 4 min, injector: 300°C, interface temperature: 220°C, ion source temperature: 200°C. Retention times of the TMS derivatives of GMP and GMS were 15.3 and 16.25 min, respectively. Helium was used as the carrier gas at a flow rate of 3 mL/min.
To prepare standard curves of GMP and GMS in biodiesel, six different concentrations of each compound were prepared as follows: to 1 mL biodiesel was added 20 mg of each, GMP (60.5 µmol) and GMS (55.78 µmol) to achieve a concentration of 2% (w/v) of each MAG in the biodiesel. Similarly 16, 12, 8, 4, and 2 mg of GMP and of GMS were jointly added to biodiesel to prepare solutions containing 1.6, 1.2, 0.8, 0.4, and 0.2% (w/v) of each of the two MAG. These were heated in a water bath at 60°C to dissolve the MAG. Derivatization was done by dissolving 50 µL of each sample in 100 µL pyridine followed by addition of 40 µL HMDS and 20 µL TMSCl. After 1 min at RT, the reaction mixture was centrifuged at 17,000g for 1 min and 10 µL of the sample was transferred into a 1.5 mL Eppendorf® tube containing 100 µL each of hexane and ethyl acetate. 10 µL of a 100 mM acetophenone solution in ethyl acetate served as internal standard. The mixture was filtered by filter tip FT 100 (Greiner Bio-one) and analyzed in triplicate by GC-MS as described above. The resulting GC-MS peak areas were divided by the peak areas for the corresponding internal standard and plotted against the known monoacylglyceride concentrations to generate standard curves for GMP and GMS.
Gas chromatographic analysis of FAME
Methyl palmitate (MP) and methyl stearate (MS), the corresponding transesterification products of GMP and GMS, were separated and measured on a Shimadzu Gas Chromatograph-2010 equipped with a FID and fitted with a Varian Select™ FAME capillary column (100 m × 0.25 mm × 0.25 µm, CP-Select CB for FAME) with the temperature program: 170°C for 5 min, then increased at 3°C/min to 250°C and held for 10 min. Injector and detector temperature were 250°C. Hydrogen was used as carrier gas at a flow rate of 1 mL/min. The retention times of MP and MS were 12.3 and 15.44 min, respectively. As the aim of this project was to convert the GMP and GMS into their methyl esters, one expects an increase in the concentration of MP and MS in the biodiesel reaction mixture over time of incubation with enzyme. To quantify this increase we prepared a standard curve by adding different amounts of the MP and MS to a fixed amount of biodiesel. The sample preparation for GC analysis was done as follows. A stock (60, 50, 30, 20, 5, and 0 µmol/mL) of MP and MS in chloroform was prepared. Each GC sample contained 192 µL chloroform, 5 µL 100 mM acetophenone in chloroform as internal standard, 1 µL biodiesel and 1 µL each of the above different concentrations of MP and MS. Samples were analyzed by GC using the above-mentioned program. Standard curves for these methyl esters were prepared as described above for MAG.
Lipase catalyzed transesterification of MAGs in biodiesel
Into a 10 mL round bottom flask was taken 60 mg each of GMP (181.5 µmol) and GMS (167.34 µmol) and 3 mL of biodiesel was added, giving a final concentration of 2% (w/v) for each MAG. The MAG were dissolved by heating the flask at 60°C. The flask was transferred to a 50°C bath equipped for magnetic mixing, 60 µL methanol (1.5 mmol) followed by 12 mg lipase G were added, and the flask was stoppered and stirred for 24 h. To determine residual GMP and GMS levels during these incubations, 50 µL aliquots were withdrawn at selected times, derivatized 18 and analyzed by GC-MS using the program described above. To ensure an adequate methanol concentration in the reactions during heating at 50°C, additional 60 µL doses of methanol were added after 2, 4, 6, and 24 h.
Results and discussion
In order to study the lipase-catalyzed conversion of the SMG in biodiesel, first a reliable analytical method needed to be established. GC-MS analysis of solutions of GMP and GMS of known concentrations indicated a strong linearity between detector response and the amount of analyte. These data are shown in Fig. 1, along with equations of best-fit lines for the data. The graphs showed linearity (R2 = 0.9955 for GMP and 0.9942 for GMS) and were used for the subsequent calculation of the residual MAGs during the enzymatic transesterification.
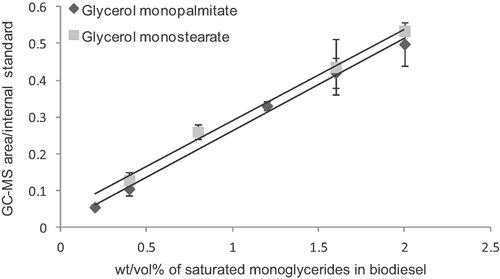
Standard curves for GC-MS analysis of GMP and GMS in biodiesel.
Similarly, Fig. 2 shows the highly linear GC response to different concentrations of MP and MS esters in biodiesel. Whereas the response curves for GMP and GMS were virtually colinear, different sensitivities were observed for the methyl esters of palmitate and stearate. For both methyl esters high linearity (R2 = 0.954 for MP and 0.973 for MS) was observed.
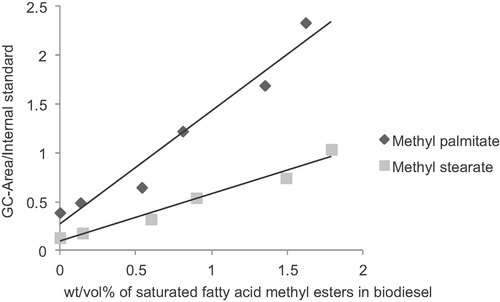
Standard curve of saturated FAME added to biodiesel.
In order to investigate the possibility that lipase-catalyzed transesterification could be employed to reduce the levels of SMG in biodiesel, we investigated the effectiveness of lipase G (selected for the reasons stated in the Introduction Section) for the transesterification of these MAG in biodiesel. To explore the efficiency and selectivity of the enzyme, SMGs of most interest in the context of biodiesel performance were added to commercial biodiesel to a concentration of 2.0% (w/v) and the lipase-catalyzed reaction was followed by GC analysis. Figure 3 shows the time course of reduction of each SMG, and a roughly corresponding increase in the corresponding methyl esters. It is obvious that almost 90% of the SMGs were converted into FAME over the course of the reaction with lipase.

Decrease in the concentration of GMP and GMS and concomitant increase in the concentrations of methyl palmitate and stearate in biodiesel by lipase-catalyzed transesterification.
To exclude that the methanol co-reactant resulted in activity loss of the lipase, the experiment was repeated using additional doses of the enzyme. The initial reaction mixture contained 2 mL biodiesel, 40 mg each of GMP and GMS, 40 µL methanol and 8 mg lipase G. Two further methanol doses (40 µL each) were added, one at 4 and the other at 10 h. Similarly, two further 8 mg doses of lipase G doses were added, one at 6 and one at 10 h. This led to a decrease in the concentration of GMP to 0.22% (w/v) and to 0.14% (w/v) for GMS after 24 h. Comparison of the results from this multiple dosing reaction with those from earlier single-dose reactions showed that only marginal differences occurred in the final concentration of the SMG in the two reactions, suggesting that the transesterification of SMG was not limited by lipase availability in the original reactions, which received only a single 12 mg dose of lipase.
Conclusions
The data show that lipase G retains enzymatic activity in FAME, and that even in a system composed predominantly of FAME it retains its activity toward MAG and can substantially reduce their concentrations via transesterification. The amount of time required to achieve this conversion, over 25 h, may seem excessive. However, it is notable that few if any potential biodiesels should contain individual SMG levels as high as the 2% used here. Also, optimization of the reaction would be expected to result in a reduced reaction time. We thus provide an alternative enzymatic method to produce a superior biodiesel quality. Furthermore, the by-product of this reaction, glycerol, can be easily removed by water washing treatment.
Acknowledgements
We thank the Alexander von Humboldt Foundation (AvH) Germany, for a fellowship to SKP and Anita Gollin (Institute of Biochemistry, Greifswald, Germany) for help with GMP synthesis.
The authors have declared no conflict of interest.




