Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax
Abstract
Four different antioxidant activity assays including 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP) and oxygen radical absorption capacity (ORAC), and thiobarbituric acid reactive substances were performed on the methanolic and ethyl acetate extracts of Camelina seeds (CS), flaxseeds (FS), Camelina meal low fat (CMLF, 9.9% fat), Camelina meal high fat (CMHF, 24.6% fat), and flaxseed meal (FSM, 2.7% fat). In addition, the fatty acid profile, and phenolic, tocopherol, flavonoid, and glucosinolate contents of CS, FS, CMLF, CMHF, and FSM were studied. The major fatty acid was α-linolenic acid (C18:3 n-3) which was 33.2, 29.4, 30.2, 60.1, and 39.3% in CS, CMLF, CMHF, FS, and FSM, respectively. The methanolic extract of CMLF showed the highest values of ABTS, DPPH and FRAP and the highest content of phenolic compounds, flavonoids, and glucosinolates. The methanolic and ethylacetate extracts of CMHF showed the highest values for ORAC and α- and γ-tocopherols. The ethylacetate extracts of seeds and meals of Camelina sativa and flax showed lower values for antioxidant activity, phenolic compounds, and flavonoids than the methanolic extracts. In general, Camelina and FS meals showed higher antioxidant activities, and phenolic and flavonoid contents than their respective seeds.
Practical applications: Camelina sativa seeds (CS) and flaxseeds (FS) are rich sources of omega 3 oils. Their by-products after oil extraction are an attractive source of proteins, lipids, fiber, and natural bioactive compounds such as antioxidants. These by-products may be used to improve nutritional value and prevent lipid oxidation in feed or food systems.
Abbreviations:
ABTS, 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid; CM, Camelina meal; CMHF, Camelina meal high fat; CMLF, Camelina meal low fat; CS, Camelina seeds; CSO, Camelina sativa oil; DPHH, 2,2-diphenyl-1-picrylhydrazyl; FRAP, ferric reducing antioxidant power; FS, flaxseeds; FSM, flaxseed meal; ORAC, oxygen radical absorption capacity; PGR, pyrogallol red; TBARS, thiobarbituric acid reactive substances; TE, trolox equivalents
Introduction
There is a lot of interest in the use of by-products from the food industry, not only for economic and environmental purposes, but also for the valuable sources of compounds with favorable technological or nutritional properties 1. Among these compounds are antioxidants which have been used to prevent lipid oxidation in food systems 2, 3 and free radical-mediated chronic diseases such as cancer and cardiovascular disease 4. Camelina sativa also known as “false flax,” is an oilseed crop from the Brassica (Cruciferae) family that has received great attention because it is a low-input crop with a great potential for feed 5 and non-feed applications such as biodiesel 6. Its seeds contain 30–40% of oil on a dry matter basis and 25–45% crude protein. Camelina sativa oil (CSO) is rich in α-linolenic (C18:3 n-3; 36–42%) acid and it has a relatively low glucosinolate content 7. CSO and its by-products have been used in animal nutrition 8-13. Camelina meal (CM), a by-product of oil extraction from the seed has been considered for animal feeding because of its content of high crude protein (35–40%), gross energy (4688 kcal/kg), and α-linolenic acid (18:3 n-3) (∼30%) 13, the parent fatty acid of the omega-3 (n-3) family. CM is also rich in antioxidant compounds and tocopherols 14, 15. Flaxseed (FS, Linum usitatissimum) is an oilseed widely studied in human and animal nutrition as a potential functional food for its high content of fat (41%), α-linolenic acid (59%), protein (21%), fiber (28%), and phenolic compounds such as the lignan secoisolariciresinol diglucoside 16. FSs and FS flour have been used for the formulation of baked cereal products, ready-to-eat cereals, fiber bars, salad toppings, and meat extenders 17-20. FS products have been used in animal feeding to increase the omega-3 content and nutritive value of eggs, milk, and meat 21-24. However, it has been reported that FSs contain antinutritional compounds (e.g., mucilage, vitamin B6 antagonist [linatine], cyanogenic glycosides, trypsin inhibitors, phytic acid, allergens, and goitrogens) that can affect bird growth and performance 25. On the other hand, FS and its by-products have been shown to have antioxidant activity in vitro 26, 27 and in vivo 28-30. The objectives of this study were to analyze the antioxidant activities, thiobarbituric acid reactive substances (TBARS), fatty acid profile, and phenolic, flavonoid, tocopherol, and glucosinolate content of Camelina seeds (CS), CM, FS, and flaxseed meal (FSM). CMs with two different levels of fat were investigated to evaluate the effect of fat content on the antioxidant activity of the meals. This study is an attempt to provide useful information to food/feed formulators or producers.
Materials and methods
Chemicals and reagents
Camelina seeds, Camelina meal low fat (CMLF), Camelina meal high fat (CMHF), FS, and FSM were supplied by Willamette Biomass Processors (Rickreall, OR). All solvents were HPLC-grade provided by EMD (Gibbstown, NJ). Chemicals and reagents used were as follows: aluminum chloride (Alfa Aesar, Ward Hill, MA); sodium nitrites (Acros Morris Plains, NJ); sodium hydroxide, potassium persulfate, sodium sulfate, and ferric chloride hexahydrate (Mallinckrodt, Phillipsburg, NJ); DL-α-tocopherol and quercetin dehydrate (MP Biomedicals, Solon, OH); rac-5,7-dimethyltocol (Matreya, Pleasant Gap, PA); sodium chloride (BHD, West Chester, PA); acetic acid glacial (Fisher, Fair Lawn, NJ); lead acetate, potassium ferricyanide, hydrochloric acid 37%, and perchloric acid (EMD, Gibbstown, NJ); barium acetate (Acros Organics, Fair Lawn, NJ). All other chemicals used were obtained from Sigma–Aldrich (St. Louis, MO).
Extracts preparation
Methanolic and ethylacetate extracts were prepared for determination of antioxidant activities, and phenolic and flavonoid contents of CS, CMLF, CMHF, FS, and FSM according to the method of Matthäus 14 with some modifications. Seed samples were ground for 20 s before extraction. Seeds (2 g) and meal samples (2 g) were placed in Erlenmeyer flasks and, after the addition of 20 mL of methanol:water (70:30, v/v), were subjected to ultrasonic treatment for 45 min at RT. The methanolic extracts were filtered, reconstituted to 20 mL and stored at −20°C. After addition of 20 mL of ethylacetate:water (70:30, v/v) the remaining meal and seed samples were subjected to ultrasonic treatment for 45 min at RT. The ethylacetate:water extracts were filtered and the ethylacetate layers were withdrawn and reconstituted to 20 mL and stored at −20°C until analysis.
Antioxidant activity assays
2,2′-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay was performed according to the method of Thaipong et al. 31. An ABTS stock solution was prepared by mixing a 7.4 mM solution of ABTS with 2.6 mM potassium perfsulfate, and leaving it to stand in the dark at RT for 12–16 h. An ABTS radical solution was prepared with 30 mL of ethanol and 1.5 mL of ABTS Stock solution to get a final absorbance higher than 1.1 ± 0.02 at 734 nm. ABTS radical solution (2850 µL) were placed in cuvettes and 150 µL of extracts or trolox standards (25–600 µM) were added and allowed to react for 2 h in the dark. Absorbance was read at 734 nm. Results were expressed in µM trolox equivalent (TE)/g meal.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay was performed according to the method of Thaipong et al. 31 with some modifications. DPPH stock solution was prepared by dissolving 24 mg DPPH in 100 mL methanol, followed by storage at −20°C. The working DPPH solution was prepared by mixing 25 mL of stock solution with 45 mL methanol to obtain an absorbance of 1.1 ± 0.02 at 515 nm. A set of trolox solutions (25–800 µM) were prepared as standard curve. DPPH working aliquots (2850 µL) were placed in cuvettes to which 150 µL of extracts or trolox standards were added and allowed to react for 24 h in the dark. Absorbance was read at 515 nm. Results were expressed in µM TE/g meal.
The ferric reducing antioxidant power (FRAP) was determined using the method of Thaipong et al. 31. Working FRAP solution was prepared by mixing 25 mL of acetate buffer 300 mM (pH 3.6), and 2.5 mL of 10 mM TPTZ (2, 4, 6, tripyridyl-s-triazine) solution in 40 mM HCl and 2.5 mL of 20 mM FeCL3·6H2O, followed by warming at 37°C before use. A set of trolox solutions (25–800 µM) were prepared as standard curve. FRAP working aliquots (2850 µL) were placed in cuvettes to which 150 µL of extracts or trolox standards were added, followed by 30 min reaction time in the dark. Absorbance was read at 593 nm. Results were expressed in µM TE/g meal.
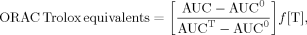
Thiobarbituric acid reactive substances (TBARS) assay
-
A = [(Abs532+TBA) − (Abs600+TBA) − (Abs532-PER − Abs600-PER]
-
B = [(Abs440+TBA − Abs600+TBA)0.0571]
-
MDA equivalents (nmol/mL) = (A − B/157 000)106
Total lipid and fatty acid analysis
Total lipids were extracted from 2 g grounded seed or meal samples using chloroform:methanol (2:1) 34 and lipid content was determined gravimetrically. FAME were prepared from the total lipid extract using methanolic HCl as the derivatizing agent as reported earlier 35. Analyses of FAME were performed with an Agilent 6890 gas chromatograph (Agilent Technologies, Inc., Palo Alto, CA) equipped with an autosampler, FID and fused silica capillary column, 30 m × 0.25 mm × 0.2 µm film thickness (Sp-2560; Supelco, Bellefonte, PA). Each sample (1 µL) was injected with helium as a carrier gas onto the column programmed for ramped oven temperatures (initial temperature of 110°C was held for 0.5 min, then ramped at 20°C/min to 190°C, held for 7 min, then ramped at 5°C/min to 210°C, and held for 8 min). Inlet and detector temperatures were both 250°C. Peak areas and fatty acid percentages were calculated using Agilent ChemStation software. FAME were identified by comparison with retention times of authentic standards (Matreya, Pleasant Gap, PA) and were expressed as percentages of total FAME. An internal standard C19:0 was used for fatty acid quantification.
Phenolic compounds
The extraction of phenolic compounds was performed according to methods of Vuorela et al. 36 with some modifications. Sample extracts and gallic acid standards (200 µL) were placed in 10-mL test tubes containing 0.2 mL of methanol–water solution (2:1, v/v), 1 mL of Folin solution and 0.8 mL of sodium bicarbonate solution 7.5%, mixed and left at RT for 30 min. Absorbances were read at 765 nm using distilled water as blank. Gallic acid solutions (0.05–0.5 µg/mL) were used as standards and results were reported as gallic acid equivalents.
Determination of total flavonoids
Total flavonoids were measured by a colorimetric assay according to Kim et al. 37. One milliliter of phenolic extract or standard solution of quercetin (0–500 mg/L, MP Biomedicals) was added to 4 mL of H2O. At zero time, 0.3 mL of 50 g/L NaNO2 was added and after 5 min, 0.3 mL of AlCl3 (100 g/L) was added. After 6 min, 2 mL of 1 mol/L NaOH was added to the mixture and then diluted with 2.4 mL of H2O. Absorbance of the mixture was read at 510 nm against a water blank. Total flavonoids were expressed as milligrams of quercetin equivalent.
Tocopherol assay
α- and γ-Tocopherol contents of CS, CMLF, CMHF, FS, and FSM were analyzed by HPLC as reported previously 35, 38. Briefly, 1 g of sample, 0.1 mL of the internal standard (rac-5,7-dimethyltocol), 2 mL of 1% ascorbic acid in ethanol, and 0.3 mL of saturated KOH were incubated in a 70°C water bath for 30 min and then cooled on ice. After addition of 2.5 mL of hexane, the samples were centrifuged and the upper hexane layer was taken and evaporated under a stream of nitrogen. The residue was dissolved in 0.2 mL of ethanol, then centrifuged at 8000×g for 5 min, after which 0.15 mL of the supernatant was taken for assay. A Shimadzu LC-2010 HT HPLC system was used with a LC2010 AHT High Speed Autosampler (Shimadzu, Columbia, MD). A SuperguardTM LC-18 guard column (Superguard TM LC-18, Supelco, Bellefonte, PA) and a mobile phase of methanol–water (95% methanol, 5% ultra pure water) at a flow rate of 1 mL/min were used. Detection was performed using a Shimadzu RF-535 ultraviolet detector at a wavelength of 295 nm. A Shimadzu EZSTART 7.3 chromatography data system was used to integrate peak areas. Concentration of α-tocopherol was calculated by comparing α-tocopherol peaks with peak areas of the internal standard (rac-5,7-dimethyltocol) and quantified by using authentic standards (dl-α-TOC).
Glucosinolate assay
The glucosinolate content of CM was determined according to Jezek 39 with simple modifications. CS, CMLF, CMHF, FS, or FSM (0.5–1.0 g) were added to 7.5 mL of boiling acetate buffer (pH 4.2, 0.2 M). The mixture was kept in a boiling water bath for 15 min. After cooling (5 min), the whole extract was mixed with a solution containing 1.5 mL of sodium sulfate and lead acetate (1.5 mL) and kept at RT for 15 min. The mixture was spun at 4000×g for 12 min, after which the supernatant (2 mL) was mixed with 0.1 g of activated charcoal and spun at 4000×g for 12 min. The supernatant (0.45 mL) was taken and mixed with 0.45 mL of 2 M NaOH. The samples were kept for 30 min at RT and 75 µL of 37% HCl was added. The samples were spun at 6000×g for 10 min and the supernatants (0.5 mL) were mixed with an equal volume of potassium ferricyanide (2 mM). The absorbance of samples was read at 420 nm (UV160U, Shimadzu Corporation) using phosphate buffer (pH 7, 0.2 M) as blank. Sinigrin hydrate (TCI, Toshima, KITA-KU, Tokyo, Japan) was used as a standard.
Results and discussion
Fatty acid profile and total lipids
Table 1 shows the fatty acid profile and total lipids of CS, CMLF, CMHF, FS, and FSM. FSM had the highest content of palmitic acid, oleic acid, and total saturated fatty acids. CMHF and CMLF showed the highest content of linoleic acid (24.63 and 24.35%, respectively). FS had the highest content of α-linolenic acid (60.08%), followed by FSM (39.29%), CS (33.21%), CMHF (30.17%), and CMLF (29.37%). The differences in content of fatty acids of seeds and meals may be explained due to the lower fat content of the meals and possible loss during the oil extraction process. CS had the highest content of eicosanoic acid (C20:1), followed by CMHF and CMLF. However, eicosenoic acid was almost negligible in FS and FSM. FS showed the highest value for total n-3 fatty acids and the lowest for n-6 which can be explained due the high content of α-linolenic acid. Consequently the ratio of n6:n3 is lowest in FS compared with the rest of meals and CS. These results are in accordance with the ones reported by Budin et al. 6. FSM showed the lowest fat content (2.72%) compared to FS (42.0%), CS (38.9%), CMLF (9.9%), and CMHF (24.6%).
| Fatty acid (%) | CS | CMLF | CMHF | FS | FSM | p-Value |
|---|---|---|---|---|---|---|
| 14:0 | 0.09 | 0.00 | 0.00 | 0.00 | 0.28a | 0.002 |
| 16:0 | 6.46 | 7.59 | 7.19 | 5.81 | 12.85a | 0.041 |
| 16:1 | 0.32b | 0.00d | 0.32b | 0.22c | 0.45a | <0.0001 |
| 18:0 | 2.49 | 2.27 | 2.48 | 3.47 | 3.69 | 0.131 |
| 18:1 | 17.54c | 20.46b | 20.25b | 15.61d | 23.31a | <0.0001 |
| 18:2 n-6 | 19.04b | 24.35a | 24.63a | 14.52c | 18.71b | <0.0001 |
| 18:3 n-3 | 33.21 | 29.37 | 30.17 | 60.08 | 39.29 | <0.0001 |
| 20:1 n-9 | 15.57a | 11.23c | 13.30b | 0.00d | 0.52d | <0.0001 |
| 20:2 n-6 | 1.67a | 1.64a | 0.00c | 0.17b | 0.00c | <0.0001 |
| 20:3 n-6 | 0.28a | 0.00c | 0.00c | 0.11b | 0.00c | <0.0001 |
| 20:4 n-6 | 0.00b | 0.91a | 0.96a | 0.00b | 0.67a | 0.008 |
| 20:5 n-3 | 0.13a | 0.00b | 0.15a | 0.00b | 0.14a | 0.032 |
| 22:4 n-6 | 0.47a | 0.19b | 0.55a | 0.00b | 0.00b | <0.0001 |
| 22:5 n-6 | 0.00 | 0.19 | 0.00 | 0.00 | 0.00 | 0.263 |
| 22:6 n-3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.479 |
| ΣSFa | 9.04 | 9.86 | 9.67 | 9.28 | 16.82a | 0.074 |
| ΣMUFa | 36.16a | 33.50b | 33.87b | 15.83d | 24.29c | <0.0001 |
| Σn-3 | 33.34c | 29.37c | 30.32c | 60.08a | 39.50b | <0.0001 |
| Σn-6 | 22.24c | 27.28a | 26.14b | 14.52e | 19.39d | <0.0001 |
| n-6:n-3 | 0.67c | 0.93a | 0.86b | 0.24e | 0.50d | <0.0001 |
| Σ Lipids | 38.90a | 9.90d | 24.60c | 42.00b | 2.72e | <0.0001 |
- CS, CMLF, CMHF, FS, and FSM represents Camelina seeds, Camelina meal low fat, Camelina meal high fat, flaxseed, and flaxseed meal. a–c Means within a row with no common superscript differ (p<0.05).
Antioxidant activity
Table 2 shows the ABTS, DPPH, FRAP, and ORAC values of CS, CMLF, CMHF, FS, and FSM. To extract antioxidant compounds with high or medium polarity, two different extracts, methanol:water for high and ethylacetate for medium polarity were prepared from the seed or meal. In addition, ethylacetate has significant selectivity in the extraction of low-molecular-weight phenolic compounds and high-molecular-weight polyphenols 14. Many of the antioxidants in oilseeds are not fat soluble as they occur in conjugation with sugar molecules. Therefore, the extraction of antioxidants with oil does not occur to any great extent because of partitioning and kinetic factors 40. In the current study, four antioxidant activity assays were performed on CS, CMLF, CMHF, FS, and FSM as antioxidants may respond in a different manner to different radical or oxidant sources. Besides, due to the diversity of antioxidant compounds in plant extracts, no single assay will accurately reflect all the antioxidant compounds present in them 41.
| Parameters | ABTS (mmol TE/g) | DPPH (mmol TE/g) | FRAP (mmol TE/g) | ORAC (µmol TE/g) | Phenolic compounds (µg/g)a) | Flavonoids (mg/g)b) |
|---|---|---|---|---|---|---|
| Methanolic extract | ||||||
| Camelina seeds | 1.94b | 1.75b | 0.06d | 3.5c | 3248.3b | 11.6c |
| Camelina meal low fat | 3.36a | 2.70a | 2.44a | 5.2c | 4591.8a | 23.0a |
| Camelina meal high fat | 1.84b | 1.42c | 1.94b | 32.0a | 3420.0b | 19.5b |
| Flaxseeds | 0.90c | 0.12e | 0.13d | 7.6b | 1538.7c | 1.7d |
| Flaxseed meal | 1.95b | 0.42d | 1.09c | 4.0c | 1728.1c | 2.8d |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Ethylacetate extract | ||||||
| Camelina seeds | 0.26b | 0.37a | 0.18b | 8.7b | 1086.8a | 1.5b |
| Camelina meal low fat | 0.38a | 0.29b | 0.23a | 4.2c | 743.5b | 2.5a |
| Camelina meal high fat | 0.26b | 0.24c | 0.18b | 20.4a | 773.3b | 2.5a |
| Flaxseeds | 0.06c | 0.06e | 0.04d | 3.7c | 252.3c | 0.0c |
| Flaxseed meal | 0.28b | 0.14d | 0.13c | 5.7bc | 291.9c | 0.2c |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
- TE, trolox equivalents.
- a–d Means within a row with no common superscript differ for each extracts (p<0.05).
- a) Gallic acid equivalents.
- b) Quercetin equivalents.
ABTS
Camelina meal low fat methanolic extract showed the highest ABTS value of all the meal and seed extracts and it was around 1.7–1.8-fold higher than the CS, CMHF, and FSM and 3.7-fold higher than the FS methanolic extracts. In the FSM, the concentration of antioxidant compounds after oil extraction can be clearly appreciated as FSM methanolic extract showed more than twofold the ABTS value of FS. However, this was not the case between CS and CMHF. Probably the use of high temperatures during the oil extraction and too long or inadequate storage caused the loss of antioxidant compounds. Also, CMHF showed the highest fat content of the meals which probably caused the use of its own antioxidants to prevent oxidation of the meal. In the case of the ethylacetate extracts, the same trend was observed as in the methanolic extracts but the ABTS values were around seven- to eightfold lower than the ones obtained for the methanolic extracts.
DPPH
Methanolic extracts DPPH antioxidant values of the seeds and meals showed similar trends as observed with the ABTS assay. However, DPPH values were low, especially for FS and FSM. Ethylacetate extracts showed a different trend in the DPPH assay. CS showed the highest value, followed by CMLF, CMHF, FSM, and FS. ABTS and DPPH assays use HAT (quench free radicals by hydrogen donation) and SET (transfer one electron to reduce, metals, carbonyls, and radicals) antioxidant mechanisms. The two mechanisms may occur at the same time but the one dominating will depend on the antioxidant structure, properties, and solubility of the compounds present in the extract as well as the partition coefficient and system solvent 41.
FRAP
Methanolic and ethylacetate extracts showed similar trends in which CMLF showed the highest FRAP value. Methanolic extract of CMHF was over tenfold higher than the ethylacetate extract of CMHF. The methanolic extracts of CS and FS showed the lowest FRAP value. This was not the case in the ethylacetate extracts of CS. FRAP uses a SET antioxidant mechanism and has been considered a reasonable screen for the ability to maintain redox status in cells or tissues. However, FRAP cannot detect compounds that act by radical quenching, especially thiols and proteins 41.
ORAC
This assay measures the protection supplied by an antioxidant to a target molecule that is being oxidized by peroxyl radicals. Fluorescein has been used as target molecule by many authors, who have estimated its decay in fluorescence by the peroxyl radicals 42. Alarcón et al. 32 developed an ORAC new method using PGR as a target molecule and then evaluating its decay in absorbance. This method was chosen because of the feasibility in our lab of measuring absorbance and not fluorescence.
Camelina meal high fat methanolic extract showed the highest ORAC value and it was around four- to eightfold higher than the rest of the extracts. A similar trend was observed for the ethylacetate extracts but in this case it was only two- to fivefold higher than the rest of the ethylacetate extracts. ORAC has been considered to report antioxidant activity of phenols in biological systems better than other methods as it uses biologically relevant free radicals and also because it integrates kinetics and degree of activity of antioxidants 42. However, it cannot be considered as a “total antioxidant activity assay” as it uses the HAT reaction mechanism, measuring only antioxidant activity against peroxyl radicals and not other relevant oxygen species such as O , HO., ONOO−, and singlet oxygen 43. According to our results, CMHF methanolic and ethylacetate extracts contained more antioxidant species capable of reacting with peroxyl radicals than the rest of the meals and seed extracts. This may be explained by the high content of tocopherols in CMHF. In our study, CMLF and CMHF extracts showed a higher content of phenolics and flavonoids as well as higher antioxidant activity than the seeds and FS extracts. A good correlation between phenolic compounds, flavonoids and antioxidant activity has been reported 44, 45. In general, the meals showed higher antioxidant activities, and higher phenolic, flavonoid, and tocopherol content than their respective seeds. This may be explained as an effect in the concentration of antioxidants after the oil extraction 14. When pressing the oil from seeds, part of the phenolic compounds is transferred to the oil. It is known that a higher content of phenolic compounds is released from the seeds when the oil is extracted at higher temperatures and higher pressure 46.
, HO., ONOO−, and singlet oxygen 43. According to our results, CMHF methanolic and ethylacetate extracts contained more antioxidant species capable of reacting with peroxyl radicals than the rest of the meals and seed extracts. This may be explained by the high content of tocopherols in CMHF. In our study, CMLF and CMHF extracts showed a higher content of phenolics and flavonoids as well as higher antioxidant activity than the seeds and FS extracts. A good correlation between phenolic compounds, flavonoids and antioxidant activity has been reported 44, 45. In general, the meals showed higher antioxidant activities, and higher phenolic, flavonoid, and tocopherol content than their respective seeds. This may be explained as an effect in the concentration of antioxidants after the oil extraction 14. When pressing the oil from seeds, part of the phenolic compounds is transferred to the oil. It is known that a higher content of phenolic compounds is released from the seeds when the oil is extracted at higher temperatures and higher pressure 46.
Phenolic compounds
Table 2 shows the phenolic content of CS, CMLF, CMHF, FS, and FSM. For the determination of phenolic compounds, the Folin–Ciocalteu method was adopted.
The content of phenolic compounds (µg/g seeds or meal) in the methanolic extract of seeds and meals was CMLF>CMHF>CS>FSM>FS. CS and meals showed more than twofold higher values of phenolic compounds than FS and its meal. In the ethylacetate extracts, the trend was somewhat different, CS>CMHF>CMLF>FSM>FS. In general, Camelina extracts showed higher phenolic content than FS extracts, which is in accordance with Wanasundara and Shahidi 20 who stated that, compared to other oilseeds, FS contains low levels of phenolic acids. On the other hand, some authors discussed that the Folin–Ciocalteu method does not quantify all phenolic constituents in the extracts and also there is the possibility of interferences from other compounds such as sugars or ascorbic acid 47.
Camelina meal low fat methanolic extract and ethylacetate extract had around 4592 and 743 µg phenolic compunds/g meal, respectively. These values are higher than the ones reported by Matthäus 14, who observed 1850 and 185 µg/g in 70% methanolic and ethylacetate extracts of CM, respectively. The differences may be explained as Matthäus 14 used two more solvents besides methanol and ethylacetate (water and acetone) to increase the selectivity of extraction, with the water extracts showing the highest content of phenolic compounds, 6001 µg/g. On the other hand, Salminen et al. 48 reported a higher value of phenolic compounds for CM, 6200 ± 490 µg/g. These authors found in CM phenolic compounds such as flavonols, flavanols, hydroxycinnamic acids and tocopherols. FS methanolic extract contained 1538.7 µg/ g seeds, a figure higher than that obtained by Kähkönen et al. 47 in an 80% methanol–water extract (800 µg/ g seeds). This difference may be explained due to variations in the extraction process (e.g., extraction time, composition extraction solvent, differences between cultivars, etc.).
In oilseeds, phenolic compounds are present as cinnamic acids, coumarins, flavonoid compounds, hydroxylated derivatives of nezoic acid, and lignins 49. FS is the richest plant source of the lignan secoisolariciresinol glucoside (SDG) which is a phenolic compound that has shown antioxidant properties 50, 51 and anticancer properties 52, 53. Harris and Haggerty 54 found that methanolic extracts of FS meal contained ferulic and chlorogenic acids that accounted for 84% of total phenolic acids.
Flavonoids
Table 2 shows the flavonoid content of methanolic and ethylacetate extracts of CS, CMLF, CMHF, FS, and FSM. CMLF methanolic extract showed the highest content of flavonoids, 23.0 mg/g, which is around eightfold higher than the values found in FSM and more than 2- and 13-fold higher than the values found in CS and FS, respectively. CMLF and CMHF ethylacetate extracts showed the same flavonoid content (2.5 mg/g), and which was more than tenfold higher than the one found for FSM.
Flavonoids were not detected in FS ethylacetate extract. It is probably that the method used was not sensitive enough to detect smaller amounts of flavonoids. Besides, flavonoids are known to be polar compounds, so lower contents of flavonoids in the ethylacetate extracts were expected.
Flaxseed methanolic extract contained 1.7 mg/g seeds which was higher than 0.35–0.71 mg/100 g reported by Oomah et al. 55. Ibrahim and Shaw 56 found flavone C- and O-glycosides as the major flavonoids present in seed cotyledons.
Tocopherols
Tocopherols are important biological antioxidants naturally present in foods. They have been strongly related with PUFA because they may counteract the potential oxidation caused by fats in the diet 57. Table 3 shows the α and γ-tocopherol content of CS, CMLF, CMHF, FS, and FSM. CMHF showed the highest content of α and γ-tocopherol, 7.2 and 338.0 µg/g, respectively, compared to the seeds and meals.
| Parameters | α-Tocopherol (µg/g) | γ-Tocopherol (µg/g) | TBARS (nmol MDA/g) | Glucosinolates (µmol/g)a) |
|---|---|---|---|---|
| Camelina seeds | 2.8b | 85.2b | 12.6a | 4.9b |
| Camelina meal low fat | 3.3b | 65.4c | 2.1 | 24.4a |
| Camelina meal high fat | 7.2a | 338.0a | 2.8 | 4.7b |
| Flaxseeds | 0.8c | 64.2c | 3.2 | 0.0 |
| Flaxseed meal | 7.0a | 6.7b | 1.1 | 0.0 |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
- MDA, malonaldehyde equivalents.
- a–c Means within a row with no common superscript differ (p<0.05).
- a) Sinigrin equivalents.
The level of antioxidants, especially tocopherols, occurring in different sources of vegetables oil is directly related to the degree of unsaturation of their fatty acids as reflected by the iodine value 40. CMHF showed more than two- to threefold higher content of α and γ-tocopherol compared to CS. CMHF showed higher γ-tocopherol content but lower α-tocopherol content than that reported by Salminen et al. 48. The CM used in the Salminen et al. 48 study contained fat content (23.5 ± 9.7%) similar to that of CMHF in this study. Meals with higher fat content showed a higher content of tocopherols, perhaps because of the lipophilic nature of these compounds.
TBARS
TBARS has been a very popular method extensively used to evaluate the antioxidant activity of a compound or group of compounds in lipid peroxidation systems 58. Evaluation of TBARS in CS, CMLF, CMHF, FS, and FSM was performed using the method of Hodges et al. 33 with slight modifications. This method was preferred as the traditional method does not take into account the interferences of sugars such as sucrose, glucose and fructose, and phenylpropanoid-type pigments such as flavonoid-like anthocyanins present in plant tissue which can contribute to the absorbance at 532 nm, resulting in an overestimation of malonaldehyde 33. Table 3 shows the TBARS content of CS, CMLF, CMHF, FS, and FSM. CS showed the highest value of TBARS compared with the meals and FS. This may be explained as CS contained fewer phenolic compounds, flavonoids, and tocopherols that could have prevented oxidation in the seed compared with the CMs. However, even FS contained a higher content of linolenic acid than CS, it showed a lower TBARS value than CS. Further research is needed to clarify this situation.
Glucosinolates
Glucosinolates are natural compounds widely spread in the Brassicaceae plant family that includes rapeseed, wintercress, C. sativa, brussels sprouts, radish, cabbage, broccoli, and cauliflower. Glucosinolate hydrolysis provides characteristic odors and flavors to these plants and may contribute to the prevention of diseases and protect plants against insects 59. Table 3 shows the glucosinolate content of Camelina and flax seeds and meals. CMLF showed the highest content of glucosinolates, 24.4 µmol/g. This is similar to the range reported by Matthäus and Zubr 60, between 14.5 and 23.4 µmol/g. CMHF showed lower glucosinolate content than CMLF, which may be explained due to differences in the oil extraction process, storage conditions and glucosinolate hydrolysis. Song and Thornalley 61 reported losses of total glucosinolates in Brassica vegetables after 7 days of storage from 11 to 27%. Besides, storage of vegetables at −85°C may cause significant loss of glucosinolates due to the freeze-thaw can fracture the vegetable cells providing accessability of myrosinase to glucosinolates with subsequent enzymatic conversion of glucosinolates to thiocyaniates during thawing. Also, shredding and steam boiling for 30 min of Brassica vegetables caused losses up to 60 and 58–77%, respectively. In our study, the oil extraction process and grinding of the meals as well as storage may have caused losses and differences between CMLF and CMHF glucosinolate content. The glucosinolate content in the seeds was 4.9 µmol/g. This value was lower compared to the values reported earlier by Schuster and Friedt 7, and Matthäus and Zubr 60. Glucosinolates were not detected in the FS and FSM.
The author has declared no conflict of interest.




